Abstract
Background
Pediatric patients with genetic disorders have a higher incidence of pulmonary arterial hypertension (PAH) regardless of their heart defects. Filamin A (FLNA) mutation is recently recognized to be associated with pediatric pulmonary disorders, however, the clinical courses of PAH related to the mutation were reported in limited cases. Here, we presented a case and pooled data for better understanding of the correlation between FLNA mutation and pediatric PAH.
Case presentation
The patient was a 8-month-old female with repeated episodes of pneumonia. Physical examination revealed cleft lip, cleft palate and developmental retardation. Imaging examination showed a small atrial septal defect (ASD), central pulmonary artery enlargement, left upper lobe of lung atelectasis, and pulmonary infiltration. Genetic test showed she carried a de novo pathogenic variant of FLNA gene (c.5417-1G > A, p.-). Oral medications didn’t slow the progression of PAH in the patient, and she died two years later.
Conclusions
FLNA mutation causes rare but progressive PAH in addition to a wide spectrum of congenital heart disease and other comorbidities in pediatric patients. We highly recommend genetic testing for pediatric patients when suspected with PAH. Given the high mortality in this group, lung transplantation may offer a better outcome.
Keywords: Pulmonary arterial hypertension, Congenital heart disease, Filamin A
Background
Pediatric pulmonary arterial hypertension (PAH) is a rare disease with high mortality. Left-to-right shunting, lung diseases and genetic disorders are most common causes leading to PAH in children[1]. Filamin A (FLNA) is a 280-kD protein widely expressed in the body and regulating cell shape and migration. Among the broad range of diseases associated with FLNA mutation, lung diseases have been seen in most patients, such as pneumonia, and respiratory failure. In addition, PAH in pediatric patients with FLNA mutation was fatal despite of their congenital heart disease (CHD), and required early lung transplantation[2]. Here we report a female patient with FLNA mutation, who presented with recurrent pneumonia, arterial septal defect (ASD), mild developmental delay and rapidly progressive PAH.
Case presentation
An 8-month-old female patient was referred to our center due to severe cough, short of breath, fatigue and fever. The patient had nine episodes of pneumonia and cardiomegaly since she was two-month-old. Physical examination revealed cleft lip, which was surgical repaired when she was 6 months old, and cleft palate. Her finger oxygen saturation was 94%. Transthoracic echocardiography showed there was a 0.5 × 0.6 cm ASD with a 2.4 cm right atrium. Laboratory test showed NT-proBNP was 963 pg/ml. Some of autoimmune antibodies, including dsDNA-antibody, SSA/Ro 60kD antibody, anti-cardiolipid antibody, and anti-β2GPI antibody, were positive. Erythrocyte sedimentation rate (ESR) and C-reaction protein (CRP) were normal. IgG was slightly elevated at 18.40 g/L, and C3 was 0.83 g/L. Significantly increased pulmonary vascular resistance (PVR, 17 WU) was seen in her first right heart catheterization despite of the slightly increased pulmonary artery pressure (PAP, 38/17/24 mmHg). Oral furosemide and antisterone were given since then. She was also suggested to inhale oxygen at home even though she maintained her daily activities without additional requirement of oxygen. The patient was re-hospitalized several times because of recurrent pneumonia and heart failure thereafter. Her finger oxygen saturation dropped to 75% at lowest, and stayed at 95% or higher when given nasal catheter oxygen inhalation. Hemodynamic parameters turned worse in the second measurements, where PAP increased along with PVR (PAP, 100/50/67 mmHg; PVR, 42 WU). Further examination included chest computed tomography (CT) scan. CT showed infiltration in upper lobes at both sides (Fig. 1a, b), and lung atelectasis in left upper lobe (Fig. 1b). Pulmonary artery and right atrium were significantly dilated (Fig. 1b, star; d). No thrombosis was seen in pulmonary artery. The patient and her parents received whole exome sequencing test. A new splicing variant (exon34: c.5417-1G > A, p.-) in the FLNA gene was found only in the patient. Diuretics, dopamine, and oral Bosentan (12.5 mg twice daily) were used to relieve her symptoms. No intubation or other advanced life supports were required during hospitalizations. Patient’s family refused any further intervention during her last hospitalization at age of 2 years. She became significantly cyanosis after last discharge. Unfortunately, the patient didn’t response well to medication therapy, and she died from a severe pneumonia 5 months later.
Fig. 1.
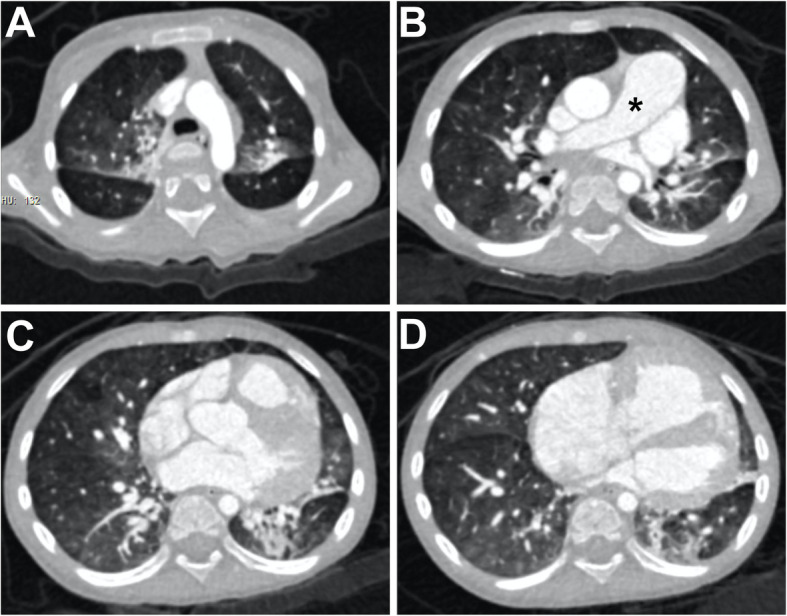
Chest CT. a Infiltration in both upper lobes of lung; b Main pulmonary artery was dilated (*). There was atelectasis in left upper lobe of lung; c Slightly infiltration in lower lobes; d Dilated right atrium.
Discussion and conclusions
PAH is a clinical symptom characterized by increased pulmonary artery pressure more than 25 mmHg. Pediatric PAH shares similarities with adult PAH in some etiology. However, specialists have addressed that pediatric patients have higher prevalence of idiopathic PAH, PAH associated with congenital heart disease (CHD), and pulmonary disorders [3]. With the attempt to explore mechanism underlying, next generation sequencing reveals the genetic defects associated with pediatric PAH.
FLNA gene was firstly related to neurologic disorder defect periventricular heterotopia (PVNH) in 1998 [4]. A broad range of diseases were observed with FLNA mutation thereafter, such as otopalatodigital syndrome (OPD) [5], frontometaphyseal dysplasia (FMD) [6], and Melnick-Needles syndrome (MNS) [5], FG syndrome (FGS), chronic idiopathic intestinal pseudoobstruction (CIIP) [7], cardiac valvular disease (CVD) [8], and others. However, lung disease was noticed in patients with FLNA mutation first by de Wit MC, et al. in 2010 [9]. Patients with lung disease related to FLNA mutation had higher incidence of pneumonia, lung developmental defects and respiratory failure, however, PAH were uncommon [10–12]. Among the reported cases, there were 19 of them having early onset PAH (including this case). Their clinical characteristics are summarized in Table 1. Developmental delay was observed in 6 patients, while CHD were seen in all. Fourteen patients had surgical correction of CHD, 6 of which had lung transplantation at the same time. Only one patient died after lung transplantation, nonetheless, mortality among pediatric PAH patients with FLNA mutation is as high as 35%.
Table 1.
Summary of pediatric PAH associated with FLNA mutation
| Mutation | Sex | Age at diagnose | CHD | Chest CT | Lung transplantation | Medicine | Outcome | |
|---|---|---|---|---|---|---|---|---|
| Masurel-Paulet 2011[13] | mosaic nonsense mutation c.994delG(p.K331X) | male | 3 months | PDA | Bilateral atelectasis; lung cysts; tracheobronchomalacia; pulmonary emphysema; congenital lobar emphysema; | N | None | ND |
| Reinstein 2013 [14] | De novo c.2193C > A (p.Tyr731X) | female | 6 months | PDA | Areas of focal hyperinflation associated with minimal patchy atelectasis | N | Sildenifil | ND |
| De novo deletion of exons 2,5 and 13 | female | 18 months | VSD | N/A | N | Bosentan | ND | |
|
De novo c.5498_5504delCACCCACinsAC |
male | 2 months |
ASD;VSD; PDA |
N/A | N | None | Died | |
| Lord 2014 [12] | Truncating filamin A mutation(c.5683G-T, p.G1895*) | female | 4 months | ASD |
Bilateral pulmonary atelectasis and cysts, tracheobronchomalacia, Areas of hyperinflation alternating with heterogeneous areas of atelectasis; alveolar simplification |
N | Inhaled nitric oxide; sidenafil; bosentan | ND |
| Eltahir 2016 [13] | c.3153dupC in exon 21 | female | 2 months | PDA | Bilateral lung emphysema with basal atelectasis; bronchospasm | N | None | Died |
| Burrage 2017 [15] |
Heterozygous c.4596dupG (p.Ser1533Glufs*12) (de novo) |
female | 4 months | PFO, PDA | Multifocal atelectasis; perinflation and hyperlucency; atelectasis; central pulmonary artery enlargement; tracheobronchomalacia | Y | Sildenafil | Alive |
|
Heterozygous c.5290G > A (p.Ala1764Thr) (de novo) |
female | 2 months | PFO, PDA, VSD | Perinflation hyperlucency; atelectasis; central pulmonary artery enlargement; tracheobronchomalacia | Y | Sildenafil | Died | |
|
Heterozygous c.4446_4447dupAT(p.Leu1483Tyrfs* 19) (de novo) |
female | 1month | PFO,PDA | Perinflation hyperlucency; atelectasis; central pulmonary artery enlargement; tracheobronchomalacia | Y | Sildenafil | Alive | |
|
Heterozygous c.4617_4618delGC(p.Leu1540Alafs*) |
female | 2 months | PFO, PDA | Perinflation hyperlucency; atelectasis; central pulmonary artery enlargement; tracheobronchomalacia | Y | Sildenafil | Alive | |
|
Heterozygous c.6585dupT (p.Pro2196Serfs*3) (de novo) |
female | 7 montsh | PFO;PDA | Perinflation hyperlucency; atelectasis; central pulmonary artery enlargement; tracheobronchomalacia | Y | Sildenafil | Alive | |
|
Heterozygous c.2807A > G (p.Lys936Arg) (VUS) |
female | 5 months | PFO, PDA | Perinflation hyperlucency; atelectasis; central pulmonary artery enlargement; tracheobronchomalacia | Y | Sildenafil | Alive | |
| Shelmerdine 2017 [10] |
Heterozygous for c.88delG, p.(Ala30fs) |
female | ND | PDA;PFO | Left lung hyperinflation; interstitial thickening in left; mediastinal shift to the right; right lobe consolidation | N | None | Died |
|
Heterozygous for c.6496dupA, p. (lle2166fs) |
female | ND | PDA | Progressive right lung hyperinflation; mediastinal shift to the left; right upper and middle lobe over inflation; coarse septal thickening; lower lobe atelectasis; patchy ground glass changes in lower lobes | N | Sildenafil | Alive | |
|
Heterozygous for c.2190_2193delTTAC, p(tyr731fs) |
female | ND | ASD;PDA | Right upper lobe hyperinflation; right middle lobe and left lower lobe atelectasis; right upper and middle, left upper lobe over-inflation; coarse septal thickening; lower lobe atelectasis | N | None | Alive | |
| Kinane 2017 [16] | c.6577delC; p.Arg2193AlafsX14[R219AfsX14 | female | 7w | PFO; VSD; PDA | Wilson–Mikity syndrome (pulmonary dysmaturity syndrome) | N | None | ND |
| Sasaki 2018 [17] | Deletion c.6670-1delG | male | neonate | PDA | Bilateral dependent and subsegmental atelectasis, scattered opacity | N | None | Died |
| Cannaerts 2018 [18] | cis-located c.7921C > G,p.Pro2641Ala,c.7923delC,p.Tyr2642Thrfs*63 | female | ND | ASD | ND | N | None | Died |
| This case | splicing c.5417-1G > A (exon 34) | female | 22 months | ASD | Central pulmonary artery enlargement; left upper lobe atelectasis | N | Bosentan | Died |
FLNA Filamin A; CT computed tomography; PDA patent ductus arteriosus; VSD ventricle septal defect; ASD atrial septal defect; N no; Y yes; ND Not provided
Interstitial lung disease (ILD) may cause PAH in pediatric patients, and FLNA mutation has been called for attention in ILD [17], but pediatric PAH patients with FLNA mutation don’t always present with characteristically pulmonary pathologic changes of ILD. Moreover, high prevalence of CHD in patients with FLNA mutation may confuse the real cause of the rapidly progressive PAH [19]. In our experience, genetic testing is more helpful to offer early-stage and accurate diagnose. Moreover, lung transplantation would bring higher survival in these patients based on previous reports.
Acknowledgements
We would like to thank the patient’s family for their consent to publish this report. We also appreciated our medical team and their efforts to treat the patient.
Abbreviations
- FLNA
Filamin A
- CHD
Congenital heart disease
- ASD
Atrial septal defect
- VSD
Ventricle septal defect
- PDA
Patent ductus arteriosus
- PFO
Patent foramen ovale
- RHC
Right heart catheterization
- PAH
Pulmonary arterial hypertension
- NT-proBNP
N-terminal prohormone of brain natriuretic peptide
- ESR
Erythrocyte sedimentation rate
- CRP
C-reaction protein
- PVR
Pulmonary vascular resistance
- WU
Wood unites
- PAP
Pulmonary artery pressure
- CT
Computed tomography
- PVNH
Periventricular heterotopia
- OPD
Otopalatodigital syndrome
- FMD
Frontometaphyseal dysplasia
- MNS
Melnick-Needles syndrome
- FGS
FG syndrome
- CIIP
Chronic idiopathic intestinal pseudoobstruction
- CVD
Cardiac valvular disease
Authors’ contributions
XD, SL, XZ management of the patient, drafting the article, critical revision of the article; QQ, BJ, MY literature review, critical revision of the article; H data collection; YW imaging evaluation; HZ, GZ critical revision of the article. All authors read and approved the final manuscript.
Funding
The authors declare that they did not receive any source of funding for the preparation of the manuscript.
Availability of data and materials
The datasets used in current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study was performed according to the Declaration of Helsinki. Written informed consent was obtained from the patient’s parents for publication of this case report and accompanying images.
Consent for publication
Written informed consent was obtained from the patient’s parents for publication of this case report and accompanying images.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Morrell NW, Aldred MA, Chung WK, Elliott CG, Nichols WC, Soubrier F, Trembath RC, Loyd JE. Genetics and genomics of pulmonary arterial hypertension. Eur Respir J. 2019;53(1). [DOI] [PMC free article] [PubMed]
- 2.Pelizzo G, Collura M, Puglisi A, Pappalardo MP, Agolini E, Novelli A, Piccione M, Cacace C, Bussani R, Corsello G, et al. Congenital emphysematous lung disease associated with a novel Filamin A mutation. Case report and literature review. BMC Pediatr. 2019;19(1):86. doi: 10.1186/s12887-019-1460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenzweig EB, Abman SH, Adatia I, Beghetti M, Bonnet D, Haworth S, Ivy DD, Berger RMF. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur Respir J. 2019;53(1). [DOI] [PMC free article] [PubMed]
- 4.Fox JW, Lamperti ED, Eksioglu YZ, Hong SE, Feng Y, Graham DA, Scheffer IE, Dobyns WB, Hirsch BA, Radtke RA, et al. Mutations in filamin 1 prevent migration of cerebral cortical neurons in human periventricular heterotopia. Neuron. 1998;21(6):1315–1325. doi: 10.1016/S0896-6273(00)80651-0. [DOI] [PubMed] [Google Scholar]
- 5.Robertson SP, Twigg SR, Sutherland-Smith AJ, Biancalana V, Gorlin RJ, Horn D, Kenwrick SJ, Kim CA, Morava E, Newbury-Ecob R, et al. Localized mutations in the gene encoding the cytoskeletal protein filamin A cause diverse malformations in humans. Nat Genet. 2003;33(4):487–491. doi: 10.1038/ng1119. [DOI] [PubMed] [Google Scholar]
- 6.Zenker M, Nahrlich L, Sticht H, Reis A, Horn D. Genotype-epigenotype-phenotype correlations in females with frontometaphyseal dysplasia. Am J Med Genet A. 2006;140(10):1069–1073. doi: 10.1002/ajmg.a.31213. [DOI] [PubMed] [Google Scholar]
- 7.Gargiulo A, Auricchio R, Barone MV, Cotugno G, Reardon W, Milla PJ, Ballabio A, Ciccodicola A, Auricchio A. Filamin A is mutated in X-linked chronic idiopathic intestinal pseudo-obstruction with central nervous system involvement. Am J Hum Genet. 2007;80(4):751–758. doi: 10.1086/513321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kyndt F, Gueffet JP, Probst V, Jaafar P, Legendre A, Le Bouffant F, Toquet C, Roy E, McGregor L, Lynch SA, et al. Mutations in the gene encoding filamin A as a cause for familial cardiac valvular dystrophy. Circulation. 2007;115(1):40–49. doi: 10.1161/CIRCULATIONAHA.106.622621. [DOI] [PubMed] [Google Scholar]
- 9.de Wit MC, Tiddens HA, de Coo IF, Mancini GM. Lung disease in FLNA mutation: confirmatory report. Eur J Med Genet. 2011;54(3):299–300. doi: 10.1016/j.ejmg.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Shelmerdine SC, Semple T, Wallis C, Aurora P, Moledina S, Ashworth MT, Owens CM. Filamin A (FLNA) mutation-A newcomer to the childhood interstitial lung disease (ChILD) classification. Pediatr Pulmonol. 2017;52(10):1306–1315. doi: 10.1002/ppul.23695. [DOI] [PubMed] [Google Scholar]
- 11.Eltahir S, Ahmad KS, Al-Balawi MM, Bukhamsien H, Al-Mobaireek K, Alotaibi W, Al-Shamrani A. Lung disease associated with filamin A gene mutation: a case report. J Med Case Rep. 2016;10:97. doi: 10.1186/s13256-016-0871-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lord A, Shapiro AJ, Saint-Martin C, Claveau M, Melancon S, Wintermark P. Filamin A mutation may be associated with diffuse lung disease mimicking bronchopulmonary dysplasia in premature newborns. Respir Care. 2014;59(11):e171–e177. doi: 10.4187/respcare.02847. [DOI] [PubMed] [Google Scholar]
- 13.Masurel-Paulet A, Haan E, Thompson EM, Goizet C, Thauvin-Robinet C, Tai A, Kennedy D, Smith G, Khong TY, Sole G, et al. Lung disease associated with periventricular nodular heterotopia and an FLNA mutation. Eur J Med Genet. 2011;54(1):25–28. doi: 10.1016/j.ejmg.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Reinstein E, Frentz S, Morgan T, Garcia-Minaur S, Leventer RJ, McGillivray G, Pariani M, van der Steen A, Pope M, Holder-Espinasse M, et al. Vascular and connective tissue anomalies associated with X-linked periventricular heterotopia due to mutations in Filamin A. Eur J Hum Genet. 2013;21(5):494–502. doi: 10.1038/ejhg.2012.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burrage LC, Guillerman RP, Das S, Singh S, Schady DA, Morris SA, Walkiewicz M, Schecter MG, Heinle JS, Lotze TE, et al. Lung transplantation for FLNA-associated progressive lung disease. J Pediatr. 2017;186:118–123 e116. doi: 10.1016/j.jpeds.2017.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinane TB, Lin AE, Lahoud-Rahme M, Westra SJ, Mark EJ. Case 4-2017. A 2-month-old girl with growth retardation and respiratory failure. N Engl J Med. 2017;376(6):562–574. doi: 10.1056/NEJMcpc1613465. [DOI] [PubMed] [Google Scholar]
- 17.Sasaki E, Byrne AT, Phelan E, Cox DW, Reardon W. A review of filamin A mutations and associated interstitial lung disease. Eur J Pediatr. 2019;178(2):121–129. doi: 10.1007/s00431-018-3301-0. [DOI] [PubMed] [Google Scholar]
- 18.Cannaerts E, Shukla A, Hasanhodzic M, Alaerts M, Schepers D, Van Laer L, Girisha KM, Hojsak I, Loeys B, Verstraeten A. FLNA mutations in surviving males presenting with connective tissue findings: two new case reports and review of the literature. BMC Med Genet. 2018;19(1):140. doi: 10.1186/s12881-018-0655-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demirel N, Ochoa R, Dishop MK, Holm T, Gershan W, Brottman G. Respiratory distress in a 2-month-old infant: is the primary cause cardiac, pulmonary or both? Respir Med Case Rep. 2018;25:61–65. doi: 10.1016/j.rmcr.2018.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used in current study are available from the corresponding author on reasonable request.


