Abstract
Recent studies have described conjunctivitis in approximately 1% of COVID-19 patients and speculated that SARS-CoV‑2 can be transmitted via the conjunctiva. In this article we recapitulate the molecular mechanisms of host cell entry of SARS-CoV‑2 and discuss the current evidence for a potential conjunctival transmission of SARS-CoV‑2. The current body of evidence indicates that SARS-CoV‑2 requires the membrane-bound angiotensin-converting enzyme 2 (ACE2) and the membrane-bound serine protease TMPRSS2 to enter cells. Recent studies suggest that COVID-19 patients rarely exhibit viral RNA in tear film and conjunctival smears and that, ACE2 and TMPRSS2 are only expressed in small amounts in the conjunctiva, making conjunctival infection with SARS-CoV‑2 via these mediators unlikely. Nevertheless, we consider the current evidence to be still too limited to provide a conclusive statement and recommend appropriate protective measures for healthcare personnel who are in close contact with suspected and confirmed COVID-19 patients.
Keywords: SARS-CoV‑2, COVID-19, ACE2, TMPRSS2, Conjunctiva
Abstract
Aktuelle Studien haben bei ca. 1 % aller COVID-19-Patienten eine Bindehautentzündung beschrieben und spekuliert, dass SARS-CoV‑2 über die Bindehaut übertragen werden kann. In der vorliegenden Arbeit rekapitulieren wir die molekularen Mechanismen des Eintritts von SARS-CoV‑2 in die Wirtszelle und diskutieren die aktuelle Studienlage zu einer möglichen konjunktivalen Transmission. Derzeit geht man davon aus, dass SARS-CoV‑2 das membrangebundene Angiotensin-konvertierende Enzym 2 (ACE2) sowie die Membran-gebundene Serinprotease TMPRSS2 benötigt, um in die Wirtszelle einzudringen. Aktuelle Studien weisen darauf hin, dass COVID-19-Patienten nur selten Virus-RNA im Tränenfilm und Bindehautabstrichen aufweisen und dass ACE2 und TMPRSS2 in der Bindehaut nur in sehr geringen Mengen gebildet werden, was eine konjunktivale Infektion durch SARS-CoV‑2 über diese Mediatoren wenig wahrscheinlich macht. Dennoch halten wir die derzeitige Studienlage für zu begrenzt, um eine abschließende Aussage treffen zu können, und empfehlen konsequente und adäquate Schutzmaßnahmen für medizinisches Personal, das in engem Kontakt mit verdächtigen und bestätigten COVID-19-Patienten steht.
Schlüsselwörter: SARS-CoV‑2, COVID-19, ACE2, TMPRSS2, Bindehaut
The WHO declared the outbreak of COVID-19 a health emergency of international concern. COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and is associated with symptoms such as fever, cough, impaired taste, fatigue, and severe pneumonia [19]. SARS-CoV‑2 is highly infectious and primarily transmitted via inhalation of aerosol droplets released by an infected individual, as well as possibly via the feco-oral route [6]. Potential conjunctival transmission of SARS-CoV‑2 has not been conclusively elucidated and would have a significant impact on public health. For example, a handful of studies postulate that SARS-CoV‑2 can be transmitted via the mucous membranes, including the conjunctiva [2, 3], and that all ophthalmologists are at increased risk and should therefore wear protective eyewear when examining suspected cases [14].
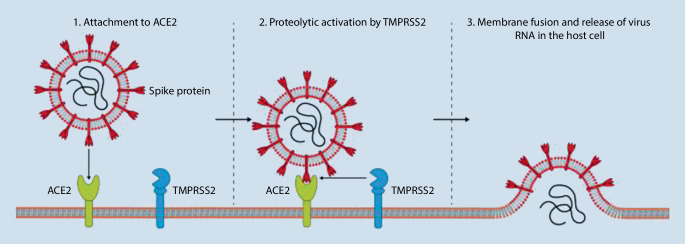
Route of infection and replication of SARS-CoV-2
Like SARS-CoV, SARS-CoV‑2 uses the membrane-bound angiotensin-converting enzyme 2 (ACE2) and the membrane-bound serine protease TMPRSS2 to enter the host cell [7]. During this process, SARS-CoV‑2 binds to ACE2 with its spike (S) glycoprotein and is able to fuse with the host membrane via TMPRSS2-mediated proteolytic activation (Fig. 1; [7, 16]). Following membrane fusion, the RNA of the virus is released, replicated, and transcribed into virus-specific proteins on the ribosomes of the host cell. It is assumed that SARS-CoV‑2, like SARS-CoV, is absorbed into the endoplasmic reticulum and can leave the host cell via exocytosis. Since the outbreak of COVID-19, a number of studies have investigated the expression of ACE2 in a variety of human tissues and, in addition to expression in lung tissue, demonstrated marked expression of the receptor in stomach, colon, liver, and kidney tissue [15, 23]. This highlights the susceptibility of a wide variety of tissues to SARS-CoV‑2 infection and explains the clinical observation of possible multiple organ involvement in SARS-CoV‑2 infection [19]. It has not yet been definitively elucidated whether ocular surface cells express ACE2 or TMPRSS2 and are therefore susceptible to SARS-CoV‑2 infection.
Fig. 1.
The molecular mechanisms and receptors of SARS-CoV‑2 cell entry. (Based on [8, 9]).
Possibilities of SARS-CoV-2 infection via the ocular surface
There are three fundamental questions related to the possibility of virus transmission via the ocular surface.
Can SARS-CoV-2 infect the conjunctiva and replicate there?
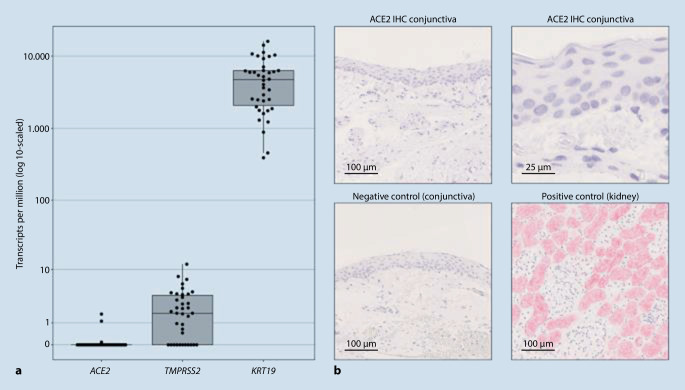
Recent studies describe subjective ocular symptoms in 7% of all COVID-19 patients [22] and signs of conjunctivitis in approximately 1% [5]. However, these observations were only rarely based on ophthalmological examinations and the inclusion criteria were heterogeneous [10]. In addition, the cited studies did not investigate adequate control cohorts and it is unclear whether the symptoms were caused by SARS-CoV‑2 or whether they were an epiphenomenon unrelated to SARS-CoV‑2, e.g., resulting from intensive care treatment. There is also controversy as to whether ocular surface cells, e.g., conjunctival epithelial cells, express ACE2 or TMPRSS2 and are therefore susceptible to SARS-CoV‑2 infection. In light of the lack of data, we recently investigated the expression levels of ACE2 and TMPRSS2 in 38 healthy and diseased conjunctival samples [11]. To this end, RNA was isolated from formalin-fixed paraffin-embedded conjunctival samples as previously described, sequenced [12, 17], and the sequencing data were then bioinformatically analyzed [1]. Whereas the conjunctival samples exhibited significant mRNA expression of the epithelial marker keratin 19, healthy and diseased conjunctival samples showed no relevant expression of the SARS-CoV‑2 receptor ACE2 (Fig. 2a). Consistent with the virtually undetectable expression of ACE2 at the transcriptional level, we also found negligible ACE2 immunoreactivity in eight healthy conjunctival samples [11], suggesting the absence of relevant ACE2 protein expression in the conjunctiva (Fig. 2b). Furthermore, our data show that the serine protease TMPRSS2 in conjunctival tissue is also not significantly transcribed (Fig. 2a). These results argue against an ACE2-mediated conjunctival route of infection for SARS-CoV‑2 and are in line with histological investigations conducted on patients having died from COVID-19 that did not detect relevant conjunctivitis [13]. Therefore, SARS-CoV‑2 appears to differ from other viruses, such as hepatitis C virus, for which conjunctival infection and transmission are described [8]. Although evidence is lacking to date, it remains to be elucidated whether individual factors such as hypoxia or smoking can trigger ACE2 expression in the conjunctiva [21]. A recent investigation using an ex-vivo model reported the possibility of cell infection, particularly conjunctival stromal cells, by SARS-CoV‑2 [9]. However, it remains unclear to what extent this observation can be extrapolated to the in vivo situation. In addition, more aggressive pretreatment of histological conjunctival specimens appears to induce ACE2 immunoreactivity of the conjunctival epithelium; however, the clinical relevance of this is unclear [4]. Other investigations, e.g., on autopsy material from COVID-19-deceased patients, are needed in order to gain insight into actual infectivity and possible sites of virus replication.
Fig. 2.
Expression of the SARS-CoV‑2 receptor ACE2 and the proteinase TMPRSS2 in the human conjunctiva. a The box plot shows low mRNA expression levels for ACE2 and TMPRSS2 compared to the conjunctival marker keratin 19 in 38 analyzed conjunctival samples. Each dot represents one sample. b Representative immunohistochemical images of ACE2 staining of conjunctival and kidney tissue. While kidney tissue shows strong ACE2 staining, healthy conjunctival samples (n = 8) show negligible immunoreactivity. For the negative control, the primary antibody was omitted. (Figure modified from [12])
Can healthy individuals become infected via the tear film?
There are currently no data on SARS-CoV‑2 infection of healthy individuals via the tear film. While some data (see above) suggest that SARS-CoV‑2 does not infect the conjunctiva, viruses in the tear film could gain access to the nasal mucosa and respiratory tract via the lacrimal drainage system, thereby triggering infection of respiratory epithelial cells. It is currently unclear to what extent rubbing the eyes with contaminated hands can cause infection via the tear film. One can only speculate at present on the importance of virus uptake via the tear ducts compared to direct uptake of virus-containing aerosols via the airways. Due to the protective blinking of the eye and the smaller surfaces, a purely ocular route of SARS-CoV‑2 infection likely plays a minor role. However, eye protection appears to be urgently required in the case of close contact with COVID-19 patients or a high risk of exposure, e.g., during intubation or extubation of COVID-19 patients.
Is the tear film in COVID-19 patients infectious?
Three recently published studies demonstrated SARS-CoV‑2 RNA in only a small proportion of conjunctival swabs from COVID-19 patients [18, 20, 22]. Zhou et al. analyzed the conjunctival swabs from 67 confirmed or suspected COVID-19 cases and reported that only one patient had a positive PCR result and two patients had likely positive results. None of the three patients had conjunctivitis [22]. Similarly, Xia et al. investigated a total of 30 patients with confirmed SARS-CoV‑2 detection in sputum samples and reported that SARS-CoV‑2 RNA was additionally detected in the conjunctival swab of only one of these patients. This patient also exhibited signs of conjunctivitis [20]. In another study, Seah et al. investigated 17 COVID-19 inpatients who had positive nasopharyngeal swabs. Despite repeated testing, they could neither isolate the virus nor detect SARS-CoV‑2 in tears from any of the patients [18]. These data suggest that even in patients with florid COVID-19 disease, the tear film only rarely contains virus RNA. The detection of viral RNA cannot be equated to the presence of infectious virus particles. Therefore, the risk of SARS-CoV‑2 infection via tear fluid from infected patients appears to be low.
Conclusion
Due to the low number of COVID-19 patients investigated, the current evidence does not permit a conclusive statement to be made on possible SARS-CoV‑2 infection of the conjunctiva. However, since COVID-19 patients only rarely exhibit clinical signs of conjunctivitis and SARS-CoV‑2 RNA has been detected only sporadically in tear fluid, conjunctival SARS-CoV‑2 infection appears to be unlikely. These clinical observations are supported by basic scientific research that describes low expression of ACE2 and TMPRSS2. This makes conjunctival SARS-CoV‑2 transmission via these mediators unlikely—but does not rule out other routes of infection via hitherto unknown receptors. In addition, inoculation of SARS-CoV‑2 could occur via tears transporting the virus through the nasolacrimal drainage system to the nose and throat region where cells get infected. Other investigations, e.g., on autopsy material from COVID-19-deceased patients, are needed in order to gain insight into actual infectiosity and possible sites of virus replication. Until these possibilities are reliably ruled out, effective prophylactic measures that protect the mouth, nose, and, where necessary, the eyes, should be used by physicians coming into close contact with COVID-19. In routine ophthalmological practice, the risk of infection via respiratory aerosols and close contact to patients during certain ophthalmological examinations is likely to be higher than via the tear film and ocular surface of patients.
Compliance with ethical guidelines
Conflict of interest
C. Lange, J. Wolf, C. Auw-Haedrich, A. Schlecht, S. Boneva, T. Lapp, H. Agostini, G. Martin, T. Reinhard, and G. Schlunck declare that they have no competing interests.
For this article no studies with human participants or animals were performed by any of the authors. The data presented here are a recapitulation of data by Lange et al. (2020) recently published by in the journal JMV.
The supplement containing this article is not sponsored by industry.
Contributor Information
Clemens Lange, Email: clemens.lange@uniklinik-freiburg.de.
Günther Schlunck, Email: guenther.schlunck@uniklinik-freiburg.de.
References
- 1.Boeck M, Thien A, Wolf J, et al. Temporospatial distribution and transcriptional profile of retinal microglia in the oxygen-induced retinopathy mouse model. Glia. 2020 doi: 10.1002/glia.23810. [DOI] [PubMed] [Google Scholar]
- 2.Chodosh J, et al. American Academy of Ophthalmology. Alert: Important coronavirus context for ophthalmologists 2020. 2020. [Google Scholar]
- 3.Dai X (2020) Peking University Hospital Wang Guangfa disclosed treatment status on Weibo and suspected infection without wearing goggles. http://www.bjnews.com.cn/news/2020/01/23/678189.html. Zugegriffen: 15.05.2020
- 4.Grajewski RS, Rokohl AC, Becker M, Dewald F, Lehmann C, Fätkenheuer G, et al. A missing link between SARS‐CoV‐2 and the eye?: ACE2 expression on the ocular surface. J Med Virol. 2020 doi: 10.1002/jmv.26136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of Coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han Q, Lin Q, Ni Z, You L. Uncertainties about the transmission routes of 2019 novel coronavirus. Influenza Other Respir Viruses. 2020 doi: 10.1111/irv.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffmann M, Kleine-Weber H, Krüger N et al (2020) The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. 10.1101/2020.01.31.929042. Zugegriffen: 15.05.2020
- 8.Hosoglu S, Celen MK, Akalin S, et al. Transmission of hepatitis C by blood splash into conjunctiva in a nurse. Am J Infect Control. 2003;31:502–504. doi: 10.1016/j.ajic.2003.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Hui KPY, Cheung M-C, Perera RAPM, et al. Tropism, replication competence, and innate immune responses of the coronavirus SARS-CoV-2 in human respiratory tract and conjunctiva: an analysis in ex-vivo and in-vitro cultures. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30193-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuo IC. A Rashomon moment? Ocular involvement and COVID-19. Ophthalmology. 2020 doi: 10.1016/j.ophtha.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lange C, Wolf J, Auw-Haedrich C, et al. Expression of the COVID-19 receptor ACE2 in the human conjunctiva. J Med Virol. 2020 doi: 10.1002/jmv.25981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lange CAK, Lehnert P, Boneva SK, et al. Increased expression of hypoxia-inducible factor-1 alpha and its impact on transcriptional changes and prognosis in malignant tumours of the ocular adnexa. Eye Lond. 2018;32:1772–1782. doi: 10.1038/s41433-018-0172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Löffler KU, Reinhold A, Herwig-Carl MC, Tzankov A, Holz FG, Scholl HPN, Meyer P. Findings in patients having died from COVID-19. Ophthalmologe. 2020 doi: 10.1007/s00347-020-01149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu C-W, Liu X-F, Jia Z-F. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet Lond Engl. 2020;395:e39. doi: 10.1016/S0140-6736(20)30313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 2020 doi: 10.1016/j.bbrc.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reinke LM, Spiegel M, Plegge T, et al. Different residues in the SARS-CoV spike protein determine cleavage and activation by the host cell protease TMPRSS2. PLoS ONE. 2017;12:e0179177. doi: 10.1371/journal.pone.0179177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlecht A, Bonvea S, Gruber M, et al. Transcriptomic characterization of human choroidal neovascular membranes identifies calprotectin as a novel biomarker for patients with age-related macular degeneration. Am J Pathol. 2020 doi: 10.1016/j.ajpath.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Seah IYJ, Anderson DE, Kang AEZ, et al. Assessing viral shedding and Infectivity of tears in Coronavirus disease 2019 (COVID-19) patients. Ophthalmology. 2020 doi: 10.1016/j.ophtha.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tu Y-F, Chien C-S, Yarmishyn AA, et al. A review of SARS-coV-2 and the ongoing clinical trials. Int J Mol Sci. 2020 doi: 10.3390/ijms21072657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia J, Tong J, Liu M, et al. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol. 2020 doi: 10.1002/jmv.25725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou L, Xu Z, Castiglione GM, et al. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Y, Duan C, Zeng Y, et al. Ocular findings and proportion with conjunctival SARS-COV-2 in COVID-19 patients. Ophthalmology. 2020 doi: 10.1016/j.ophtha.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zou X, Chen K, Zou J, et al. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020 doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]