Abstract
A 61-year-old Caucasian woman presented to an outpatient otolaryngology clinic with increased bleeding from a dorsal tongue telangiectasia for 3 weeks. Her history was significant for hereditary haemorrhagic telangiectasia (HHT), a rare condition that causes vascular dysplasia, and recent symptomatic anaemia requiring blood transfusions. After failing medical management with topical haemostatic agents, she was offered and underwent surgical intervention to remove the tongue telangiectasia with duel therapy potassium titanyl phosphate (KTP) laser coblation and bevacizumab injections. A team of otolaryngologists removed the lesion without complications, and the patient denied bleeding, had minimal pain, and endorsed increased quality of life postoperatively. Tongue telangiectasias can cause life-threatening bleeding in some patients with HHT, and no surgical management guidelines exist to treat them. This case demonstrates the efficacy of KTP laser followed by bevacizumab injections in treating tongue telangiectasias in a patient with HHT.
Keywords: ear, nose and throat/otolaryngology, nose and throat, therapeutic indications, head and neck surgery, otolaryngology / ENT
Background
Hereditary haemorrhagic telangiectasia (HHT) is an autosomal dominant disorder that causes vascular dysplasia affecting various organ systems.1 HHT commonly manifests as epistaxis, mucocutaneous telangiectasias, gastrointestinal bleeding and iron deficiency anaemia, but can also cause arteriovenous malformations (AVMs) in the brain, lungs and liver.2 Because of its rarity, HHT treatment is not well studied and much existing research addresses the management of epistaxis, the most common manifestation of HHT.1 Therapy for intranasal telangiectasias includes systemic, topical and surgical options, but little evidence guides treatment of tongue telangiectasias. A number of case studies describe the efficacy of laser or bevacizumab injection monotherapy for recurrent bleeding from tongue telangiectasias in patients with HHT,3 4 but no evidence-based treatment guidelines exist to our knowledge. The current case report describes the treatment of tongue telangiectasias using potassium titanyl phosphate (KTP) laser and bevacizumab and demonstrates the benefit of duel therapy in patients with HHT with tongue telangiectasias.
Case presentation
A 61-year-old Caucasian woman presented to an outpatient otolaryngology clinic with increased bleeding from a dorsal tongue telangiectasia. Her medical history includes HHT with oral telangiectasias, gastric AVMs and epistaxis controlled after sinus surgery. She denied a family history of HHT. On presentation, she reported increased bleeding from her tongue telangiectasia for 3 weeks, occurring three to four times a day with an estimated daily blood loss of 40 mL. Most episodes were provoked by eating, while others occurred without incitement. She also noted increased fatigue and shortness of breath consistent with anaemia.
On examination, multiple telangiectasias of varying sizes were observed on the anterior, lateral and dorsal portions of the tongue, as well as along the floor of the nose, right inferior turbinate and lateral wall, and superior nasal cavity bilaterally. The telangiectasia that bled most frequently was approximately 4 mm in diameter on the dorsal anterior portion of the tongue deep to the mucosa. Complete blood count was notable for haemoglobin (Hb) of 11.4 g/dL, haematocrit of 32.8% and red blood cell distribution width of 20.3%. All other lab values, blood pressure and heart rate were within normal limits. The patient’s lingual bleeding was well controlled with Surgifoam for 2 months prior to presentation without use of systemic therapies, but her quality of life suffered from recurrent bleeding with eating, which prompted the use of surgical intervention.
Investigations
The patient first experienced recurrent epistaxis at age 18 and noticed telangiectasias on her tongue and fingertips at age 42. She was definitively diagnosed with HHT at age 51 by meeting three of four Curaçao diagnostic criteria for HHT; the fourth criterion of family history was negative in this patient, suggesting a spontaneous genetic mutation unidentified by chart review or genetic testing, and unknown to the patient. This patient suffered from long-standing history of symptomatic anaemia, which required multiple blood transfusions since her diagnosis, and she received two prior treatments of KTP laser ablation of nasal telangiectasias for epistaxis in 2009 and 2011. Five months prior to presentation, her symptomatic anaemia worsened due to continuous haemorrhage of her tongue telangiectasia for which she received three units of blood and three iron transfusions to correct an Hb of 7.4 g/dL. Her symptoms and Hb levels improved since that time.
Treatment
The patient agreed to surgery to remove the tongue and nasal telangiectasias after weighing the risks and discomfort of continued haemorrhage against those of the proposed procedure. After obtaining informed consent and administering anaesthesia, the telangiectasias along the floor of the nose, right inferior turbinate and lateral wall, and superior nasal cavity bilaterally were addressed with coblation at a setting of 3 Watts, and submucosal regions were treated with 100 mg bevacizumab injection. Small lateral and anterior tongue telangiectasias were ablated with the KTP laser on setting 2 Watts, while the larger dorsal telangiectasias were ablated with the KTP laser on setting 3 and 4 Watts with a total of 206 joules. The large dorsal haemorrhagic telangiectasia most bothersome to the patient was ablated using the KTP laser at a setting of 4 Watts; laser energy was allocated peripherally to centrally in a circular motion until the telangiectasia was removed (figure 1). Bevacizumab was then conservatively injected into telangiectasias in the submucosa of the tongue to prevent soft tissue necrosis (figure 2). Finally, several telangiectasias on the hard palate were addressed with continuous KTP laser at a setting of 2 Watts followed by bevacizumab injection. There was no significant bleeding during the procedure. The patient was discharged home in stable condition after surgery.
Figure 1.
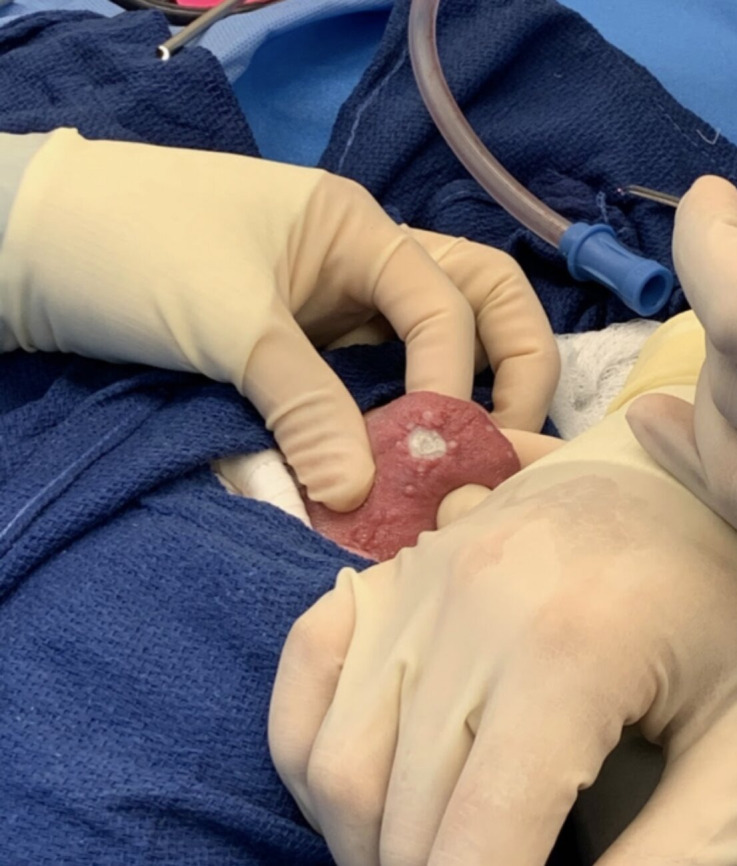
Dorsal tongue telangiectasia following potassium titanyl phosphate ablation at a setting of 4 Watts.
Figure 2.
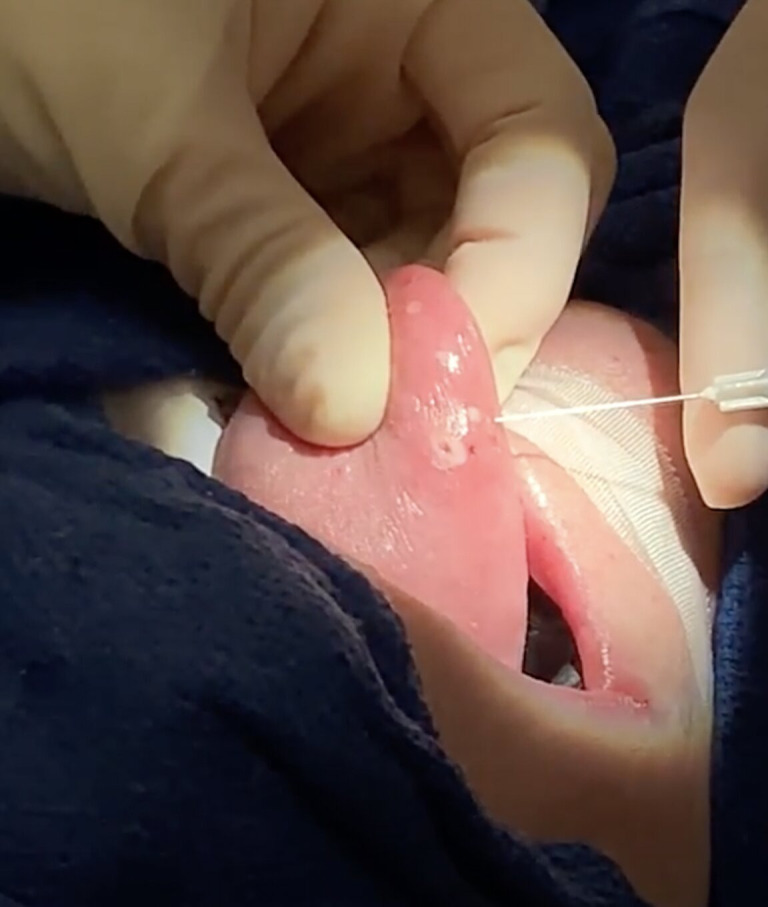
Submucosal bevacizumab injections of tongue telangiectasias.
Outcome and follow-up
The patient’s tongue bleeding stopped immediately. She denied bleeding events or complications at any point postoperatively. On postoperative day 12, she rated her tongue pain a 3 out of 10 without use and reported no additional complaints. She denied fatigue and shortness of breath and endorsed a subjectively improved quality of life compared to her preoperative status.
Discussion
HHT, also known as Rendu-Osler-Weber syndrome, is a rare autosomal dominant disease characterised by small mucocutaneous telangiectasias and larger AVMs of the brain, lungs and liver.2 The prevalence of this disease is thought to be approximately 1 in 5000,5 but this may be an underestimation due to variable penetrance as well as later-onset presentation in some patients. HHT typically presents by age 21, and the most common clinical manifestation is epistaxis, which occurs in over 90% of patients.6 7 Telangiectasias are present in over 74% of all HHT cases and typically manifest prior to age 30.6 HHT is associated with increased risk of bleeding, with epistaxis and gastrointestinal bleeding accounting for over two-thirds of urgent medical attention in this patient population.7 Patients have increased rates of iron deficiency anaemia requiring blood transfusions, and they report decreased quality of life compared with the general population.8 Overall, 90% of patients with telangiectasias report having nasal mucosa telangiectasias, and 50%–80% report having some form of mucocutaneous telangiectasias of the tongue, lips, palate or fingers.9 While considerably less likely to cause serious bleeding than nasal mucosa telangiectasias,9 tongue telangiectasias may be an under-recognised complication of HHT.
To be definitely diagnosed with HHT, patients must meet three of the four Curaçao criteria1: recurrent, spontaneous epistaxis2 a first-degree relative with HHT3 multiple telangiectasias at characteristic sites (nose, oral cavity, lips, fingers)4 presence of gastrointestinal lesions and AVMs of the brain, lungs or liver.10 A diagnosis may be suspected if a patient meets two of the four criteria and is unlikely if fewer than two criteria are met.10 Although the exact pathogenesis of HHT is unclear, 85% of HHT cases stem from mutations of three genes encoding proteins which mediate transforming growth factor beta signalling.11 Patients with HHT express significantly elevated levels of vascular endothelial growth factor (VEGF) when compared with controls,12 and there is consensus that impaired blood vessel formation in addition to a discrepancy of proangiogenic and antiangiogenic factors in patients with HHT considerably contribute to the disease pathogenicity.11 Treatment, therefore, is aimed at intervening on the genetic and cellular mechanisms of the disease and managing symptoms.
Heavy, recurrent bleeding of specific vascular lesions is most common in patients over 40 and significantly contributes to the associated morbidity of the disease.2 6 These lesions are initially managed with diet modification and topical therapies such as ointments, saline sprays and topical haemostatic agents.1 13 Tamoxifen and tranexamic acid have been shown to be effective systemic treatments of vascular lesions when local therapy fails, but both carry increased risk of venous thromboembolism.14–16 Refractory telangiectasias are treated via surgery with laser photocoagulation, coblation or bipolar cautery, among other therapies.
Evidence-based surgical guidelines in treating HHTs are lacking, but laser photocoagulation is commonly used since it spares surrounding mucosa and has limited potential for thermal depth injury.17 18 The KTP laser is relatively specific for treating cutaneous vascular lesions due to its wavelength (585 nm) that corresponds to the absorption peak of oxyhaemoglobin (542 nm), and it effectively controls moderate-to-severe cases of telangiectasia bleeding.19 KTP laser is well tolerated by patients is not associated with perioperative and postoperative complications, and its specificity for blood vessels allows for repeated procedures. While evidence in support of the KTP laser in patients with HHT stems from studies addressing epistaxis,20 it appears to be an appropriate treatment modality for tongue telangiectasias due to its proven effectiveness in safely ablating small, punctate vascular lesions as well as reported success in limited case reports.3
Mucosal injection of bevacizumab is an additional therapy used to treat moderate-to-severe telangiectasia bleeding and is thought to be of increased value when combined with surgery.17 Bevacizumab is a recombinant, humanised, monoclonal antibody that prevents angiogenesis by binding to and inhibiting the actions of VEGF, causing the regression of aberrant blood vessels. It is conventionally used to reduce abnormal retinal blood vessels in patients with age-related macular degeneration and to treat patients with metastatic colorectal cancer;21 its use in treating HHT is currently off-label. Bevacizumab treats telangiectasias by blocking increased levels of VEGF observed in those with HHT and represents a promising option for treating epistaxis in such patients.22 Notably, bevacizumab has been shown to disrupt angiogenesis-dependent wound healing of irritated mucosa, and therefore should be injected into soft tissue conservatively to avoid tissue necrosis secondary to deficient blood supply.23
While valuable as a monotherapy,24 a study by Simonds et al concluded that patients who received submucosal injections of bevacizumab in addition to KTP laser therapy had less bleeding, fewer transfusion requirements and showed improvement in quality of life compared with those who received KTP laser treatment alone.23 Injected bevacizumab is considered safe when avoiding the cartilaginous septum and is effective in reducing telangiectasias and iron deficiency anaemia.23 25 Based on its mechanism and success in one case study treating mucocutaneous lesions,4 bevacizumab appears to be a viable option in treating tongue telangiectasias especially in combination with KTP laser, though randomised clinical trials are still needed to confirm its efficacy. Two cases reported the success of surgical wedge resection to remove glossal lesions caused by HHT.26 27 Surgical resection may be a reasonable treatment for deep vascular lesions impenetrable by KTP laser or those that rebleed after laser therapy. A longitudinal investigation comparing surgical management of tongue telangiectasias would be valuable.
While tongue telangiectasias are typically of cosmetic concern, they have the potential to cause serious bleeding that may require blood transfusions and contribute to decreased quality of life in some patients. Our patient tolerated duel KTP laser and bevacizumab therapy without postsurgical tongue bleeding. She reported limited pain, improvements in fatigue, shortness of breath, and quality of life postoperatively. KTP laser with bevacizumab injections controlled this patient’s bleeding successfully and represented a safe and beneficial treatment option in a patient with tongue telangiectasias refractory to medical management.
Learning points.
This is a case of a rare and understudied disease. Hereditary haemorrhagic telangiectasia (HHT) is an autosomal dominant disorder that causes vascular dysplasia in various organ systems.
While uncommon, tongue telangiectasias can cause significant morbidity in patients with HHT. No treatment guidelines exist to direct surgical care for patients suffering from haemorrhagic tongue telangiectasias.
Dual-therapy potassium titanyl phosphate laser coblation and bevacizumab injections were effective in curing a tongue telangiectasia in a woman with HHT. This surgery represents a safe and effective treatment option for patients with tongue telangiectasias refractory to medical management.
Footnotes
Twitter: @evebowers_
Contributors: EMRB collected and interpreted the patient data regarding the genetic disease and surgery and was the major contributor in writing the manuscript. SL was the attending surgeon in this patient’s case and provided edits and oversight to this manuscript. All authors read and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Silva BM, Hosman AE, Devlin HL, et al. Lifestyle and dietary influences on nosebleed severity in hereditary hemorrhagic telangiectasia. Laryngoscope 2013;123:1092–9. 10.1002/lary.23893 [DOI] [PubMed] [Google Scholar]
- 2.Lesca G, Olivieri C, Burnichon N, et al. Genotype-Phenotype correlations in hereditary hemorrhagic telangiectasia: data from the French-Italian HHT network. Genet Med 2007;9:14–22. 10.1097/GIM.0b013e31802d8373 [DOI] [PubMed] [Google Scholar]
- 3.Masuda Y, Tanabe T, Ohmae Y, et al. Using the KTP laser to control epistaxis in patients with hereditary hemorrhagic telangiectasia. Pract Otorhinolaryngol 2004;97:1075–81. 10.5631/jibirin.97.1075 [DOI] [Google Scholar]
- 4.Kochanowski J, Sobieszczańska M, Tubek S, et al. Successful therapy with bevacizumab in a case of hereditary hemorrhagic telangiectasia. Hum Vaccin Immunother 2015;11:680–1. 10.1080/21645515.2015.1011960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kjeldsen AD, Vase P, Green A. Hereditary haemorrhagic telangiectasia: a population-based study of prevalence and mortality in Danish patients. J Intern Med 1999;245:31–9. 10.1046/j.1365-2796.1999.00398.x [DOI] [PubMed] [Google Scholar]
- 6.Plauchu H, de Chadarévian JP, Bideau A, et al. Age-Related clinical profile of hereditary hemorrhagic telangiectasia in an epidemiologically recruited population. Am J Med Genet 1989;32:291–7. 10.1002/ajmg.1320320302 [DOI] [PubMed] [Google Scholar]
- 7.Gallitelli M, Pasculli G, Fiore T, et al. Emergencies in hereditary haemorrhagic telangiectasia. QJM 2006;99:15–22. 10.1093/qjmed/hci148 [DOI] [PubMed] [Google Scholar]
- 8.Ingrand I, Ingrand P, Gilbert-Dussardier B, et al. Altered quality of life in Rendu-Osler-Weber disease related to recurrent epistaxis. Rhinology 2011;49:155–62. 10.4193/Rhino09.138 [DOI] [PubMed] [Google Scholar]
- 9.Shovlin CL, Letarte M. Hereditary haemorrhagic telangiectasia and pulmonary arteriovenous malformations: issues in clinical management and review of pathogenic mechanisms. Thorax 1999;54:714–29. 10.1136/thx.54.8.714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shovlin CL, Guttmacher AE, Buscarini E, et al. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am J Med Genet 2000;91:66–7. [DOI] [PubMed] [Google Scholar]
- 11.Dupuis-Girod S, Bailly S, Plauchu H. Hereditary hemorrhagic telangiectasia: from molecular biology to patient care. J Thromb Haemost 2010;8:1447–56. 10.1111/j.1538-7836.2010.03860.x [DOI] [PubMed] [Google Scholar]
- 12.Sadick H, Naim R, Gössler U, et al. Angiogenesis in hereditary hemorrhagic telangiectasia: VEGF165 plasma concentration in correlation to the VEGF expression and microvessel density. Int J Mol Med 2005;15:15–19. 10.3892/ijmm.15.1.15 [DOI] [PubMed] [Google Scholar]
- 13.Cooper B. William Osler on telangiectatic syndromes. Baylor University Medical Center Proceedings 1999;12:238–40. 10.1080/08998280.1999.11930183 [DOI] [Google Scholar]
- 14.Hsu Y-P, Hsu C-W, Bai C-H, et al. Medical treatment for epistaxis in hereditary hemorrhagic telangiectasia: a meta-analysis. Otolaryngol Head Neck Surg 2019;160:22–35. 10.1177/0194599818797316 [DOI] [PubMed] [Google Scholar]
- 15.Livesey JA, Manning RA, Meek JH, et al. Low serum iron levels are associated with elevated plasma levels of coagulation factor VIII and pulmonary emboli/deep venous thromboses in replicate cohorts of patients with hereditary haemorrhagic telangiectasia. Thorax 2012;67:328–33. 10.1136/thoraxjnl-2011-201076 [DOI] [PubMed] [Google Scholar]
- 16.Gaillard S, Dupuis-Girod S, Boutitie F, et al. Tranexamic acid for epistaxis in hereditary hemorrhagic telangiectasia patients: a European cross-over controlled trial in a rare disease. J Thromb Haemost 2014;12:1494–502. 10.1111/jth.12654 [DOI] [PubMed] [Google Scholar]
- 17.Karnezis TT, Davidson TM. Efficacy of intranasal bevacizumab (Avastin) treatment in patients with hereditary hemorrhagic telangiectasia-associated epistaxis. Laryngoscope 2011;121:636–8. 10.1002/lary.21415 [DOI] [PubMed] [Google Scholar]
- 18.Adamič M, Pavlović MD, Troilius Rubin A, et al. Guidelines of care for vascular lasers and intense pulse light sources from the European Society for laser dermatology. J Eur Acad Dermatol Venereol 2015;29:1661–78. 10.1111/jdv.13177 [DOI] [PubMed] [Google Scholar]
- 19.Sautter NB, Smith TL. Hereditary hemorrhagic telangiectasia-related epistaxis: innovations in understanding and management. Int Forum Allergy Rhinol 2012;2:422–31. 10.1002/alr.21046 [DOI] [PubMed] [Google Scholar]
- 20.Harvey RJ, Kanagalingam J, Lund VJ. The impact of septodermoplasty and potassium-titanyl-phosphate (KTP) laser therapy in the treatment of hereditary hemorrhagic telangiectasia-related epistaxis. Am J Rhinol 2008;22:182–7. 10.2500/ajr.2008.22.3145 [DOI] [PubMed] [Google Scholar]
- 21.Kovach JL, Schwartz SG, Flynn HW, et al. Anti-Vegf treatment strategies for wet AMD. J Ophthalmol 2012;2012:1–7. 10.1155/2012/786870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riss D, Burian M, Wolf A, et al. Intranasal submucosal bevacizumab for epistaxis in hereditary hemorrhagic telangiectasia: a double-blind, randomized, placebo-controlled trial. Head Neck 2015;37:783–7. 10.1002/hed.23655 [DOI] [PubMed] [Google Scholar]
- 23.Simonds J, Miller F, Mandel J, et al. The effect of bevacizumab (Avastin) treatment on epistaxis in hereditary hemorrhagic telangiectasia. Laryngoscope 2009;119:988–92. 10.1002/lary.20159 [DOI] [PubMed] [Google Scholar]
- 24.Karnezis TT, Davidson TM. Treatment of hereditary hemorrhagic telangiectasia with submucosal and topical bevacizumab therapy. Laryngoscope 2012;122:495–7. 10.1002/lary.22501 [DOI] [PubMed] [Google Scholar]
- 25.Epperla N, Hocking W. Blessing for the bleeder: bevacizumab in hereditary hemorrhagic telangiectasia. Clin Med Res 2015;13:32–5. 10.3121/cmr.2013.1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ichimura K, Kikuchi H, Imayoshi S. How to deal with oral telangiectasis in hereditary hemorrhagic telangiectasia patients. Stomato-pharyngology 2013;26:85–8. [Google Scholar]
- 27.Caldwell TA, Schweber SJ, Lucchesi FJ. Resection of tongue lesion associated with hereditary telangiectasia (Osler-Weber-Rendu disease): report of case. J Oral Surg 1970;28:299–301. [PubMed] [Google Scholar]


