Abstract
We report a case of a 76-year-old British man living in Malta who presented with a 7-month history of recurrent epistaxis and an enlarging right nasal vestibular lesion. Of note, his medical history included rheumatoid arthritis for which he was on long-term methotrexate. Blood results were unremarkable other than a mild lymphopaenia. Despite the use of various antibiotics and intranasal steroids, the lesion failed to resolve. This was eventually biopsied, and the histological picture was that of mucosal leishmaniasis. Leishmania donovani complex was detected by PCR. The patient was treated with liposomal amphotericin B on alternate days for a total of 20 doses. The lesion was found to have healed well at follow-up and the patient denied any further episodes of epistaxis.
Keywords: ear, nose and throat, infections, infectious diseases
Background
Leishmaniasis is a disease that is caused by the Leishmania protozoan, transmitted by an infected, female phlebotomine sandfly. Humans, dogs and rodents act as reservoirs. Subtypes include visceral, cutaneous, mucocutaneous and mucosal leishmania (ML), with the latter being the least prevalent. ML is most often caused by the Leishmania (Viannia) subgenus in South America. Only a few cases of ML per annum are reported in Europe, less so in Malta. It typically involves the upper respiratory tract mucosa from the nostrils to the larynx and oral cavity and can lead to severe deformity if untreated. ML can manifest as a consequence of cutaneous leishmaniasis metastasising to the mucosa (also known as espundia). The cutaneous lesions need not be present concurrently, but may have occurred years prior to the patient developing mucosal lesions. ML can also coexist with cutaneous/visceral leishmaniasis as well as rarely occurring as a primary infection.
Case presentation
A 76-year-old retired British male (RW), who has been living in Malta for 25 years presented to the ENT specialist with recurrent episodes of epistaxis and an enlarging right vestibular lesion. He had a medical history of rheumatoid arthritis, treated long term with methotrexate, Paget’s disease and osteopaenia. Despite antibiotics and intranasal steroids, the lesion failed to improve and the epistaxis persisted. RW denied a history of trauma to the nose. He was previously well and did not report fever, chills and rigours. He denied weight loss, weakness, arthralgias or rashes. In fact, RW could not recall ever having unexplained skin lesions. His vision, smell and taste were not impaired. There was no family history of note. RW never smoked and never had contact with dogs, only having a pet cat that was healthy. He did admit to going for walks in the Maltese countryside, where rubble walls are quite prevalent. Other than going to England regularly, he had no other travel history of note.
On review, vital signs were normal. Examination of the nose revealed a 1 cm diameter right vestibular ulcerated lesion. The left vestibule and oral cavity were unremarkable. There was no cervical lymphadenopathy. Examination of the face, scalp, trunk and limbs revealed no other rashes or lesions. Abdominal examination was normal with no obvious organomegaly.
Investigations
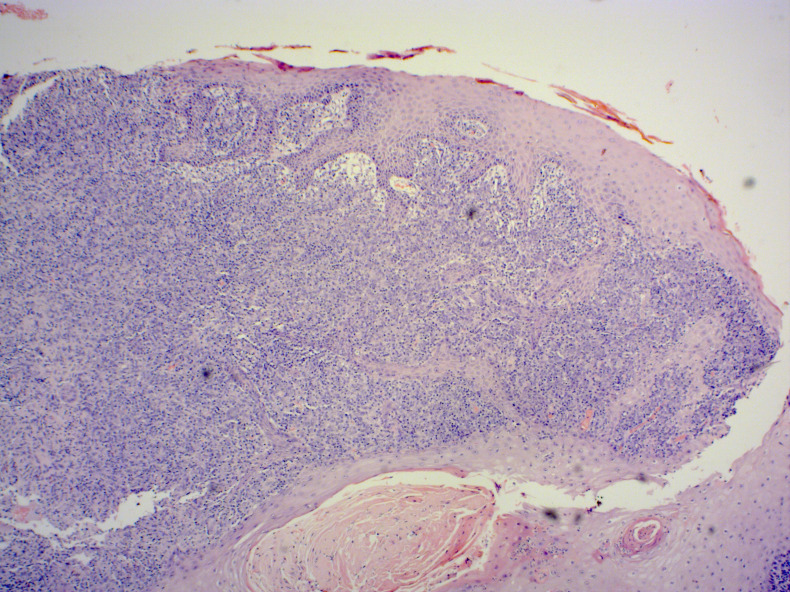
Initial laboratory values showed a low lymphocyte count of 1×109 cells/L. The haemoglobin, platelet count, C-reactive protein and erythrocyte sedimentation rate were normal. Liver function tests showed a chronically elevated alkaline phosphatase secondary to Paget’s disease. HIV status was found to be negative and serum protein electrophoresis was normal. Rheumatoid factor was 179, however this had been down-trending over the previous years. A biopsy was performed and the histology confirmed the presence of non-necrotising granulomatous inflammatory infiltration of the dermis. The infiltrate was composed of sheets of epithelioid histiocytes, occasional multinucleated giant cells, lymphocytes and plasma cells. Numerous Leishmania amastigotes were identified within the cytoplasm of the histiocytes (figures 1 and 2). There were no atypical features hence malignancy was excluded. PCR for Leishmania subspecies was then done and this revealed the presence of Leishmania donovani complex subgenus as the causative organism.
Figure 1.
Histology of the nasal vestibular lesion showing features of leishmaniasis.
Figure 2.
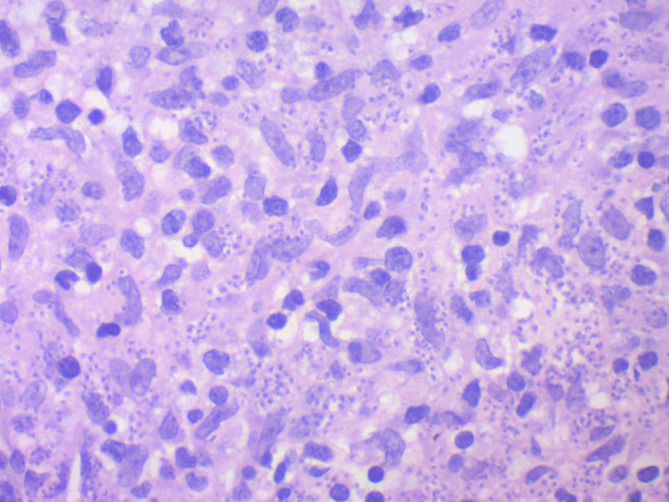
Histology of the nasal vestibular lesion showing features of leishmaniasis.
RW was reviewed by the infectious diseases team with an intention to treat. Due to immunosuppression from the methotrexate he was taking for his arthritis, it was decided to treat him with liposomal amphotericin B at a dose of 75 mg on alternate days for a total of 20 doses. By the end of treatment, the epistaxis stopped and the lesion had completely healed.
Outcome and follow-up
On follow-up, the lesion had resolved and did not recur. RW denied any further episodes of epistaxis. He was advised to report back immediately with any new unexplained lesions.
Discussion
Leishmaniasis refers to the infection by the Leishmania protozoan, transmitted by an infected, female phlebotomine sandfly.1 Although the majority of cases of ML occur in Latin America, it can be found worldwide. Poverty and crowded housing increase sites for sandfly breeding and malnourishment increases the severity of the disease. Urbanisation of rural areas also increases the risk of Leishmania infection.2
ML can manifest after years of cutaneous leishmaniasis that would have metastasised to the mucosa (also known as espundia). It can also coexist with cutaneous/visceral leishmaniasis and rarely as in this case, ML can occur in isolation as a primary infection.1 ML is a form of tegumentary leishmaniasis that is associated with L. braziliensis, L. panamensis and less frequently with L. amazonensis. These are subspecies of Leishmania (Viannia) that are most commonly found in Mexico, Central and South America, the New western world. In contrast, only a few cases of ML have been reported in the Old Eastern World comprising Asia, the Middle East, Africa and Southern Europe. The Leishmania species subtypes in the Old World causing ML are L. infantum in the Mediterranean Basin, L. tropica and L. donovani mostly in India, Sudan and Sri Lanka.1 In Malta, Leishmania is endemic with cutaneous cases being especially prevalent. The only other reported case of ML in Malta was of laryngeal ML that was also a primary infection,3 but the causative subspecies was not reported. Our case is the first reported case of ML caused by L. donovani in Malta.
The nasal vestibule and septum, the lower turbinates and the floor of the nose are the preferred sites for Leishmania, however, the mouth, pharynx and larynx may also be involved. If it is left untreated, it can cause severe disease and deformity.4 No CT or MRI of the paranasal sinuses was done. It would have been useful in checking complications resulting from deeper infiltration of the infection such as perforations. However, our patient responded very well to treatment and there was no evidence of such complications hence such imaging was not deemed necessary.
ML treatment options include pentavalent antimonial sodium stibogluconate, liposomal amphotericin B, azoles and miltefosine.5 Sodium stibogluconate can be administered intravenously or intramuscularly, however, care should be taken when prescribing it in individuals over 50 years of age in view of the risk of serious complications including prolonged QTc interval on ECG and hyperkalaemia.6 In this case given the patient’s immunosuppression, thus, requiring a stronger agent, and old age liposomal amphotericin B was deemed to be a more suitable and safer option.
Treatment failure or disease reactivation can occur after several years, however, this is more often seen in cases of visceral leishmaniasis. The cause of reactivation can be multifactorial including the Leishmania species, patient’s genetic background, age, weight, comorbidities, lesion duration, number of lesions and drug metabolism.7
Leishmania incidence is higher in patients receiving chemotherapy or other immunosuppressants, or who are HIV positive. These patients also tend to have a worse prognosis and a greater chance of reactivation. It has been found that in patients with rheumatoid arthritis on methotrexate, the risk of reactivation is higher than for those on anti-tumour necrosis factor-α treatment.8–10 We believe that this rarely described condition in Malta developed in our patient mainly because of his immunosuppressed state. For this reason, the possibility of Leishmania reactivation should be kept in mind when prescribing methotrexate in endemic areas such as Malta. Immunosuppressed patients with ML need a more intensive treatment regimen to ensure complete treatment of the disease.11 12 In this case, the patient was treated with intravenous liposomal amphotericin B for a total of 20 doses.
Patient’s perspective.
I was very anxious to find out what was the cause of the recurrent epistaxis. Once leishmaniasis was diagnosed and I was informed that there is treatment for it I was more reassured. After the first few doses of liposomal amphotericin the epistaxis stopped. I am now asymptomatic and glad that the infection has been treated.
Learning points.
Mucosal leishmaniasis (ML) can manifest as the reactivation of previous cutaneous leishmaniasis (espundia), it can also coexist with cutaneous or visceral forms or rarely as a primary infection.
ML is rare in Malta and Leishmania donovani rarely causes ML in the Old World, but this is the first such case report in Malta.
Immunosuppressed patients are at the greatest risk of leishmaniasis infection and reactivation.
ML reactivation is rare, but methotrexate use in rheumatoid arthritis is linked to a higher incidence of reactivation compared with other biological agents.
Leishmania should always be part of the differential diagnosis of skin and mucosal lesions in areas where the disease is endemic
Footnotes
Contributors: PG accepted full responsibility of the work, the conduct of the study, data collection and controlled the decision to publish. SMV contributed also in the planning, conduction and acquisition of data. TP contributed in the interpretation of data, critical revision of the case report and approved the final version to be published.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Strazzulla A, Cocuzza S, Pinzone MR, et al. Mucosal leishmaniasis: an underestimated presentation of a neglected disease. Biomed Res Int 2013;2013:1–7. 10.1155/2013/805108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marsden PD. Mucosal leishmaniasis ("espundia" Escomel, 1911). Trans R Soc Trop Med Hyg 1986;80:859–76. 10.1016/0035-9203(86)90243-9 [DOI] [PubMed] [Google Scholar]
- 3.Fsadni C, Fsadni P, Piscopo T, et al. Laryngeal leishmaniasis in Malta. J Infect 2007;54:e61–3. 10.1016/j.jinf.2006.04.003 [DOI] [PubMed] [Google Scholar]
- 4.Faucher B, Pomares C, Fourcade S, et al. Mucosal Leishmania infantum leishmaniasis: specific pattern in a multicentre survey and historical cases. J Infect 2011;63:76–82. 10.1016/j.jinf.2011.03.012 [DOI] [PubMed] [Google Scholar]
- 5.World Health Organisation Leishmaniasis, 2020. Available: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis
- 6.Neumayr ALC, Walter C, Stoeckle M, et al. Successful treatment of imported mucosal Leishmania infantum leishmaniasis with miltefosine after severe hypokalemia under meglumine antimoniate treatment. J Travel Med 2012;19:124–6. 10.1111/j.1708-8305.2011.00572.x [DOI] [PubMed] [Google Scholar]
- 7.Souza RMde, Andrade HFde, Duarte MIS, et al. Reactivation of cutaneous and mucocutaneous tegumentary leishmaniasis in rheumatoid arthritis patients: an emerging problem? Rev Inst Med Trop Sao Paulo 2017;59:e6. 10.1590/S1678-9946201759006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conceição-Silva F, Leite-Silva J, Morgado FN, et al. The binomial parasite-host immunity in the healing process and in reactivation of human tegumentary leishmaniasis. Front Microbiol 2018;9:1308. 10.3389/fmicb.2018.01308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Ruiter MHT, Stijnis C, Nolte JW, et al. Fulminant presentation of oral mucosal leishmaniasis as severe stomatitis and periodontitis. Neth J Med 2018;76:40–2. [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention CDC Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the infectious diseases Society of America (IDSA) and the American Society of tropical medicine and hygiene, 2020. Available: https://www.cdc.gov/parasites/leishmaniasis/health_professionals/index.html
- 11.Miranda Lessa M, Andrade Lessa H, Castro TWN, et al. Mucosal leishmaniasis: epidemiological and clinical aspects. Braz J Otorhinolaryngol 2007;73:843–7. 10.1016/S1808-8694(15)31181-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aliaga L, Cobo F, Mediavilla JD, et al. Localized mucosal leishmaniasis due to Leishmania (Leishmania) infantum. Medicine 2003;82:147–58. 10.1097/01.md.0000076009.64510.b8 [DOI] [PubMed] [Google Scholar]