Abstract
Discrepancies between actigraphic and self-reported sleep measures are common. Studies of people with insomnia, in whom both sleep disturbances and discrepancy are common, suggest disturbances and discrepancy reflect differences in the sleeping brain’s activity measurable using spectral electroencephalogram (EEG). Disentangling effects of discrepancy and disturbance on sleep EEG could help target research on the consequences and treatments of different sleep phenotypes. We therefore categorized participants in a cohort study including 2,850 men (average age = 76 years, standard deviation = 5.5) into four groups using median splits on actigraphic and self-reported sleep efficiency (SE). We compared spectral power between these groups in 1-Hz bins up to 24 Hz. Compared with the concordant-high SE group: (a) the group with high actigraphic and low self-reported SE had higher spectral power from 11–15 Hz across the night; (b) both groups with low actigraphic SE had higher power across the 15–24 Hz range, predominantly in early cycles, and greater slow frequency power in later cycles. These findings suggest that perceived wakefulness undetected by actigraphy may result from or drive activity corresponding to spindles. We also found, consistent with hyperarousal models, that low SE detectable via actigraphy was related to higher frequency power in the beta range; actigraph-measured inefficiency was also associated with later slow oscillations, potentially representing attempts to dissipate homeostatic drive elevated from earlier hyperarousal. These distinct spectral EEG markers (of low SE measured with actigraphy vs. low perceived SE that is not captured by actigraphy) may have different causes or consequences.
Keywords: beta, hyper-arousal, misperception, sigma, sleep perception
1 |. INTRODUCTION
Sleep research commonly uses both self-report and actigraphy measures, owing to their relatively low costs and ability to estimate sleep parameters. However, discrepancies between these subjective and actigraphic sleep measures are common (Hughes et al., 2018; Van Den Berg et al., 2008). Compared with actigraphic measures, the majority of patients with insomnia disorder underestimate the amount of time they sleep (Te Lindert et al., 2019). The co-occurrence (or lack thereof) of self-report and actigraphic sleep disturbance measures may reflect different processes or phenotypes with different mechanisms and consequences. Sleep discrepancy in people with insomnia (i.e., where self-reported sleep time is lower than objectively measured sleep) is thought to reflect worry, brief awakenings and sleep being misinterpreted as wake (Harvey & Tang, 2012). Pre-sleep cognitive activity predicts underestimation of objectively recorded sleep measures (Herbert, Pratt, Emsley, & Kyle, 2017). However, the biological mechanisms underlying such sleep discrepancies are not fully understood (Rezaie, Fobian, McCall, & Khazaie, 2018).
Past studies have linked subjective–objective sleep discrepancies to differences in spectral electroencephalogram (EEG) activity. In a sample of 26 individuals, Perlis et al. (Perlis, Smith, Andrews, Orff, & Giles, 2001) reported that discrepancies in self-report and polysomnograpy measures of sleep duration positively correlated with greater beta EEG power. Kang et al. (2018) studied 36 people with major depressive disorder and found that discrepancy in sleep latency measured with self-report and polysomnography correlated with greater high-frequency EEG power in alpha, beta and sigma ranges. However, because discrepancies in sleep measures are more common in people with insomnia (Te Lindert et al., 2019), higher sigma and beta activity in these studies could be characteristics of prevalent sleep disturbances, discrepancy, or both. Indeed, without regard to sleep discrepancy, Spiegelhadler et al. compared 25 patients with primary insomnia with 29 controls with good sleep (Spiegelhalder et al., 2012); the findings showed that people with insomnia disorder had relatively greater non-rapid eye movement (NREM) power in sigma and beta bands, which were thought to reflect ongoing sleep-protective mechanisms and hyperarousal (respectively). Furthermore, although actigraphy is a common and reliable method for assessing sleep characteristics (Ancoli-Israel et al., 2015), it is not a direct measure of the brain’s (potentially sleeping) activity comparable with polysomnographic measures (Danzig, Wang, Shah, & Trotti, 2020). Thus, little evidence exists regarding what discrepancies between these two common methods for estimating sleep (self-report and actigraphy) reflect about the brain. Disentangling effects of discrepancies and disturbances (measured with these two methods) on brain function (measured using sleep EEG) would indicate what the perception of sleep disturbances, movement-related sleep disturbances and their combinations reflect mechanistically.
Few existing studies have compared spectral EEG signals in people with similar levels of sleep disturbances that differ in terms of discrepancy, and most studies have been small. Krystal, Edinger, Wohlgemuth, and Marsh (2002) compared patients with insomnia who had “subjective insomnia” (complaints and relatively long objectively measured sleep; n = 12) with insomnia patients who had both self-reported and polysomnography-recorded short sleep (n = 18). The findings showed that the “subjective only insomnia group” had relatively lower delta and increased alpha, sigma and beta power during non-rapid eye movement sleep. We are unaware of prior large-scale studies on this topic in samples that were not selected based on sleep characteristics. As such, how the activity of sleeping brains differs in phenotypes that have objective movement-based disturbances only, self-reported disturbances only, both, or neither remains unclear.
We therefore examined the spectral EEG correlates of sleep disturbances, and discrepancy between self-report and actigraphy, in a large community-based study of older men. Given the size of this study (n > 2,800), it was possible to compare groups with similar sleep characteristics that differed on whether self-report/actigraphy discrepancy was present. We focused on identifying the spectral EEG measures that correlate with sleep efficiency (SE) levels and discrepancies comparing actigraphy and self-report measures. SE captures both sleep onset latency and fragmentation and is expressed as the proportion of total time in bed spent asleep, thereby enabling measurement of overall sleep disturbances while standardizing for differences in sleep duration. Discrepancies between self-reported and actigraphic measures of SE are common (e.g., one study found half of older adults reported lower SE than was objectively measured) (Hughes et al., 2018). We chose to study these measurement modalities (self-report and actigraphy) because they are commonly used in sleep research (due to their ease and relatively low expense/subject burden) and therefore understanding what these measures signal (separately and together) about sleep is a priority. Thus, our overarching aim was to characterize the spectral EEG correlates of high and low SE with and without discrepancy between these common measurement modalities.
2 |. METHODS
2.1 |. Participants
Participants were 2,850 older men recruited from six study sites in the USA as part of the Osteoporotic Fractures in Men Sleep Study (MrOS Sleep; Birmingham, AL; Minneapolis, MN; Palo Alto, CA; the Monogohela Valley near Pittsburgh, PA; Portland, OR; and San Diego, CA). All participants provided informed consent under the Institution Review Boards of these research sites. To be eligible for the parent study, individuals had to be ≥65 years old, be able to walk without assistance, and be without bilateral hip replacement. For the MrOS Sleep Study, men were excluded if they used positive pressure or oral appliances regularly during sleep or used overnight nocturnal oxygen therapy. The analytic sample for this work was restricted to the 2,850 men in MrOS Sleep who contributed adequate data for spectral EEG measures.
2.2 |. Sleep efficiency and discrepancy group definitions
Participants completed self-reported and actigraphy measures designed to reflect usual sleep. Subjective SE was calculated from a widely used measure of subjective sleep disturbances and quality, the Pittsburgh Sleep Quality Index (PSQI), by dividing the response to the question “How many hours of actual sleep do you get at night? (This may be different than the number of hours you spend in bed)” by the total amount of time in bed (calculated as the difference between the time the participant reported getting into and out of bed). Participants were also asked to wear an actigraph on their non-dominate wrist for five consecutive days (mean recording length = 4.7 days, standard deviation = 0.8). Objective SE was calculated using actigraphy scoring algorithms described previously (Blackwell et al., 2005) as the percentage of time sleeping after “lights off”.
We dichotomized each SE measure at the median value and created four groups: (a) high objective and subjective SE (HighO-HighS); (b) high objective and low subjective SE (HighO-LowS); (c) low objective and high subjective SE (LowO-HighS); and (d) low objective and objective SE (LowO-LowS). We recognize the terms “low” and “high” are arbitrary and they are only used here for simplicity in referring to relative differences across the range of SE values observed in this population-based study. In contrast to this categorization approach, using a simple subtraction of subjective and objective measures could result in similar discrepancy levels despite different SE levels; for example, two people could both have a subjective–objective discrepancy subtraction of −25% SE while having very different actigraphic sleep efficiency (e.g., of 90% and 70%).
2.3 |. Spectral analysis
Within 30 days of the PSQI and actigraphy measurements, participants also underwent unattended polysomnography (Safiro, Compumedics, Inc.) in their own homes. Spectral analysis of the EEG data was performed using the SpectralTrainFig tool of the National Sleep Research Resource (sleepdata.org, https://github.com/nsrr/SpectralTrainFig). The tool has been described extensively previously (Mariani et al., 2018) and includes automated detection and removal of tall spikes on the power density spectra. For each lead, the absolute power in log10(μV^2/Hertz) was computed in 1-Hz frequency bins and time epochs of 30 s. To define sleep cycles, we employed a modified version of the rules detailed by Fenberg and Floyd (Feinberg & Floyd, 1979). We defined a sleep cycle as a period of at least 15 min of NREM sleep starting with stage N2 or N3 and terminated by the end of a period of REM sleep lasting at least 5 min for all cycles except cycle 1, or a period of wake or stage N1 lasting 15 min or longer. Two epochs of REM sleep separated by no more than 15 min of NREM sleep or wake were merged into a single REM period. For each frequency bin, power values corresponding to epochs in the same cycles were averaged together.
2.4 |. Covariates
We assessed whether any correlations between discrepancy and EEG spectral components were independent of demographic and health status covariates. These include age, race, educational attainment, weekly alcohol use, smoking status, body mass index, the apnea–hypopnea index (defined as the number of respiratory events with oxygen desaturation ≥3% per hr), self-reported health professional diagnoses of chronic diseases (including diabetes, hypertension and cardiovascular disease) and medication use (use of antidepressants, non-benzodiazepine non-barbiturate sedatives/hypnotics, or benzodiazepines).
2.5 |. Statistical modeling
We studied the association between spectral power and sleep discrepancy groups via linear random-intercept models, where the spectral power was the dependent variable and sleep efficiency and discrepancy groups were independent variables of interest. The reference group was the HighO-HighS group (i.e., the set of individuals in which SE estimated by both accelerometry and self-report was high). To normalize power, we standardized the log transformed power spectral density with a mean of 0 and a standard deviation of 1. We fit separate models for each frequency band and adjusted for study site, age, cycle, and the interaction effects between group and cycle. We estimated the cycle-specific differences in spectral power, comparing each group to the reference group, by summing the estimate of the main group effect and the interaction effect between group and cycle. Finally, to test whether there are indeed differences in the spectral power in a specific frequency band across groups, we applied Wald tests on the hypotheses of both the main group effect and interaction effect between group and cycle being zero. To account for multiple comparisons, we adjusted the Wald test p-values using the Benjamini & Hochberg method. Analyses were conducted in SAS 9.4 (SAS Institute) and R 3.5.3 (R Foundation for Statistical Computing).
3 |. RESULTS
3.1 |. Phenotype description
The categorization scheme (Figure 1) was chosen to assess effects of both SE level and discrepancy. The median subjective (PSQI) SE measure was 87.5 (interquartile range, 80–94; mean = 85, standard deviation = 12.4), whereas the median objective (actigraphy) SE measure was 81.1 (interquartile range, 73.2–86.4; mean = 78.2, standard deviation = 11.9). Stratifying by the median values of these SE measures produced groups that differed as expected: distributions of PSQI-measured SE closely overlapped in the LowO-HighS and HighO-HighS groups (restricted to the higher range) but these groups differed in terms of actigraph-derived SE (restricted to a lower range in the LowO-HighS group only); similarly, distributions of PSQI-measured SE closely overlapped for the LowO-LowS and HighO-LowS groups (in the lower range), but the HighO-LowS group also had actigraphy-derived SE values restricted to a higher range.
FIGURE 1.
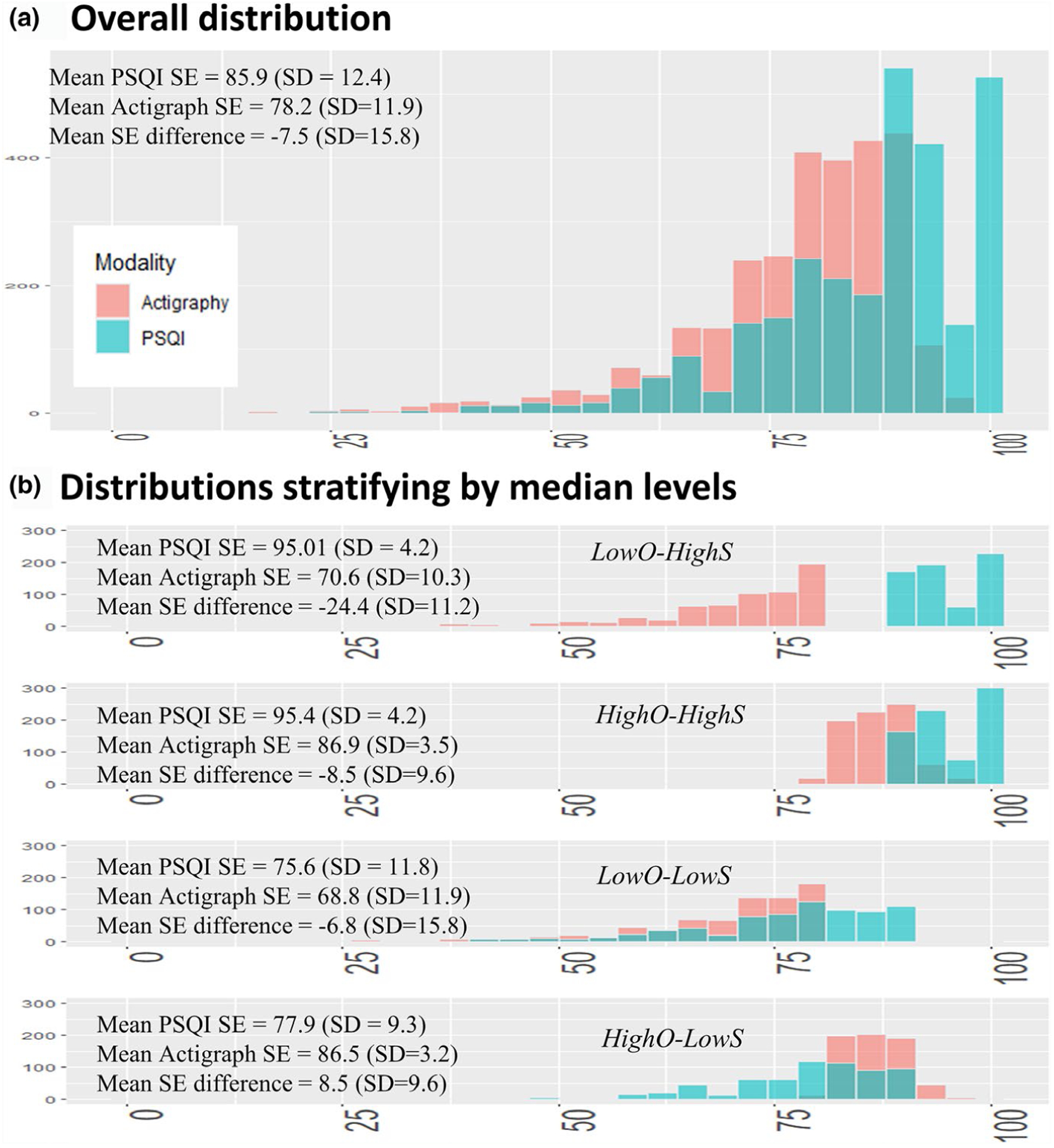
Descriptive information showing the four groups with distinct sleep efficiency (SE) and discrepancy values. As opposed to a simple subtraction of the two SE measures, stratifying by median values of SE measures and creating four groups isolates the effect of SE levels and discrepancy. PSQI, Pittsburgh Sleep Quality Index
Having low objective SE tended to be associated with being non-white, having less educational attainment, higher having body mass and apnea–hypopnea indices, and a greater prevalence of diabetes (Table S1). Prevalence rates of hypertension and cardiovascular disease were somewhat higher in groups in which either SE measures was low. The prevalence of sleep medications was higher in the groups that had subjectively low SE.
3.2 |. Comparison of HighO-HighS with HighO-LowS
These groups were similar on the objective measure; therefore, this contrast reflects the effects of having low PSQI SE and/or discrepancy where people reported low SE that was not captured on the actigraph. We found differences in spectral power between these groups in the 11–15 Hz range (Figure 1, left). As illustrated in the heatmap (Figure 1, right), among men with high objective SE, the presence of low subjective SE was associated with greater power in the band from 11 to 15 Hz (most strongly at around 13 Hz). These associations were numerically consistent across the four NREM cycles examined and there was no significant interaction between the effect of HighO-HighS versus HighO-LowS group and NREM cycle (e.g., at 13 Hz p = .44).
3.3 |. Comparison of HighO-HighS with LowO-HighS
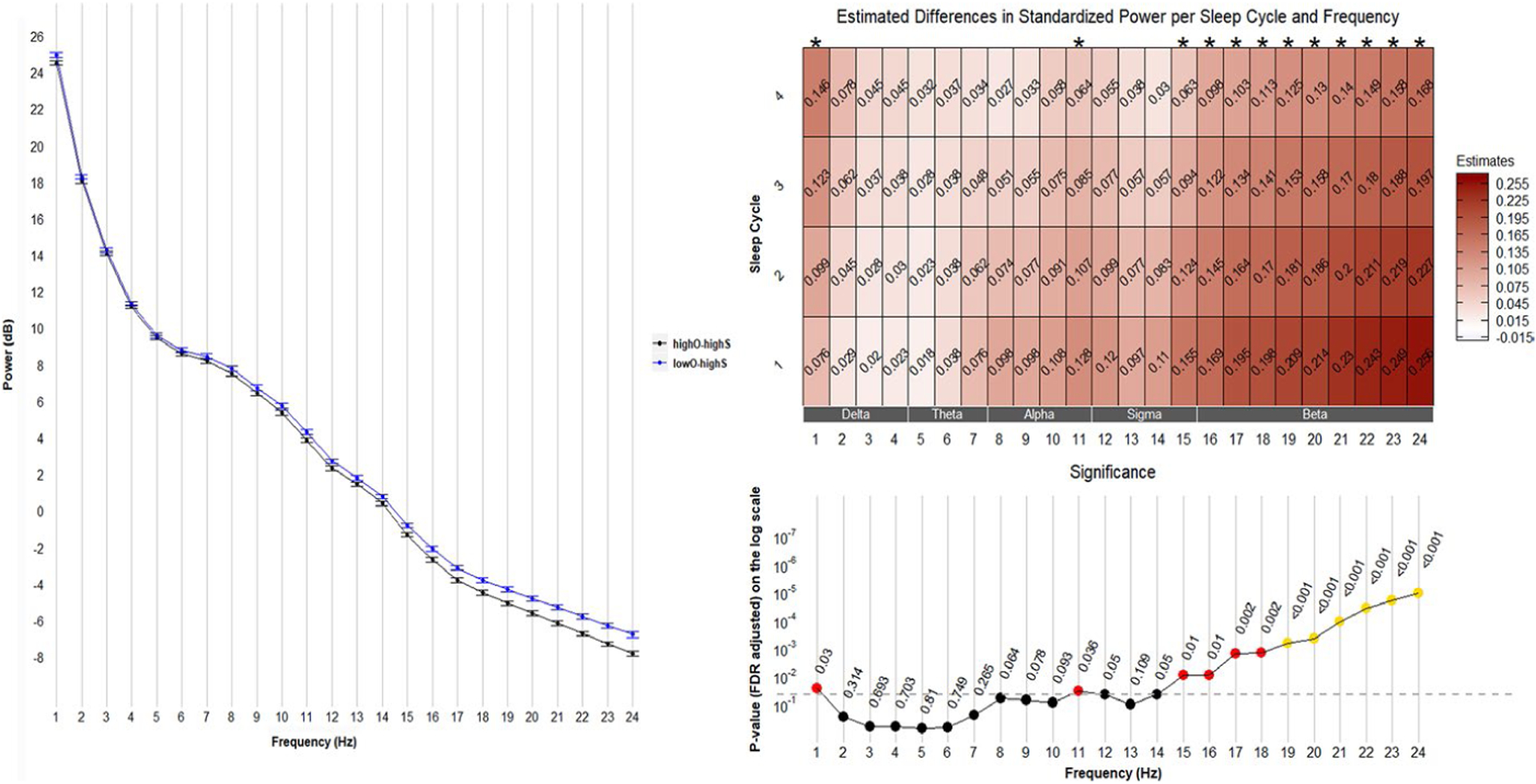
Because these groups were similar on the subjective measure, this contrast reflects the effect of having low actigraphy SE and/or discrepancy where people reported relatively high SE but actigraphy found it to be relatively low. Spectral power differed in these groups in the 15–24 Hz range (Figure 2, left). Among men with high subjective SE, the presence of low objective SE was most robustly associated with greater power, predominately from 15–24 Hz (Figure 2, right). Heat maps illustrate that these effects were numerically larger in earlier NREM cycles and there was also some statistical evidence that the effect of HighO-HighS versus LowO-HighS became smaller in successive NREM cycles (e.g., effect by cycle interaction at 22 Hz: β = −0.03, p = .05).
FIGURE 2.
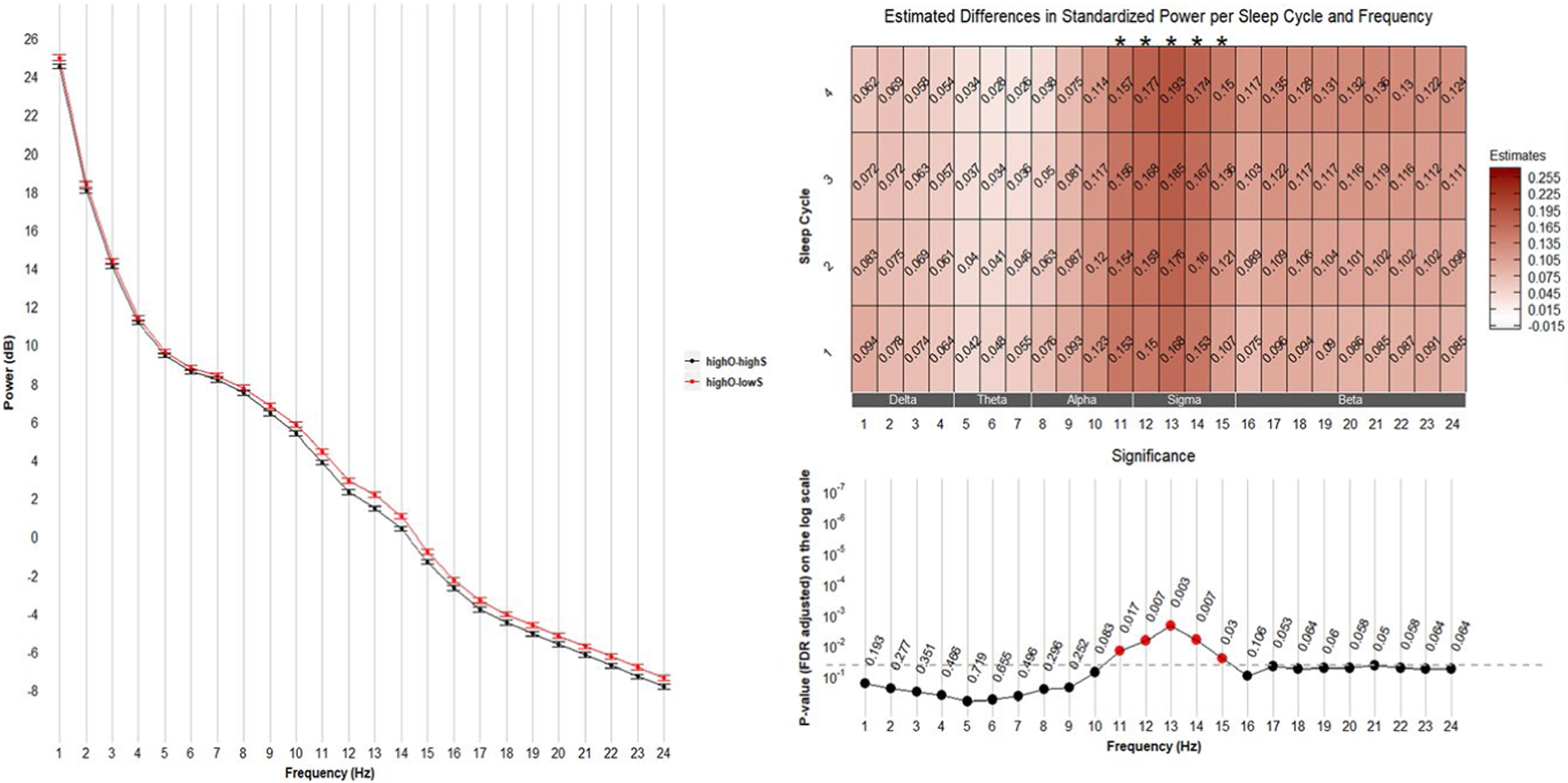
Comparisons between HighO-LowS and HighO-HighS groups. Left: mean (± standard error) of power averaged over four sleep cycles. Right: frequency-specific and sleep cycle-specific differences in standardized power comparing HighO-LowS with HighO-HighS. p-values were adjusted for multiple comparisons (72 comparisons from 24 frequency bins and 3 sleep efficiency groups) using the Benjamini & Hochberg method. Positive estimates indicate higher power in the HighO-LowS group than in the HighO-HighS group. FDR, False Discovery Rate
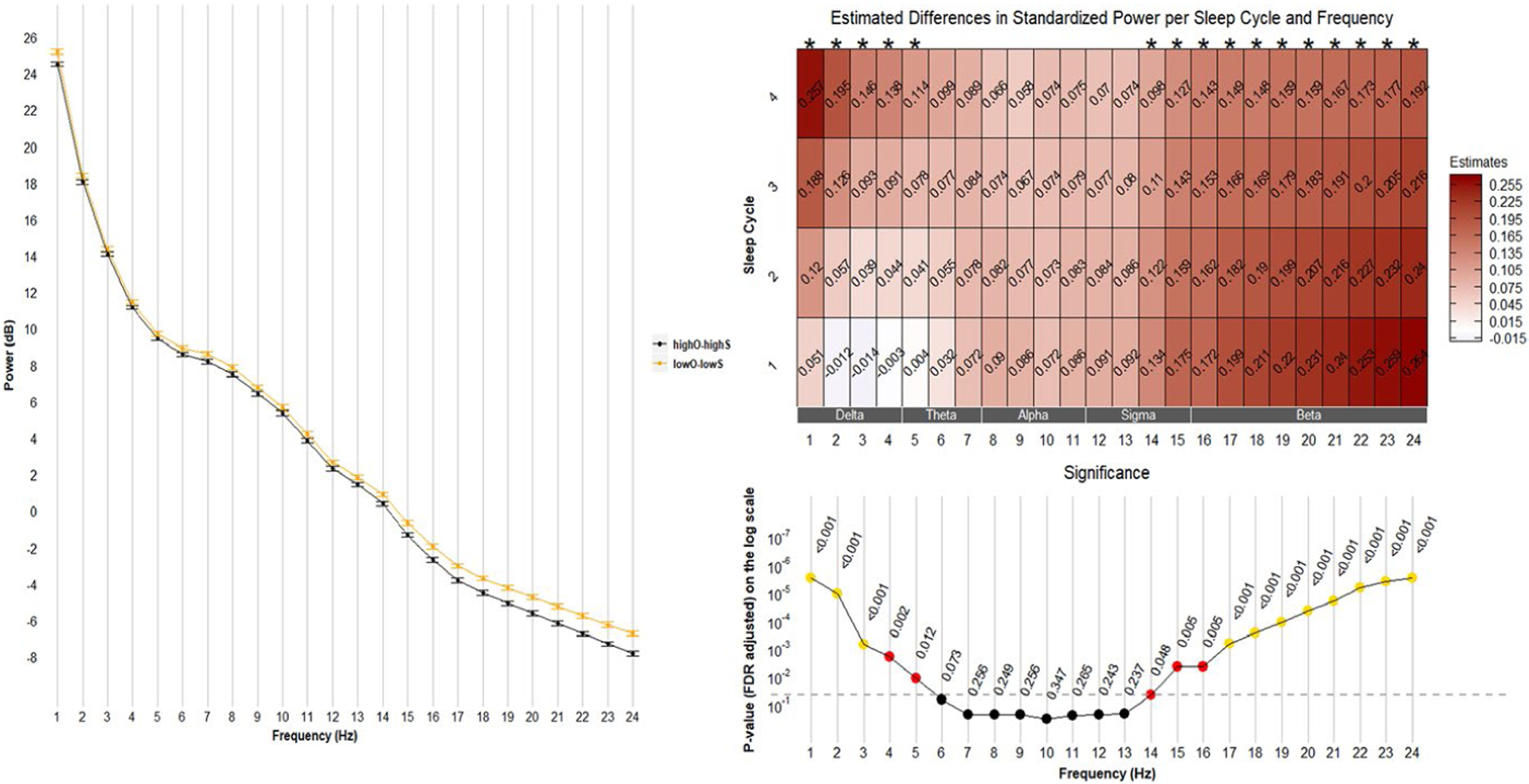
3.4 |. Comparison of HighO-HighS with LowO-LowS
This contrast reflects differences in SE levels among people with generally concordant subjective and objective measures. Spectral power differed in these groups in the 15–24 Hz range (Figure 3, left). Heat maps (Figure 3, right) illustrate that the group with concordant-low SE had markedly higher power around the 15–24 Hz range; again, these effect sizes were numerically larger in earlier REM cycles and there was some statistical evidence that the effect of HighO-HighS versus LowO-LowS became smaller in successive NREM cycles (e.g., effect by cycle interaction at 22 Hz: β = −0.03, p = .08). In addition, compared with men who had concordant-high SE, men with concordant-low SE had higher power around the 1–3 Hz range; these effects appeared larger in the later NREM cycles (effect by cycle interaction at 1 Hz: β = 0.06, p < .0001).
FIGURE 3.
Comparisons between LowO-HighS and HighO-HighS groups. Left: mean (± standard error) of power averaged over four sleep cycles. Right: frequency-specific and sleep cycle-specific differences in standardized power comparing LowO-HighS with HighO-HighS. p-values were adjusted for multiple comparisons (72 comparisons from 24 frequency bins and 3 sleep efficiency groups) using the Benjamini & Hochberg method. Positive estimates indicate higher power in the LowO-HighS group than in the HighO-HighS group. FDR, False Discovery Rate
3.5 |. Additional modelling
We observed that the HighO-LowS group had higher power than the HighO-HighS group in the range corresponding to the traditional sigma power band. The other groups with LowS did not have differences in the sigma range, suggesting this was an effect specific to the combination of HighO-LowS. We performed additional analyses to confirm whether the statistical effect of HighO-LowS was due to having low PSQI sleep efficiency or, indeed, measurement discrepancy. We modelled the NREM-1 sigma power (12.25–15 Hz) as predicted by PSQI SE category (above or below the median), actigraphy SE category (above or below the median), and their interaction; this model was fully adjusted for study site and covariates listed above and in the Supplemental Table. This model confirmed a statistically significant interaction of these SE measures on sigma power (p = .046). To illustrate these findings (Figure 4), we stratified base models by actigraphy SE and PSQI SE, and show predicted means and 95% confidence intervals for sigma. In a model restricted to people with low actigraphy SE, low PSQI versus high had no statistically significant effect (β = 0.003, standard error = 0.01, p = .80). In contrast, in a model restricted to people with high actigraphy SE, we observed the statistical effect of having low PSQI (vs. high, reference) on sigma power (β = 0.04, standard error = 0.01, p = .003). There were no statistically significant interactions between PSQI and actigraphy SE for the effect of low (vs. high) actigraphy SE on NREM cycle 1 beta or NREM cycle 3 slow oscillation power (interaction p-values were, respectively, .32 and .46). These same findings were observed when excluding sleep medication users as well as when excluding the 1.5% of the sample who contributed <3 days of actigraphy data.
FIGURE 4.
Comparisons between LowO-LowS and HighO-HighS groups. Left: mean (± standard error) of power averaged over four sleep cycles. Right: frequency-specific and sleep cycle-specific differences in standardized power comparing LowO-LowS with HighO-HighS. p-values were adjusted for multiple comparisons (72 comparisons from 24 frequency bins and 3 sleep efficiency groups) using the Benjamini & Hochberg method. Positive estimates indicate higher power in the LowO-LowS group than in the HighO-HighS group. FDR, False Discovery Rate
4 |. DISCUSSION
In this large community-based sample of older men, we identified spectral EEG characteristics associated with: (a) low actigraphy-measured SE (in both LowO-LowS and LowO-HighS groups); and (b) having discrepancy in SE where participants reported low SE despite normative actigraphy SE (HighO-LowS vs. HighO-HighS). Specifically, compared to the HighO-HighS group, both groups with low SE that was objectively detectable on actigraphy (LowO regardless of discrepancy) had greater power in the higher frequency range, roughly corresponding to beta power (16–24 Hz), suggesting an effect of low actigraphy SE rather than discrepancy. We also found greater slow oscillation power when objectively measured SE was lower; this effect size was strongest in later sleep cycles in the concordant-low SE group (LowO-LowS). Finally, we found that compared with the HighO-HighS group, men who reported low SE that was not detected by wrist actigraphy (HighO-LowS) had greater EEG power around 13 Hz. This effect (of HighO-LowS) appeared to be specific to this type of sleep discrepancy: the other group with low PSQI SE (LowO-LowS) did not have relatively higher power around the sigma range, and the effect was only apparent when actigraphy SE was high but PSQI SE was low (see Figure 5). As discussed below, these findings confirm and extend prior work while also highlighting unanswered questions regarding the functional neurobiology of sleep.
FIGURE 5.
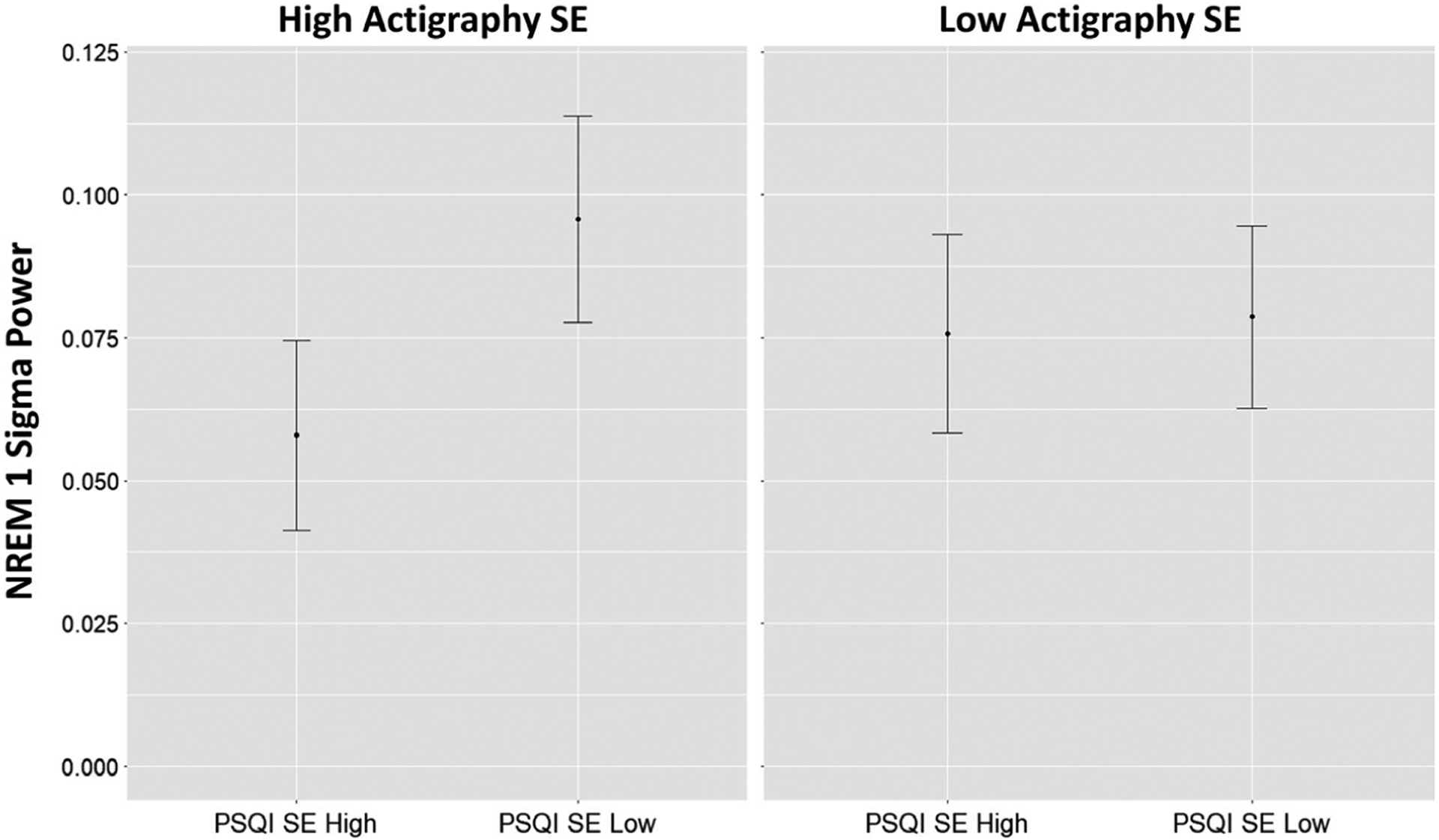
Predicted means illustrating the interaction detected. Having low Pittsburgh Sleep Quality Index (PSQI) sleep efficiency (SE) was associated with higher sigma power only when actigraphy SE was high (interaction p-value = .04). NREM, non-rapid eye movement
Our main finding related to sleep discrepancy was that men who perceived low SE that was not detected by actigraphy (HighO-LowS) had greater power around the sigma range. Again, this did not appear to be an effect of low subjective SE, because it was only observed when PSQI SE was low and actigraphy SE was high. This is consistent with previous work reporting that higher sigma power is related to “paradoxical insomnia” (Krystal et al., 2002). Sigma power may correspond to k-complexes and subsequent sleep spindles, which are thought to help maintain sleep (Dang-Vu, McKinney, Buxton, Solet, & Ellenbogen, 2010) and/or have a role in consolidating memories (Holz et al., 2012). Correspondingly, higher levels of sigma power may reflect a compensatory function to maintain sleep in spite of unmeasured arousing stimuli (either from the environment or an aspect of brain arousal not captured on EEG).
We also found that higher-frequency EEG activity was present when men had actigraphy-measured low SE (LowS), particularly in early NREM cycles. This did not appear to be an effect of discrepancy between measures, but rather an effect of having low actigraph-measured SE. Past studies found that people with insomnia tend to have more high-frequency electric activity (e.g., in the beta range; Buysse et al., 2008; Perlis et al., 2001; Spiegelhalder et al., 2012), suggesting heightened cortical arousal related to sleep disturbance (supporting hyperarousal models of insomnia) (Perlis, Giles, Mendelson, Bootzin, & Wyatt, 1997; Riemann et al., 2010). Our findings show that persistent activity in these higher frequency ranges may be due to arousals linked with detectable movement. Furthermore, low actigraphy was associated with greater slow-wave power, markedly in later sleep cycles. Potentially, hyperarousal and objectively measurable sleep disturbances lead to greater accumulation of, or more persistent, homeostatic drive that requires more ongoing slow waves to dissipate it.
Several limitations of this work should be noted. First, we are unable to test the mechanisms underlying these associations. As discussed above, findings related to slow-wave, sigma and beta activity can be interpreted in the context of past literature and current models, but we did not directly measure the level of cognitive arousal, spindles around 13 Hz or homeostatic drive. Second, our measure of sleep (SE) was chosen because it provides an overall integration of sleep onset and continuity disturbances, and because both self-report and actigraphic estimates of SE were available. Future work is required to isolate how these spectral-, movement- and perception-based features of sleep interrelate with specific types of sleep disturbances. In addition, although the sample was large and based in several locations in the USA, it only included men and was mostly composed of white individuals; these findings will not necessarily generalize to other groups and life stages. Finally, note that the PSQI and actigraphy were not reflecting the same night. Although PSQI and actigraphy are both designed to reflect “usual” sleep habits, the effect sizes (of discrepancy) we observed may be diminished by the requirement to have these measures within 30 days (rather than on the same single night).
Nevertheless, this work contributes to the existing literature by beginning to specify neurophysiological correlates of sleep perceptions and sleep disturbances. Low objective SE appears to be associated with higher levels of power in the range thought to reflect cortical hyperarousal, and when this low SE is accurately perceived, higher late-night slow waves may ensue in an attempt to dissipate sleep drive. In contrast, when low SE is perceived but not captured by actigraphy, it may reflect processes associated with spindle activity. Because this sample was not selected on the basis of a sleep disorder, these may be general phenomena (not necessarily specific to insomnia disorders).
Future research is needed to clarify the basic mechanisms underlying low perceived and measured SE. Future studies are needed to test: (a) whether high-frequency activation reflects cognitive arousal, which, when perceived, leads to perpetuation of slow-wave activity throughout the night; and (b) whether, and in what cases, k-complexes and spindles are perceived as wake (or perceptions of wakefulness may trigger spindles). Pending replication and further mechanistic evidence, these convenient measurement modalities (actigraphy and self-report) could be used to extrapolate information on brain functioning during sleep. At a minimum, our current findings indicate that research aimed at understanding the mechanisms of sleep perception (e.g., Gabryelska et al., 2019; Kaplan et al., 2017)) should use several measures of sleep, including subjective and objective measurements. Future work is also needed to determine whether these composite phenotypes, and their functional bases, have particular consequences on brain health throughout the lifespan.
Supplementary Material
Funding information
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160 and UL1 TR000128. The National Heart, Lung, and Blood Institute (NHLBI) provides funding for the MrOS Sleep ancillary study “Outcomes of Sleep Disorders in Older Men” under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839. SFS is supported by K01MH112683.
Footnotes
CONFLIC T OF INTEREST
The authors have no conflicts of interest to declare.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- Ancoli-Israel S, Martin JL, Blackwell T, Buenaver L, Liu L, Meltzer LJ, … Taylor DJ (2015). The SBSM guide to actigraphy monitoring: Clinical and research applications. Behavioral Sleep Medicine, 13(Suppl 1), S4–S38. 10.1080/15402002.2015.1046356 [DOI] [PubMed] [Google Scholar]
- Blackwell T, Ancoli-Israel S, Gehrman PR, Schneider JL, Pedula KL, & Stone KL (2005). Actigraphy scoring reliability in the study of osteoporotic fractures. Sleep, 28(12), 1599–1605. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Germain A, Hall ML, Moul DE, Nofzinger EA, Begley A, … Kupfer DJ (2008). EEG spectral analysis in primary insomnia: NREM period effects and sex differences. Sleep, 31(12), 1673–1682. 10.1093/sleep/31.12.1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang-Vu TT, McKinney SM, Buxton OM, Solet JM, & Ellenbogen JM (2010). Spontaneous brain rhythms predict sleep stability in the face of noise. Current Biology, 20(15), R626–R627. 10.1016/j.cub.2010.06.032 [DOI] [PubMed] [Google Scholar]
- Danzig R, Wang M, Shah A, & Trotti LM (2020). The wrist is not the brain: Estimation of sleep by clinical and consumer wearable actigraphy devices is impacted by multiple patient- and device-specific factors. Journal of Sleep Research, 29(1), e12926. 10.1111/jsr.12926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg I, & Floyd TC (1979). Systematic trends across the night in human sleep cycles. Psychophysiology, 16(3), 283–291. 10.1111/j.1469-8986.1979.tb02991.x [DOI] [PubMed] [Google Scholar]
- Gabryelska A, Feige B, Riemann D, Spiegelhalder K, Johann A, Bialasiewicz P, & Hertenstein E (2019). Can spectral power predict subjective sleep quality in healthy individuals? Journal of Sleep Research, 28(6), e12848. 10.1111/jsr.12848 [DOI] [PubMed] [Google Scholar]
- Harvey AG, & Tang NKY (2012). (Mis)perception of sleep in insomnia: A puzzle and a resolution. Psychological Bulletin, 138(1), 77–101. 10.1037/a0025730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert V, Pratt D, Emsley R, & Kyle SD (2017). Predictors of nightly subjective-objective sleep discrepancy in poor sleepers over a seven-day period. Brain Sciences, 7(3), 29. 10.3390/brainsci7030029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz J, Piosczyk H, Feige B, Spiegelhalder K, Baglioni C, Riemann D, & Nissen C (2012). EEG sigma and slow-wave activity during NREM sleep correlate with overnight declarative and procedural memory consolidation. Journal of Sleep Research, 21(6), 612–619. 10.1111/j.1365-2869.2012.01017.x [DOI] [PubMed] [Google Scholar]
- Hughes JM, Song Y, Fung CH, Dzierzewski JM, Mitchell MN, Jouldjian S, … Martin JL (2018). Measuring sleep in vulnerable older adults: A comparison of subjective and objective sleep measures. Clinical Gerontologist, 41(2), 145–157. 10.1080/07317115.2017.1408734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SG, Mariani S, Marvin SA, Ko KP, Redline S, & Winkelman JW (2018). Sleep EEG spectral power is correlated with subjective-objective discrepancy of sleep onset latency in major depressive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 85, 122–127. 10.1016/j.pnpbp.2018.04.010 [DOI] [PubMed] [Google Scholar]
- Kaplan KA, Hirshman J, Hernandez B, Stefanick ML, Hoffman AR, Redline S, … Zeitzer JM (2017). When a gold standard isn’t so golden: Lack of prediction of subjective sleep quality from sleep polysomnography. Biological Psychology, 123, 37–46. 10.1016/j.biopsycho.2016.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal AD, Edinger JD, Wohlgemuth WK, & Marsh GR (2002). NREM sleep EEG frequency spectral correlates of sleep complaints in primary insomnia subtypes. Sleep, 25(6), 630–640. [PubMed] [Google Scholar]
- Mariani S, Tarokh L, Djonlagic I, Cade BE, Morrical MG, Yaffe K, … Aeschbach D (2018). Evaluation of an automated pipeline for large-scale EEG spectral analysis: The National Sleep Research Resource. Sleep Medicine, 47, 126–136. 10.1016/j.sleep.2017.11.1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis ML, Giles DE, Mendelson WB, Bootzin RR, & Wyatt JK (1997). Psychophysiological insomnia: The behavioural model and a neurocognitive perspective. Journal of Sleep Research, 6(3), 179–188. 10.1046/j.1365-2869.1997.00045.x [DOI] [PubMed] [Google Scholar]
- Perlis ML, Smith MT, Andrews PJ, Orff H, & Giles DE (2001). Beta/Gamma EEG activity in patients with primary and secondary insomnia and good sleeper controls. Sleep, 24(1), 110–117. [DOI] [PubMed] [Google Scholar]
- Rezaie L, Fobian AD, McCall WV, & Khazaie H (2018). Paradoxical insomnia and subjective-objective sleep discrepancy: A review. Sleep Medicine Reviews, 40, 196–202. 10.1016/j.smrv.2018.01.002 [DOI] [PubMed] [Google Scholar]
- Riemann D, Spiegelhalder K, Feige B, Voderholzer U, Berger M, Perlis M, & Nissen C (2010). The hyperarousal model of insomnia: A review of the concept and its evidence. Sleep Medicine Reviews, 14(1), 19–31. 10.1016/j.smrv.2009.04.002 [DOI] [PubMed] [Google Scholar]
- Spiegelhalder K, Regen W, Feige B, Holz J, Piosczyk H, Baglioni C, … Nissen C (2012). Increased EEG sigma and beta power during NREM sleep in primary insomnia. Biological Psychology, 91(3), 329–333. 10.1016/j.biopsycho.2012.08.009 [DOI] [PubMed] [Google Scholar]
- Te Lindert BHW, Blanken TF, van der Meijden WP, Dekker K, Wassing R, van der Werf YD, … Van Someren EJW (2019). Actigraphic multi-night home-recorded sleep estimates reveal three types of sleep misperception in insomnia disorder and good sleepers. Journal of Sleep Research, 29, e12937, 10.1111/jsr.12937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Berg JF, Van Rooij FJ, Vos H, Tulen JH, Hofman A, Miedema HM, … Tiemeier H (2008). Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons. Journal of Sleep Research, 17(3), 295–302. 10.1111/j.1365-2869.2008.00638.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


