Abstract
Background
The long-term complication rates of open repair and thoracic endovascular aortic repair (TEVAR) have not yet been determined. Therefore, this study aimed to compare the long-term outcomes and aortic reintervention rates between open repair and TEVAR in patients with descending thoracic aortic pathologies.
Methods
Between January 2002 and December 2017, 230 patients with descending thoracic aortic pathologies underwent surgery. Of these, 136 patients were included in this retrospective study: 45 patients (10, 2, and 33 with dissection, penetrating atherosclerotic ulcer, and pseudoaneurysm, respectively) underwent open repair and 91 patients (27, 1, and 63 with dissection, penetrating atherosclerotic ulcer, and pseudoaneurysm, respectively) underwent TEVAR. The primary end points were in-hospital mortality, and short-term complications. The secondary end points were long-term mortality and reintervention rates. Based on the propensity score matching (PSM), 35 patients who underwent open repair were matched to 35 patients who underwent TEVAR (ratio = 1:1).
Results
The mean follow-up period was 70.2 ± 51.9 months. Shorter intensive care unit and hospital stay were seen in the TEVAR group than in the open repair group before and after PSM (p < 0.001 and p < 0.001, respectively). However, in-hospital mortality, and spinal cord ischemia were not significantly different among the two groups (before PSM: p = 0.068 and p = 0.211, respectively; after PSM: p = 0.303 and p = 0.314, respectively). The cumulative all-cause death and aorta-related death showed no significant differences between the two groups (before PSM: p = 0.709 and p = 0.734, respectively; after PSM: p = 0.888 and p = 0.731, respectively). However, aortic reintervention rates were higher in the TEVAR group than in the open repair group before and after PSM (p = 0.006 and p = 0.013, respectively).
Conclusion
The TEVAR group was superior in short-term recovery outcomes but had higher reintervention rates compared to the open repair group. However, there were no significant differences in long-term survival between the two groups.
Keywords: Descending aorta, Endovascular repair, Graft replacement, Outcomes, Reintervention
Background
The treatment of descending thoracic aortic pathologies is challenging and depends on its pathologies. Since the introduction of thoracic endovascular aortic repair (TEVAR), many reports have demonstrated that it is a safe and feasible alternative to the conventional open repair [1–3]. Typically, the success of this procedure is dictated by its favorable outcomes and ease of use [4]. Although the use of TEVAR has rapidly increased due to improved perioperative morbidity rates, significant postoperative complications associated with TEVAR contribute to its relatively poor results; these complications include, endoleak, stent-graft migration, retrograde type aortic dissection, new-onset dissection, and stent-graft infection [3, 5, 6]. In early clinical results with open repair versus TEVAR covered in previous reports [3, 6, 7], there are little long-term data comparing two procedures. In particular, the durability and long-term complication rates of open repair and TEVAR have not yet been determined. We hypothesized that the advantages of TEVAR, which included less invasive and ease of use, will result in improved long-term outcomes for patients.
The purpose of this study was to compare long-term outcomes and reintervention rates between open repair and TEVAR in patients with descending aortic pathologies. To neutralize the effects of confounding independent variables such as unbalanced numbers (45:91) and age discrepancy, a propensity matched subsample of patients was created for an adequately powered analysis.
Methods
Study population
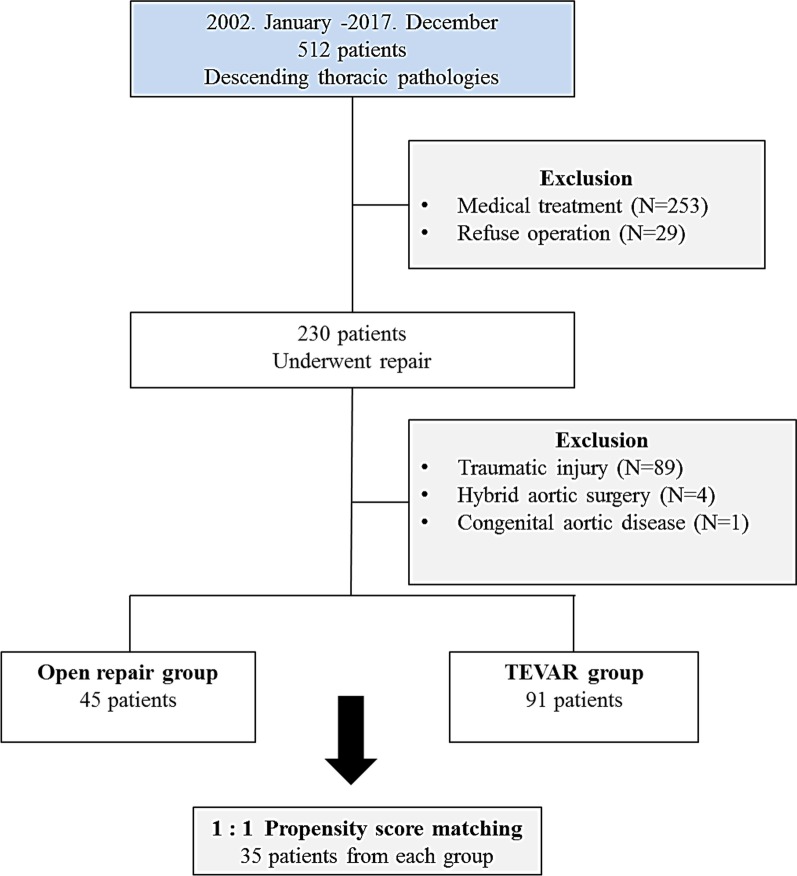
Between January 2002 and December 2017, 512 patients were diagnosed with descending thoracic aortic pathologies, comprising mainly dissection, pseudoaneurysm and penetrating atherosclerotic ulcer (PAU) at Kyungpook National University. Among them, 253 patients with uncomplicated descending aortic pathologies and who underwent medical therapy aimed at maintaining hemodynamic stability for promoting aortic stability were excluded; antihypertensive agents were administered to prevent aortic expansion. Furthermore, 29 additional patients who refused operation due to old age, financial problems, and poor general conditions were excluded as well. Out of the remaining 230 patients, 93 with traumatic aortic injury and hybrid aortic surgery who experienced cardiac arrest were excluded. Additional exclusion criteria were congenital aortic surgery including repair of coarctation of the aorta. The remaining 136 patients were included: Of these, 45 patients (10, 2, and 33 with dissection, PAU, and pseudoaneurysm, respectively) underwent open repair (the open repair group) and 91 patients (27, 1, and 63 with dissection, PAU, and pseudoaneurysm, respectively) underwent TEVAR (the TEVAR group). Telephonic clinical assessments and outpatient clinical records were reviewed for these patients. The study design flowchart is demonstrated in Fig. 1.
Fig. 1.
Study design flowchart
Acute dissection was defined when the occurrence developed within 14 days from the first symptom. Complicated aortic pathologies were defined as the presence of one or more of the following conditions: aortic maximum size > 5.5 cm; resistant hypertension despite adequate medical therapy; recurrent or refractory pain; impending rupture; rupture with end-organ malperfusion; and extension of dissection. Aortic reintervention was delineated as the need for any surgical or endovascular interventions following the initial procedure during follow-up. In the TEVAR group, an endoleak represents as radiological evidence of blood flow outside the stent -graft according to published guidelines [8].
Operative strategies
Contrast-enhanced computed tomography (CT) was performed for the preoperative assessment of descending thoracic aortic pathologies. Surgery was performed for patients with complicated acute or chronic descending aortic pathologies. The treatment modality was decided collaboratively by the cardiologist and cardiac surgeons who were involved in the patients’ care; the decision was based on the patients’ co-morbidities, functional status, anatomical feature of the lesion, and the appropriate of vascular access [9].
Open aortic repair was performed via left thoracotomy or median sternotomy. All procedures were performed with sequential clamping to minimize ischemic times. In case of left atrial-left femoral artery partial bypass, blood was drained from the left atrium via the inferior pulmonary vein and returned through the femoral artery. In case of circulatory arrest, bypass was initiated via the femoral artery and vein or the ascending aorta and right atrium. After the distal aortic arch was cross-clamped, the aorta was opened and a proximal anastomosis was constructed. If there was severe calcification, and friable tissue of the aorta or proximal clamp technically impossible, the patient was put under total circulatory arrest. Moderate hypothermia to 24 °C was achieved by gradual cooling, and circulation was arrested [10].
Preoperative CT were reviewed for the preoperative assessment of access routes for the feasibility of TEVAR. The scans were also reviewed to investigate the bilateral vertebral artery for assessing the subclavian steal syndrome with a policy of selective subclavian artery revascularization. All TEVAR procedures were performed via the transfemoral approach under general anesthesia. Perioperative anticoagulation with heparin was prescribed at a dose of 3000–5000 units. For patients with a healthy native aorta, stent-graft oversizing was considered 5–10%, and excessive oversizing was considered over 20%. The proximal landing zones in the aortic arch were classified as 0 to 4 according to Ishimaru’s classification [11]. The proximal landing zone length was maintained at least 2 cm away from the lesion, and bypass was performed if necessary, through a preoperative assessment. TEVAR was performed using S&G SEAL Thoracic Stent-Grafts (S&G Biotech, Seongnam, Korea) and Valiant Thoracic Stent-Grafts (Medtronic Vascular, Santa Rosa, CA, USA).
When comparing continuous variables, the Student’s t-test and Wilcoxon test were used for parametric and nonparametric data, respectively, and are presented with the mean ± standard deviation (SD) or as median and interquartile range (IQR). Categorical variables were reported as absolute numbers or percentages and the Fisher’s exact test or Chi-square test was used for comparison. The Kaplan–Meier method was used to estimate survival. For statistical analyses, p-values < 0.05 were deemed significant. Univariate and multivariate logistic regression models were utilized to determine independent risk factors. Hazard ratios (HRs) are presented with 95% confidence intervals (CIs).
To reduce the effect of selection bias and potential confounding in this retrospective cohort study, estimated propensity scores were used to match two groups. This was computed for each patient using a logistic regression model including the following variables: age, proximal maximal aortic size, aortic pathology, and proximal aortic tear site. The propensity score model was well-calibrated (Hosmer–Lemeshow goodness‐of‐fit test; p = 0.784) with good discrimination (c‐statistic = 0.712). To neutralize the effects of confounding variables, 35 patients in the open repair group were matched to 35 patients who underwent TEVAR using propensity score matching (PSM). The data were analyzed using SAS/STAT software, v. 9.4 (SAS Institute Inc., NC, USA) and SPSS 25 (IBM, Armonk, NY, USA).
Results
Baseline characteristics of patients
Baseline characteristics of the patients who underwent open repair and TEVAR are shown in Table 1. The mean follow-up duration was 70.2 ± 51.9 months (range: 0.0–212.0 months). The median age of the patients differed significantly between the open repair and TEVAR groups, and were 56.0 years (range: 43.0–64.0 years) and 65.0 years (range: 57.0–72.0 years), respectively (p < 0.001). Moreover, the incidence of connective tissue diseases was significantly higher in the open repair group than in the TEVAR group (p < 0.001). After PSM, the baseline characteristics of the patients exhibited no significant differences between the two groups.
Table 1.
Baseline characteristics of patients
| Characteristics | Overall series | After matching | ||||
|---|---|---|---|---|---|---|
| Open repair N = 45 |
TEVAR N = 91 |
p-value | Open repair N = 35 |
TEVAR N = 35 |
p-value | |
| Age (years) | 56.0 (43.0–64.0) | 65.0 (57.0–72.0) | < 0.001 | 60.0 (49.0–69.0) | 59.0 (51.0–68.0) | 0.967 |
| Men | 31 (30.1) | 72 (69.9) | 0.279 | 25 (71.4) | 30(85.7) | 0.145 |
| Height (cm) | 167 ± 10.0 | 166 ± 9.0 | 0.623 | 164.8 ± 10.1 | 168.3 ± 7.6 | 0.112 |
| Weight (kg) | 66.1 ± 13.2 | 64.9 ± 11.0 | 0.584 | 65.7 ± 12.9 | 68.9 ± 12.4 | 0.283 |
| Initial SBP (mmHg) | 133.5 ± 34.2 | 141.3 ± 29.3 | 0.208 | 135.7 ± 37.3 | 145.5 ± 27.1 | 0.215 |
| Hypertension | 30 (66.7) | 66 (72.5) | 0.480 | 26 (74.3) | 25 (71.4) | 0.788 |
| Diabetes | 5 (11.1) | 12 (13.2) | 0.731 | 5 (14.3) | 1 (2.9) | 0.088 |
| Current smoking | 20 (44.4) | 36 (39.6) | 0.586 | 16 (45.7) | 16 (45.7) | 1.000 |
| Obesity | 2 (4.4) | 3 (3.3) | 0.738 | 2 (5.7) | 2 (5.7) | 1.000 |
| CAOD | 4 (8.9) | 10 (11.0) | 0.705 | 3 (8.6) | 6 (17.1) | 0.284 |
| PAOD | 4 (18.2) | 18 (81.8) | 0.105 | 4 (11.4) | 5 (14.3) | 0.721 |
| COPD | 2 (4.4) | 4 (4.4) | 0.990 | 2 (5.7) | 2 (5.7) | 1.000 |
| Cerebrovascular accident | 1 (2.2) | 11 (12.1) | 0.056 | 1 (2.9) | 4 (11.4) | 0.164 |
| Acute kidney injury | 2 (4.4) | 5 (5.5) | 0.794 | 2 (5.7) | 1 (2.9) | 0.555 |
| Chronic renal failure | 4 (8.9) | 7 (7.7) | 0.810 | 2 (5.7) | 3 (8.6) | 0.643 |
| Previous cardiac operation | 10 (22.2) | 18 (19.8) | 0.740 | 3 (8.6) | 6 (17.1) | 0.284 |
| Connective tissue disease | 8 (17.8) | 1 (1.1) | < 0.001 | 2 (5.7) | 0 (0.0) | 0.151 |
| Preoperative status | ||||||
| Shock | 7 (15.6) | 5 (5.5) | 0.052 | 5 (14.3) | 3 (8.6) | 0.654 |
| Hemoptysis | 6 (13.3) | 11 (12.1) | 0.836 | 4 (11.4) | 4 (11.4) | 1.000 |
| Persistent pain | 37 (82.2) | 73 (80.2) | 0.780 | 30 (85.7) | 27 (77.1) | 0.356 |
| Neurologic deficit | 3 (6.7) | 3 (3.3) | 0.368 | 2 (5.7) | 0 (0.0) | 0.151 |
Values are presented as the median (interquartile range), mean ± standard deviation or number of patients (%)
TEVAR thoracic endovascular aortic repair, SBP systemic blood pressure, CAOD coronary artery occlusive disease, PAOD peripheral artery occlusive disease, COPD chronic obstructive pulmonary disease
Descending thoracic aortic pathologies details of the patients are shown in Table 2. No variables differed significantly between the two groups, both before and after PSM.
Table 2.
Descending thoracic aortic diseases details of patients
| Characteristics | Overall series | After matching | ||||
|---|---|---|---|---|---|---|
| Open repair N = 45 |
TEVAR N = 91 |
p-value | Open repair N = 35 |
TEVAR N = 35 |
p-value | |
| Acute | 15 (33.3) | 20 (22.0) | 0.154 | 13 (37.1) | 7 (20.0) | 0.112 |
| Rupture | 19 (42.2) | 21 (23.1) | 0.021 | 16 (45.7) | 9 (25.7) | 0.081 |
| Preoperative diagnosis | ||||||
| Dissection | 10 (22.2) | 27 (29.7) | 0.358 | 8 (22.9) | 10 (28.6) | 0.584 |
| PAU | 2 (4.4) | 1 (1.1) | 0.211 | 2 (5.7) | 0 (0.0) | 0.151 |
| Pseudoaneurysm | 33 (73.3) | 63 (69.2) | 0.621 | 25 (71.4) | 25 (71.4) | 1.000 |
| Arch involvement | 12 (26.7) | 14 (15.4) | 0.115 | 9 (25.7) | 4 (11.4) | 0.124 |
| Maximal aortic size (mm) | 54.9 ± 4.7 | 51.5 ± 4.5 | 0.471 | 52.8 ± 4.8 | 51.8 ± 3.7 | 0.985 |
| Aortic tear site | ||||||
| Arch | 5 (11.1) | 4 (4.4) | 0.138 | 4 (11.4) | 1 (2.9) | 0.164 |
| Isthmus | 8 (17.8) | 34 (37.4) | 0.020 | 6 (17.1) | 12 (34.3) | 0.101 |
| Descending | 32 (71.1) | 53 (58.2) | 0.145 | 25 (71.4) | 22 (62.9) | 0.445 |
| Malperfusion | 5 (11.1) | 3 (3.3) | 0.068 | 3 (8.6) | 0 (0.0) | 0.077 |
| CSF drainage | 14 (31.1) | 17 (18.7) | 0.104 | 8 (22.9) | 7 (20.0) | 0.771 |
| Emergency | 17 (37.8) | 28 (30.8) | 0.414 | 16 (45.7) | 9 (25.7) | 0.081 |
Values are presented as mean ± standard deviation or number of patients (%)
TEVAR thoracic endovascular aortic repair, PAU penetrating atherosclerotic ulcer, CSF cerebrospinal fluid
Operative details are shown in Table 3. In the open repair group, circulatory arrest perfusion was performed most frequently (60.0%), and thoracotomy was the most common approach (60.0%). In the TEVAR group, zone 3 TEVAR was performed most frequently (40.7%).
Table 3.
Operative details
| Open repair | (N = 45) |
|---|---|
| Perfusion method | |
| Left atrium- Femoral artery partial bypass | 15 (33.3) |
| Partial Shunta | 3 (6.67) |
| Circulatory arrest | 27 (60.0) |
| Femoral artery-Femoral vein | 9 (20.0) |
| Aorta-Right atrium | 18 (40.0) |
| Operative Approach | |
| Median sternotomy | 10 (22.2) |
| Median sternotomy + Thoracotomy | 8 (17.7) |
| Thoracotomy | 27 (60.0) |
| Cardiopulmonary bypass time (min) | 191.1 ± 104.3 |
| Cross clamp time (min) | 60.5 ± 49.9 |
| Circulatory arrest time (min) | 25.5 ± 31.4 |
| TEVAR | (N = 91) |
| Number of devices | 1.2 ± 0.4 |
| Proximal stent size (mm) | 35.1 ± 4.7 |
| Stent Length (mm) | 140.2 ± 28.2 |
| Zone 0 |
6 (6.6) Total debranching bypass with TEVAR |
| Zone 1 |
1 (1.1) BCA to LCCA and LCCA to LSCA bypass |
| Zone 2 |
25 (27.5) LCCA to LSCA bypass in 5 patients |
| Zone 3 | 37 (40.7) |
| Zone 4 | 22 (24.2) |
Values are presented as mean ± standard deviation or number of patients (%)
TEVAR thoracic endovascular aortic repair, BCA brachiocephalic artery, LCCA left common carotid artery, LSCA left subclavian artery
aGraft placed proximal and distal to the injury site
Postoperative outcomes and complications
Postoperative outcomes and complications are shown in Table 4. The patients in the open repair group significantly required more operative time, needed longer ventilator care, stayed longer in the intensive care unit, and had longer periods of hospitalization than those in the TEVAR group (all p < 0.05, respectively), before and after PSM, respectively.
Table 4.
Postoperative outcomes and complications
| Characteristics | Overall series | After matching | ||||
|---|---|---|---|---|---|---|
| Open repair N = 45 |
TEVAR N = 91 |
p-value | Open repair N = 35 |
TEVAR N = 35 |
p-value | |
| Outcomes | ||||||
| Operative time | 420.6 ± 182.9 | 149.3 ± 92.9 | < 0.001 | 386.7 ± 124.5 | 150.9 ± 88.7 | < 0.001 |
| Postop Hospital stay (day) | 41.5 ± 42.7 | 18.3 ± 16.0 | < 0.001 | 34.2 ± 26.5 | 15.1 ± 8.6 | < 0.001 |
| Total ICU stay (day) | 19.6 ± 31.2 | 5.7 ± 14.0 | < 0.001 | 17.7 ± 24.6 | 3.7 ± 5.4 | 0.002 |
| Total ventilator care (min) | 145.7 ± 334.1 | 33.6 ± 158.2 | 0.009 | 111.9 ± 225.4 | 11.9 ± 48.6 | 0.008 |
| Reintubation | 7 (15.6) | 4 (4.4) | 0.025 | 5 (14.3) | 1 (2.9) | 0.088 |
| Complications | ||||||
| Acute kidney injury | 19 (42.2) | 18 (19.8) | 0.006 | 15 (42.9) | 6 (17.1) | 0.019 |
| Dialysis | 6 (13.3) | 6 (6.6) | 0.192 | 3 (8.6) | 1 (2.9) | 0.303 |
| Hematologic complications | 1 (2.2) | 2 (2.2) | 0.993 | 1 (2.9) | 0 (0.0) | 0.314 |
| Bleeding | 9 (20.0) | 3 (3.3) | 0.001 | 6 (17.1) | 2 (5.7) | 0.133 |
| Spinal cord injury | 2 (4.4) | 1 (1.1) | 0.211 | 1 (2.9) | 0 (0.0) | 0.314 |
| Stroke | 3 (6.7) | 1 (1.1) | 0.071 | 2 (5.7) | 0 (0.0) | 0.151 |
| Pulmonary complications | 9 (20.0) | 7 (7.7) | 0.036 | 7 (20.0) | 2 (5.7) | 0.074 |
| Wound complications | 12 (26.7) | 6 (6.6) | 0.001 | 9 (25.7) | 3 (8.6) | 0.057 |
| In-hospital mortality | 5 (11.1) | 3 (3.3) | 0.068 | 3 (8.6) | 1 (2.9) | 0.303 |
| 30-day mortality | 5 (11.1) | 4 (4.4) | 0.138 | 3 (8.6) | 2 (5.7) | 0.643 |
Values are presented as mean ± standard deviation or number of patients (%)
TEVAR thoracic endovascular aortic repair, Postop postoperative, ICU intensive care unit
Postoperative acute kidney injury (AKI) was higher in the open repair group before and after PSM (p = 0.006 and p = 0.019, respectively); however, there was no difference between the two groups in the AKI requiring dialysis before and after PSM (p = 0.192 and p = 0.303, respectively). AKI progressed to chronic renal failure (CRF) in only two patients in the TEVAR group. Bleeding and wound complications including wound dehiscence and infection, were frequently observed in the open repair group before PSM (p = 0.001 and p = 0.001, respectively); however, there were no statistical difference between the two groups after PSM (p = 0.133 and p = 0.057, respectively). In addition, there was no statistical difference in occurrence of spinal cord ischemia (SCI) between the two groups before and after PSM (p = 0.211 and p = 0.314, respectively).
There were no statistical difference in-hospital mortality and 30-day mortality between the two groups before PSM (p = 0.068 and p = 0.138, respectively). Moreover, same as before PSM, in-hospital mortality and 30-day mortality showed no statistical difference between the two groups (p = 0.303 and p = 0.643, respectively).
In-hospital mortality was observed in five patients in the open repair group (11.1%), of whom four and one died of acute aorta-related complications and hospital-acquired pneumonia, respectively. In the TEVAR group, among three patients (3.3%), two patients died of delayed rupture after stent insertion and one patient died of aspiration pneumonia.
Reintervention
Reintervention data associated with specific treatments are depicted in Table 5. In the open repair group, five patients (11.1%) required reinterventions; and four patients experienced new onset aortic expansion. Among these patients, secondary open repair surgery was performed in three patients, and TEVAR was performed in one patient. In the TEVAR group, 22 patients (48.9%) required reintervention; twelve patients had endoleaks and four patient developed fistula, such as aortobronchial fistula or aortoesophageal fistula.
Table 5.
Aortic reintervention details
| Cause of Reintervention | Open repair (N = 5, 11.1%) | TEVAR (N = 22, 48.9%) |
|---|---|---|
| New onset dissection/expansion |
Aorta replacement:3 TEVAR:1 |
Aorta replacement:2 TEVAR:1 |
| Fistula formation | Aorta replacement and lobectomy:1 |
Aorta replacement and lobectomy:4 Aorta replacement and esophagotomy:1 TEVAR:1 |
| Endoleak |
TEVAR:12 LSCA plug obliteration:1 |
|
| Infection | Aorta replacement:1 |
TEVAR thoracic endovascular aortic repair, LSCA left subclavian artery
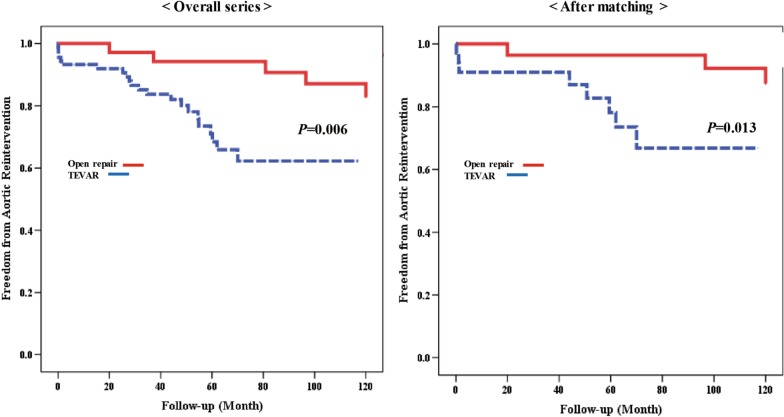
In the overall series, freedom from aortic reintervention at 1, 5, and 10 years in the open repair group was 97.1% ± 0.1%, 94.2% ± 0.1%, and 87.1% ± 0.0%, respectively. The corresponding rates in the TEVAR group were 93.2% ± 0.1%, 68.4% ± 0.1%, and 62.2% ± 0.0%, respectively. After PSM, the rate of freedom from aortic reintervention at 1, 5, and 10 years in the open group was 100% ± 0.0%, 96.4% ± 0.1%, and 92.2% ± 0.1%, respectively. In the TEVAR group, the corresponding values were 91.0% ± 0.1%, 78.1% ± 0.1%, and 66.8% ± 0.1%, respectively. Freedom from aortic reintervention (Fig. 2) was lower in the TEVAR group than in the open repair group before and after PSM (p = 0.006 and p = 0.013, respectively).
Fig. 2.
Freedom from aortic reintervention before propensity matching and after propensity matching
A multivariable Cox proportional hazard model is shown in Table 6.
Table 6.
Cox proportional hazard regression analysis for reintervention
| Variable | Overall series | After matching | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Male | 2.0 (0.6–5.8) | 0.191 | 0.2 (0.0–2.2) | 0.230 | ||||
| Hemoptysis | 2.8 (1.3–6.9) | 0.026 | 3.8 (1.2–11.3) | 0.016 | 8.5 (2.4–29.3) | 0.001 | 6.8 (1.3–34.7) | 0.021 |
| Hoarseness | 2.2 (0.8–9.0) | 0.102 | 1.6 (0.2–13.2) | 0.619 | ||||
| Hypertension | 0.2 (0.5–2.9) | 0.595 | 4.4 (0.5–34.9) | 0.151 | 7.9 (0.8–73.1) | 0.048 | ||
| Diabetes | 2.0 (0.7–5.4) | 0.148 | 1.3 (0.2–10.7) | 0.766 | ||||
| CAOD | 2.5 (0.9–6.7) | 0.059 | 2.2 (0.5–10.6) | 0.210 | ||||
| PAOD | 1.7 (0.7–4.4) | 0.210 | 1.9 (0.4–8.9) | 0.400 | ||||
| COPD | 3.4 (1.0–11.4) | 0.046 | 4.4 (0.9–21.2) | 0.063 | ||||
| Cerebrovascular accident | 2.2 (0.7–6.4) | 0.144 | 1.7 (0.2–14.2) | 0.579 | ||||
| Acute kidney injury | 1.1 (0.1–7.4) | 0.994 | 5.1 (0.6–41.2) | 0.124 | ||||
| Previous cardiac operation | 1.1 (0.6–3.4) | 0.388 | 3.2 (0.8–12.4) | 0.082 | 6.81 (1.3–34.9) | 0.021 | ||
HR hazard ratio, CI confidence interval, CAOD coronary artery occlusive disease, PAOD peripheral artery occlusive disease, COPD chronic obstructive pulmonary disease
Change in the aorta size after the repair
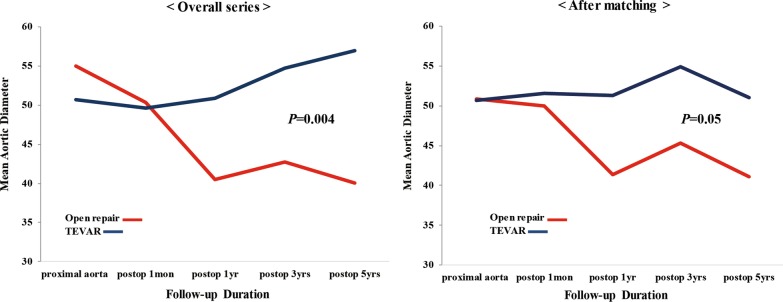
Figure 3 shows the changes in the aorta size after the repair. During the following 5 years, the maximal aortic diameter was more reduced in the open repair group as compared to that in the TEVAR group before and after PSM (p = 0.004 and p = 0.05, respectively). The maximal aortic diameter decreased from 55.4 ± 18.2 mm to 40.07 ± 11.0 mm in the open repair group as we expected. Meanwhile the maximal aortic diameter increased from 52.0 ± 14.7 mm to 56.8 ± 19.1 mm in the TEVAR group.
Fig. 3.
Changes in aortic diameters over time before propensity matching and after propensity matching
Survival
In the open repair group, the overall mortality was 42.2% (19/45), and the aorta-related mortality was 17.8% (8/45) during follow-up. Aorta-unrelated death secondary to cancer occurred in three patients, while three patients died of pneumonia. Late deaths secondary to unknown causes occurred in four patients. In the TEVAR group, the overall mortality was 35.2% (32/91), and the aorta-related mortality was 12.1% (11/91) during follow-up. Aorta-unrelated death secondary to cancer occurred in three patients, while two patients died of pneumonia. Late deaths secondary to unknown causes occurred in 10 patients.
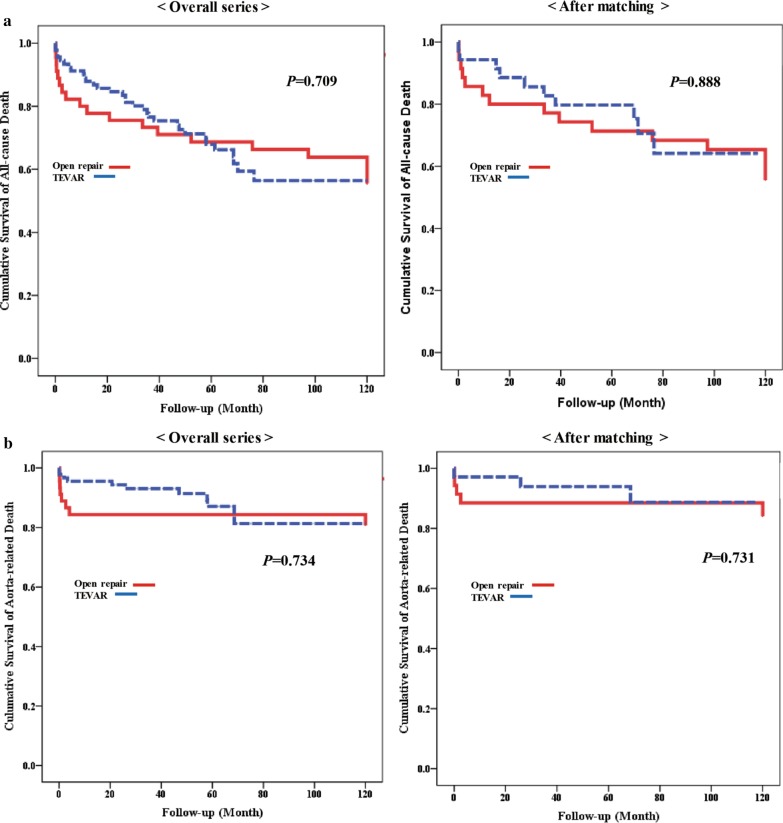
The cumulative survival of all-cause death did not differ significantly between the two groups, neither before nor after PSM. Additionally, the cumulative survival rates from aorta-related deaths were also not significantly different between the two groups, before and after PSM (Fig. 4a, b).
Fig. 4.
Cumulative survival of all-cause death and aorta-related death
A multivariable Cox proportional hazard model identified that age > 80 years, systolic blood pressure < 90 mmHg, diabetes, preoperative chronic renal failure, and aortic arch involvement were the predictive factors in the in overall series; after PSM analysis, age > 80 years, and aortic arch involvement (p = 0.024 and p = 0.048, respectively) were the independent predictors of aorta-related mortality (Table 7).
Table 7.
Cox proportional hazard regression analysis for aorta-related mortality
| Variable | Overall series | After matching | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age > 80 years | 5.9 (1.3–25.9) | 0.018 | 16.7 (2.7–104.3) | 0.002 | 9.9 (1.2–83.4) | 0.031 | 24.0 (1.5–378.1) | 0.024 |
| SBP < 90 mmHg | 2.9 (0.9–8.9) | 0.058 | 6.0 (1.6–22.4) | 0.008 | 2.6 (0.5–13.3) | 0.241 | ||
| Hemoptysis | 2.5 (0.9–7.0) | 0.077 | 5.2 (1.2–22.0) | 0.023 | ||||
| Dissection | 0.7 (0.3–1.4) | 0.356 | 0.4 (0.0–3.3) | 0.392 | ||||
| Pseudoaneurysm | 1.4 (0.7–2.6) | 0.333 | 2.8 (0.3–23.2) | 0.329 | ||||
| Arch involvement | 3.5 (1.4–8.7) | 0.007 | 5.4 (1.8–16.1) | 0.002 | 5.1 (1.3–20.9) | 0.022 | 14.5 (1.0–211.5) | 0.048 |
| Maximal aortic size > 50 mm | 3.1 (0.9–10.8) | 0.068 | 3.8 (0.5–31.9) | 0.200 | ||||
| Malperfusion | 2.3 (0.5–10.1) | 0.210 | 5.2 (0.6–43.9) | 0.128 | ||||
| Chronic renal failure | 7.0 (2.4–20.3) | 0.000 | 5.7 (1.2–26.8) | 0.027 | 8.4 (1.5–46.7) | 0.015 | ||
| eGFR | 1.2 (1.1–1.3) | 0.001 | 1.1 (0.9–1.5) | 0.211 | ||||
| Diabetes | 2.8 (1.0–7.8) | 0.047 | 3.1 (0.9–10.7) | 0.049 | 4.2 (0.8–20.9) | 0.080 | ||
| COPD | 2.7 (0.6–11.9) | 0.178 | 0.1 (0.0–34.8) | 0.654 | ||||
HR hazard ratio, CI confidence interval, SBP systolic blood pressure, eGFR estimated glomerular filtration rate, COPD chronic obstructive pulmonary disease
Discussion
The prevalence of descending thoracic aortic pathologies, comprising mainly of aneurysm, dissection, and PAU, which eventually rupture if not recognized and treated appropriately, are increasing among our population [12]. Since, the report on the first successful open repair of a descending thoracic aortic aneurysm with a prosthetic graft in 1953 by De Bakey and Cooley [13], an open surgical repair for treating descending thoracic aortic disease has been the gold standard for 50 years [13–16]. Despite remarkably improved operative techniques and maximized organ protection, open repair of the descending thoracic aorta is still associated with high complications, including intraoperative and postoperative death, hemorrhage, stroke, and paraplegia [9, 17].
Dake et al. [14] proposed an alternate method of TEVAR which sought to provide better clinical outcomes in patients who were deemed to be at high risk for open repair or were typically considered nonsurgical candidates. Therefore, TEVAR has shown significantly improved early quality of life versus open repair and a general trend toward better short-term perioperative survival and freedom from major complications [1, 3, 4, 18]. However, TEVAR has anatomic restrictions such as severe thoracic aortic tortuosity, short landing and sealing zones, and extensive mural thrombus. These are the limiting factors, although a seemingly infinite variety of debranching and bypass procedures can be applied to extend either the proximal or distal sealing zones [6, 19]. Furthermore, significant complications related to stent-grafts were always implied [3, 5, 17].
Patients who underwent TEVAR have a tendency to have a worse prognosis and older age, with multiple comorbidities, than patients who underwent open repair. Due to the relative lack of data supporting the long-term reliability of TEVAR, open repair procedure has been preferentially offered to younger patients [4, 12]. Therefore, in this study, to neutralize the effects of age difference that could potentially unmask a mortality benefit, PSM was performed between the two groups to perform an adequately powered analysis.
In the present study, the operative time, postoperative length of stay, and procedure-related complications showed better results with TEVAR before and after PSM. Not surprisingly, TEVAR was considering as the procedure involved no aortic cross-clamping, ischemic time, or thoracotomy [4]. In open repair cases, some disadvantages of deep hypothermia, including coagulopathy which caused difficulty in controlling bleeding, retraction injury to a heparinized lung, cold injury to the lung, and a profound inflammatory response from the bypass circuit [20]. Regarding the in-hospital mortality in the open repair group, in the present study, one patient died of pneumonia. AKI is another important complication and regarded as a marker of increased early or late morbidity and mortality after open repair or TEVAR [21]. Patients who underwent TEVAR were older and tended to receive larger amounts of contrast agent, which was not safe considering the risk of AKI. In the present study, postoperative AKI was higher in the open repair group (42.2%), and dialysis was performed in 13.3% of the patients in this group. Eighteen patients who underwent TEVAR (19.8%) had an AKI, six patients in the TEVAR group experienced AKI requiring dialysis. Before and after PSM, AKI was higher in the open repair group; however, there was no statistical difference in the requirement of dialysis between the two groups.
Although a short-term hospital outcome is more favorable for TEVAR, aorta-related complications are more frequent for TEVAR. Five patients (11.1%) in the open repair group underwent reintervention, and the most common cause of reintervention was new-onset aortic dissection or expansion. In the case of TEVAR, the most common cause of reintervention was endoleaks. Twenty-two (48.9%) patients who underwent reintervention showed no in-hospital mortality in the TEVAR; however, seven patients showed late mortality, one patient died of aortobronchial fistula and one patient died of sepsis due to stent-graft infection. Ascending aortic replacement was performed in one patient with retrograde aortic dissection, four patients with aortabronchial fistula underwent aorta replacement and lobectomy, and one patient with aortoesophageal fistula underwent aorta replacement and esophagotomy, and showed late mortality. Additionally, eight patients who were initially offered TEVAR, later crossed over to open repair due to difficult anatomy or other reasons.
In the open repair cases, high-volume centers reported mortality and neurological morbidity rates ranging from 5.4% to 7.2% for mortality, 2.1% to 6.2% for permanent stroke, 5.7% for permanent paraparesis, and 0.8% to 2.3% for permanent paralysis [22, 23]. In the TEVAR cases, the perioperative results for the three stent-graft trials showed 1.9 to 2.1% for mortality, 2.4 to 4% for stroke, 4.4 to 7.2% for permanent paraparesis, and 1.3 to 3% for permanent paralysis [1, 2, 24]. In the present study, the open repair group showed 63.8% of overall 10-year survival rate, 84.3% of aorta-related 10-year survival rate, 6.7% of stroke, and 4.4% of SCI (after PSM, 65.4%, 88.5%, 5.7%, and 2.9%, respectively). In TEVAR, overall 10-year survival rate was 56.5%, aorta-related 10-year survival rate 81.3%, 1.1% stroke, and 1.1% SCI (after PSM, 64.2%, 88.7%, 0.0%, 0.0%, respectively). Although mortality was higher than previous large-scale studies, it was difficult to compare our results because previous reports did not have long-term follow-up data.
The existing literature on TEVAR versus open surgical repair suggests that the short-term benefit of TEVAR is lost as early as within 1–2 years from the surgery [3–7]. For mid-term outcomes, analysis of the Medicare database from 2004 to 2007 showed that TEVAR offered a significant perioperative survival advantage when compared to open repair, regardless of the indication for repair. However, the 5-year survival in the Medicare population was similar between the two cohorts [25]. In another study, Goodney et al. [26] reported patients with intact thoracic aortic aneurysm who underwent TEVAR had a significantly worse survival at 1 year than patients who underwent open repair (87% for open repair and 82% for TEVAR; p = 0.001) and 5 years (72% for open repair and 62% for TEVAR; p = 0.001) than patients who underwent open repair.
Chiu et al. [27] reported that open surgical repairs of descending thoracic aortic aneurysms between 1999 and 2010 were associated with increased odds of early postoperative mortality, but reduced late hazard of death. Despite the improved durability of open surgical repair, the initial mortality advantage of TEVAR over open surgical repair persisted for until nine years post-operatively, resulting in a significant survival benefit associated with TEVAR. They proposed TEVAR ought to be considered as first-line therapy among Medicare beneficiaries, and open surgical repair be restricted to high-volume centers and patients with a low risk of perioperative mortality. However, Desai et al. [13] reviewed their series of patients who had descending thoracic aortic aneurysms repaired by either TEVAR or open repair. They concluded that 105 patients in the TEVAR group clearly had undergone more reinterventions than 45 patients who underwent open repair. The TEVAR cohort had a trend towards a decreased 30-day mortality as well as decreased rates of neurologic complications; however, none of these findings reached statistical significance. During a long-term follow-up from 1995 to 2007, the choice between TEVAR and open approach did not influence survival (p = 0.5).
In our study, TEVAR was more prevalent in terms of postoperative short-term outcomes. However, we could not conclude that TEVAR was superior to open repair in terms of SCI, 30-day mortality, and in-hospital mortality. Furthermore, there was no significant difference in the long-term follow-up survival between the two approaches. We hypothesized that these advantages of TEVAR, i.e., less invasiveness and ease of use, will provide improved long-term results for the patients.
Similar to the previous study, our findings indicated that TEVAR was better than open repair in terms of short-term outcomes; however, the long-term results were similar in both groups. Considering the fact that the previous studies had data older than in this study and there were discrepancies in the age or number of patients between the two groups, we believe that this study, which used PSM to neutralize the effects of confounding independent variables, presents more accurate data on the long-term results of the two groups.
In case of reintervention rates, there were significantly reinterventions in the TEVAR group than in the open repair group. Moreover, although long-term survival of the two groups did not differ significantly, more reinterventions occurred in the TEVAR group; the costs of additional graft modules to treat endoleaks and follow-up CT scans increase hospital cost, attributing to the disadvantage of TEVAR. Furthermore, while TEVAR had lesser procedure-related complications than open repair; patients had more adverse events, such as re-dissection, fistula formation and stent-graft infection. This should be considered in the choice of approach.
Some authors have proposed that TEVAR does not change the natural history of the disease, and although less invasive, may be inferior to open therapies [27]. In our present study, the maximal aortic size decreased more in the open repair group than in the TEVAR group, but not dramatically. This supports the report that it does not alter natural history of aortic pathologies, and, emphasizes the importance of long -term follow up. For this reason, in patients requiring TEVAR, the establishment of a precise TEVAR indication will reduce the requirement for further reintervention; better results are expected with improvements in debranching skills and stent- graft development.
Our study has several limitations. First, it was a single-center retrospective study that included a small number of patients, with a possible selection bias that might have affected our results. Second, the difference of follow-up duration and frequency can affect the survival rate of both groups. We performed PSM attempts to reduce the bias due to confounding variables. However, since TEVAR was introduced in 2007, it has a relatively short follow-up duration, whereas more frequent follow-up to monitor stent- grafts is expected to affect the results. Finally, the functional status of patient information influenced treatment strategy; there was no data interpretation, and studies on cost analysis, which is an increasingly important consideration for treatment strategy, have not been conducted. The results, which include all of these, may influence the final assessment of quality of life and longer life expectancy.
Conclusions
In conclusion, our study showed that a long-term comparison between open repair and TEVAR demonstrated similar results in patients with descending aortic pathologies. However, patients who underwent TEVAR showed superior short-term recovery outcomes and a higher reintervention rate than the open repair group. Larger multicenter population studies that consider quality of life could support our results.
Acknowledgements
We certify that this research is our own work and all sources of information used in this research have been fully acknowledged.
Abbreviations
- TEVAR
Thoracic endovascular aortic repair
- PSM
Propensity score matching
- PAU
Penetrating atherosclerotic ulcer
- CT
Computed tomography
- SD
Standard deviation
- IQR
Interquartile range
- HRs
Hazard ratios
- CIs
Confidence intervals
- AKI
Acute kidney injury
- CRF
Chronic renal failure
- SCI
Spinal cord ischemia
Authors’ contributions
SS collected the case data and drafted the original manuscript. JC and HJ operated and treated the patients and critically reviewed the manuscript. All authors read and approved the final manuscript.
Funding
None of the authors have any financial interests to disclose.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
The datasets used and/or analyzed during the current study are available from the Department of Thoracic and Cardiovascular Surgery, Kyungpook National University, Kyungpook National University Hospital, on reasonable request.
Ethics approval and consent to participate
The institutional review board (IRB) of Kyungpook National University Hospital approved this retrospective study and waived the requirement for individual patient consent (IRB approval No. 2019–10-051).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fairman RM, Criado F, Farber M, Kwolek C, Mehta M, White R, Lee A, Tuchek JM, Investigators V. Pivotal results of the Medtronic Vascular Talent Thoracic Stent Graft System: the VALOR trial. J Vasc Surg. 2008;48(3):546–554. doi: 10.1016/j.jvs.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 2.Matsumura JS, Cambria RP, Dake MD, Moore RD, Svensson LG, Snyder S, Investigators TXCT. International controlled clinical trial of thoracic endovascular aneurysm repair with the Zenith TX2 endovascular graft: 1-year results. J Vasc Surg. 2008;47(2):247–257. doi: 10.1016/j.jvs.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 3.Makaroun MS, Dillavou ED, Kee ST, Sicard G, Chaikof E, Bavaria J, Williams D, Cambria RP, Mitchell RS. Endovascular treatment of thoracic aortic aneurysms: results of the phase II multicenter trial of the GORE TAG thoracic endoprosthesis. J Vasc Surg. 2005;41(1):1–9. doi: 10.1016/j.jvs.2004.10.046. [DOI] [PubMed] [Google Scholar]
- 4.Gopaldas RR, Huh J, Dao TK, LeMaire SA, Chu D, Bakaeen FG, Coselli JS. Superior nationwide outcomes of endovascular versus open repair for isolated descending thoracic aortic aneurysm in 11,669 patients. J Thorac Cardiovasc Surg. 2010;140(5):1001–1010. doi: 10.1016/j.jtcvs.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L, Zhao Z, Chen Y, Sun Y, Bao J, Jing Z, Zhou J. Reintervention after endovascular repair for aortic dissection: A systematic review and meta-analysis. J Thorac Cardiovasc Surg. 2016;152(5):1279–1288. doi: 10.1016/j.jtcvs.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 6.Hua HT, Cambria RP, Chuang SK, Stoner MC, Kwolek CJ, Rowell KS, Khuri SF, Henderson WG, Brewster DC, Abbott WM. Early outcomes of endovascular versus open abdominal aortic aneurysm repair in the National Surgical Quality Improvement Program-Private Sector (NSQIP-PS) J Vasc Surg. 2005;41(3):382–389. doi: 10.1016/j.jvs.2004.12.048. [DOI] [PubMed] [Google Scholar]
- 7.Hansen CJ, Bui H, Donayre CE, Aziz I, Kim B, Kopchok G, Walot I, Lee J, Lippmann M, White RA. Complications of endovascular repair of high-risk and emergent descending thoracic aortic aneurysms and dissections. J Vasc Surg. 2004;40(2):228–234. doi: 10.1016/j.jvs.2004.03.051. [DOI] [PubMed] [Google Scholar]
- 8.Fillinger MF, Greenberg RK, McKinsey JF, Chaikof EL. Society for Vascular Surgery Ad Hoc Committee on TRS: reporting standards for thoracic endovascular aortic repair (TEVAR) J Vasc Surg. 2010;52(4):1022–1033. doi: 10.1016/j.jvs.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Lee HC, Joo HC, Lee SH, Lee S, Chang BC, Yoo KJ, Youn YN. Endovascular repair versus open repair for isolated descending thoracic aortic aneurysm. Yonsei Med J. 2015;56(4):904–912. doi: 10.3349/ymj.2015.56.4.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desai ND, Burtch K, Moser W, Moeller P, Szeto WY, Pochettino A, Woo EY, Fairman RM, Bavaria JE. Long-term comparison of thoracic endovascular aortic repair (TEVAR) to open surgery for the treatment of thoracic aortic aneurysms. J Thorac Cardiovasc Surg. 2012;144(3):604–609. doi: 10.1016/j.jtcvs.2012.05.049. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell RS, Ishimaru S, Ehrlich MP, Iwase T, Lauterjung L, Shimono T, Fattori R, Yutani C. First International Summit on Thoracic Aortic Endografting: roundtable on thoracic aortic dissection as an indication for endografting. J Endovasc Ther. 2002;9(Suppl 2):II98–105. [PubMed] [Google Scholar]
- 12.Harky A, Kai Chan JS, Ming Wong CH, Bashir M. Open versus endovascular repair of descending thoracic aortic aneurysm disease: a systematic review and meta-analysis. Ann Vasc Surg. 2019;54(304–315):e305. doi: 10.1016/j.avsg.2018.05.043. [DOI] [PubMed] [Google Scholar]
- 13.Adams JD, Angle JF, Matsumoto AH, Peeler BB, Arslan B, Cherry KJ, Kern JA, Dake MD. Endovascular repair of the thoracic aorta in the post-FDA approval era. J Thorac Cardiovasc Surg. 2009;137(1):117–123. doi: 10.1016/j.jtcvs.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Dake MD, Miller DC, Semba CP, Mitchell RS, Walker PJ, Liddell RP. Transluminal placement of endovascular stent-grafts for the treatment of descending thoracic aortic aneurysms. N Engl J Med. 1994;331(26):1729–1734. doi: 10.1056/NEJM199412293312601. [DOI] [PubMed] [Google Scholar]
- 15.Cambria RP, Clouse WD, Davison JK, Dunn PF, Corey M, Dorer D. Thoracoabdominal aneurysm repair: results with 337 operations performed over a 15-year interval. Ann Surg. 2002;236(4):471–479. doi: 10.1097/00000658-200210000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huynh TT, Miller CC, 3rd, Estrera AL, Porat EE, Safi HJ. Thoracoabdominal and descending thoracic aortic aneurysm surgery in patients aged 79 years or older. J Vasc Surg. 2002;36(3):469–475. doi: 10.1067/mva.2002.127348. [DOI] [PubMed] [Google Scholar]
- 17.Zhang H, Wang ZW, Zhou Z, Hu XP, Wu HB, Guo Y. Endovascular stent-graft placement or open surgery for the treatment of acute type B aortic dissection: a meta-analysis. Ann Vasc Surg. 2012;26(4):454–461. doi: 10.1016/j.avsg.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Orandi BJ, Dimick JB, Deeb GM, Patel HJ, Upchurch GR., Jr A population-based analysis of endovascular versus open thoracic aortic aneurysm repair. J Vasc Surg. 2009;49(5):1112–1116. doi: 10.1016/j.jvs.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 19.Stone DH, Brewster DC, Kwolek CJ, Lamuraglia GM, Conrad MF, Chung TK, Cambria RP. Stent-graft versus open-surgical repair of the thoracic aorta: mid-term results. J Vasc Surg. 2006;44(6):1188–1197. doi: 10.1016/j.jvs.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Coselli JS, Bozinovski J, Cheung C. Hypothermic circulatory arrest: safety and efficacy in the operative treatment of descending and thoracoabdominal aortic aneurysms. Ann Thorac Surg. 2008;85(3):956–963. doi: 10.1016/j.athoracsur.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 21.Huynh TT, van Eps RG, Miller CC, 3rd, Villa MA, Estrera AL, Azizzadeh A, Porat EE, Goodrick JS, Safi HJ. Glomerular filtration rate is superior to serum creatinine for prediction of mortality after thoracoabdominal aortic surgery. J Vasc Surg. 2005;42(2):206–212. doi: 10.1016/j.jvs.2005.03.062. [DOI] [PubMed] [Google Scholar]
- 22.Wong DR, Parenti JL, Green SY, Chowdhary V, Liao JM, Zarda S, Huh J, LeMaire SA, Coselli JS. Open repair of thoracoabdominal aortic aneurysm in the modern surgical era: contemporary outcomes in 509 patients. J Am Coll Surg. 2011;212(4):569–579. doi: 10.1016/j.jamcollsurg.2010.12.041. [DOI] [PubMed] [Google Scholar]
- 23.Estrera AL, Miller CC, 3rd, Chen EP, Meada R, Torres RH, Porat EE, Huynh TT, Azizzadeh A, Safi HJ. Descending thoracic aortic aneurysm repair: 12-year experience using distal aortic perfusion and cerebrospinal fluid drainage. Ann Thorac Surg. 2005;80(4):1290–1296. doi: 10.1016/j.athoracsur.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 24.Bavaria JE, Appoo JJ, Makaroun MS, Verter J, Yu ZF, Mitchell RS, Gore TAGI. Endovascular stent grafting versus open surgical repair of descending thoracic aortic aneurysms in low-risk patients: a multicenter comparative trial. J Thorac Cardiovasc Surg. 2007;133(2):369–377. doi: 10.1016/j.jtcvs.2006.07.040. [DOI] [PubMed] [Google Scholar]
- 25.Conrad MF, Ergul EA, Patel VI, Paruchuri V, Kwolek CJ, Cambria RP. Management of diseases of the descending thoracic aorta in the endovascular era: a Medicare population study. Ann Surg. 2010;252(4):603–610. doi: 10.1097/SLA.0b013e3181f4eaef. [DOI] [PubMed] [Google Scholar]
- 26.Goodney PP, Travis L, Lucas FL, Fillinger MF, Goodman DC, Cronenwett JL, Stone DH. Survival after open versus endovascular thoracic aortic aneurysm repair in an observational study of the Medicare population. Circulation. 2011;124(24):2661–2669. doi: 10.1161/CIRCULATIONAHA.111.033944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elefteriades JA, Farkas EA. Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J Am Coll Cardiol. 2010;55(9):841–857. doi: 10.1016/j.jacc.2009.08.084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
The datasets used and/or analyzed during the current study are available from the Department of Thoracic and Cardiovascular Surgery, Kyungpook National University, Kyungpook National University Hospital, on reasonable request.