Abstract
Background
Vitamins’ deficiency in humans is an important threat worldwide and requires solutions. In the concept of natural biofactory for bioactive compounds production, microalgae represent one of the most promising targets filling many biotechnological applications, and allowing the development of an eco-sustainable production of natural bioactive metabolites. Vitamins are probably one of the cutting edges of microalgal diversity compounds.
Main text
Microalgae can usefully provide many of the required vitamins in humans, more than terrestrial plants, for instance. Indeed, vitamins D and K, little present in many plants or fruits, are instead available from microalgae. The same occurs for some vitamins B (B12, B9, B6), while the other vitamins (A, C, D, E) are also provided by microalgae. This large panel of vitamins diversity in microalgal cells represents an exploitable platform in order to use them as natural vitamins’ producers for human consumption. This study aims to provide an integrative overview on vitamins content in the microalgal realm, and discuss on the great potential of microalgae as sources of different forms of vitamins to be included as functional ingredients in food or nutraceuticals for the human health. We report on the biological roles of vitamins in microalgae, the current knowledge on their modulation by environmental or biological forcing and on the biological activity of the different vitamins in human metabolism and health protection.
Conclusion
Finally, we critically discuss the challenges for promoting microalgae as a relevant source of vitamins, further enhancing the interests of microalgal “biofactory” for biotechnological applications, such as in nutraceuticals or cosmeceuticals.
Keywords: Vitamin D, Vitamin K, Microalgae, Biotechnology, Antioxidants, Nutraceuticals
Background
The class of vitamins includes a diversity of organic compounds that represent essential micro-nutrients for life. These molecules cover a plethora of biological functions, such as coenzymes, hormones, antioxidants, mediators of cell signalling and regulators of cell and tissue growth or differentiation. Vitamins can be divided in two large groups, the water-soluble and fat-soluble compounds. Vitamins A, D, E and K are the four fat-soluble molecules, while the vitamin C and the vitamins B [B1 (thiamin), B2 (riboflavin), B3 (niacin = nicotinic acid), B5 (pantothenic acid), B6 (pyridoxine), B7 (biotin), B9 (folic acid) and B12 (cobalamin)] are water-soluble. Most of the vitamins are synthetized by photosynthetic organisms, while others (some vitamins B and vitamin K) are bioaccumulated through diet and mainly produced by bacteria [1]. Accumulation and/or synthesis of vitamins in photosynthetic organisms is highly variable [2], and strongly related to physiological responses to environmental cues [3]; the magnitude of these responses being dependent on the fitness between organism and the environment [4].
Although crucial for life, vitamins are either not or little synthetized in animals and humans, thus requiring their continuous assimilation through diet, e.g. from plants, fruits or seeds. In order to avoid vitamins deficit in humans, it is strongly recommended to follow diets with high content in the different vitamins. However, not all plants contain all vitamins, and some of them (vitamins D, K or some B) are scarcely present.
Among plant kingdom, marine algae produce or/and accumulate a large diversity of vitamins (Fig. 1), and microalgae—photosynthetic unicellular and fast dividing rate organisms—potentially could be extremely helpful as vitamins’ producers, as the already known “super food” vitamins-rich Spirulina platensis [5]. Microalgae can contain vitamins such as vitamins B12 [3, 6], vitamin K [7] or D [8] that are not present in higher plants. Vitamin D is known to be highly concentrated in sea edible organisms (e.g., fishes, [9]) which accumulate it through algal based diets, being not able to synthetize it [10]. The content in the other vitamins, generally provided by higher plants, can be also significant if we consider that are unicellular organisms. For instance, the green microalga Dunaliella is known to highly accumulate the vitamins B2, B12, B9, B3 as well as the vitamins C and E [11] while high content of vitamin C has been reported in the diatom Skeletonema marinoi [12, 13].
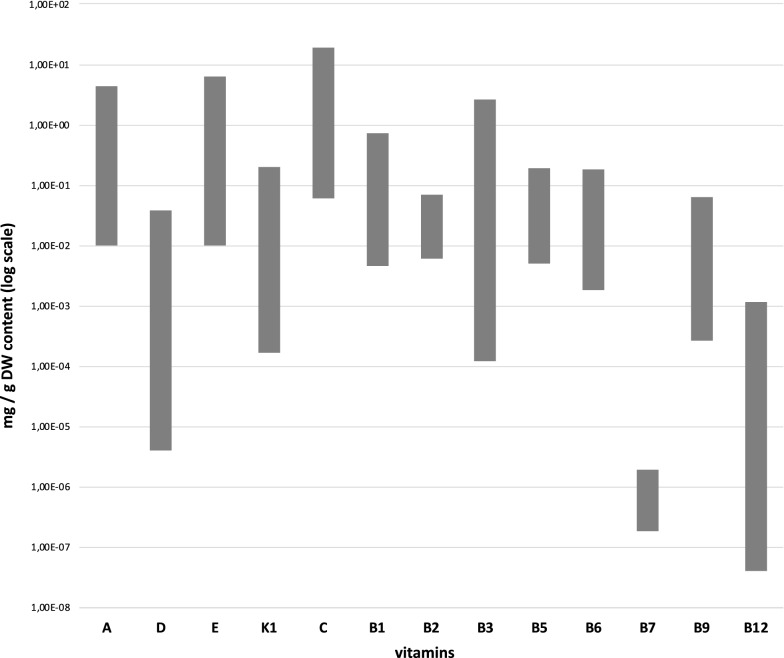
Fig. 1.
Vitamin content (mg g−1 of dry weight biomass) variability in microalgae. Axis Y in logarithmic scale
The general aim of this study is to critically discuss if and why microalgae can become a functional source of vitamins to fill human requirements through food complements or nutraceuticals. In biotechnology, microalgae can be defined a real and natural biofactory of bioactive compounds as well as vitamins for dietary intake [14–16]. It is noteworthy that some vitamins (e.g., vitamins E, A or C) have been the object of numerous studies, although other vitamins were poorly investigated in microalgae. Here, we update an integrated state-of-art on the vitamins A, D, E, K, C, B1, B2, B3, B5, B6, B7, B9 and B12 contents in microalgae, as well as on their biological roles and modulation by environmental or biological forcing in microalgae. We also report on the biological activity of the different vitamins in human metabolism and health protection.
Functions and roles of vitamins in microalgae
In living organisms, vitamins are involved in numerous processes and functions, being required for example as precursors or coenzymes in key metabolic pathways, controlling and regulating tissue growth and cell functioning, or acting as antioxidants. Some vitamins have few specific biological functions (e.g., vitamin B7), while others display multiple roles (e.g., vitamin C).
In microalgae, vitamin A is mainly synthetized from the provitamin A carotenoids (e.g., β-carotene, β-cryptoxanthin, and α-carotene) [17]. The well-known β–carotene is the precursor of many carotenoids, such as those belonging to the photoprotective xanthophyll cycle (Arianna [13] and acts as antioxidant in photosynthetic organisms [17]. Indeed, the efficacy of β-carotene against the reactive oxygen species singlet oxygen is greater than vitamin E and vitamin C [18].
The involvement of the vitamins D2 and D3 in cellular functions in microalgae remains unclear. In higher plants that are able to synthesize vitamin D2, the latter exerts a role as a growth factor [19]. Provitamin D has been hypothesized to act as a UV-B receptor in plants [19]. It has been speculated that the vitamin D: provitamin D ratio might be a proxy of UV-B assimilation in plants [20]. In microalgae, vitamin D production might be the result of damage or degradation of biological membranes under the action of UV radiation [21, 22].
Vitamin E presents roles as antioxidant or also against photooxidative stress [23]. When tocopherol synthesis is chemically blocked in algae exposed to high light, in association with PS II inactivation, the tocopherol pool undergoes to rapid depletion due to its action against photooxidative stress [24]. It has been hypothesized a complementary role of tocopherol with the photoprotective xanthophyll cycling-pigments [25]. Other studies showed that in microalgae tocopherol production is often associated with polyphenols production in response to abiotic stress, such as light, nutrient or metals [26–28].
Vitamin K1 has a function of redox cofactor in plants, green algae and some cyanobacteria [29–31]. Specifically, vitamin K1 is the secondary electron acceptor of photosystem (PS I), also known as A1 [32]. However, it is now accepted that at least half of vitamin K1 is not bound to PS I [33, 34], suggesting a role of phylloquinone in redox reactions distinct from that of the one-electron transfer in PS I. Likewise, menaquinone (vitamin K2) is a secondary electron acceptor of PS I in red algae, diatoms, cyanobacteria and archaeal species [35–38].
Vitamin C acts as a cofactor of many enzymes, and is involved in photosynthesis, hormone biosynthesis and regeneration of antioxidants [39, 40]. Ascorbic acid plays a relevant role in algal photoprotection, being used as a co-factor for the de-epoxidase enzyme operating in the photoprotective xanthophyll cycle (violaxanthin-antheraxanthin-zeaxanthin or diadinoxanthin-diatoxanthin) for the synthesis of the photoprotective xanthophyll [13, 41]. Indeed, in the diatom Skeletonema marinoi, high intensity blue light induced a parallel increase of ascorbic acid and xanthophyll cycle activity [13]. Inside the chloroplast, ascorbic acid plays a key role in photosynthesis by removing hydrogen peroxide formed by oxygen photoreduction in PSI (Mehler reaction) via ascorbate peroxidase [42]. In coordination with glutathione and enzymatic antioxidants in chloroplasts, mitochondria, peroxisomes and cytosol, ascorbic acid controls the amount of hydrogen peroxide formed within the cell [43]. The expression of the VTC2 gene (GDP-L-galactose phosphorylases catalyzing the first step in the L-ascorbate biosynthesis) in Chlamydomonas reinhardtii is rapidly induced by hydrogen peroxide and singlet oxygen: the subsequent response, resulting in a manifold increase in ascorbate content, conversely to plants does not require circadian regulation nor photosynthesis [44].
Although in plants ascorbic acid controls a number of processes including cell division and cell expansion [17, 18], this role is not confirmed in microalgae, at the exception of a study in red algae [45].
Vitamin B1 is ubiquitously involved in the acetyl-CoA synthesis, tricarboxylic acid cycle, pentose phosphate pathway, Calvin–Benson cycle and isoprenoid biosynthesis pathway [46]. This vitamin is known to exert a defense function against abiotic and biotic stressors in plants [46] and in several microalgae [47]. A putative role of vitamin B1 as antioxidant has been hypothesized [48], even though the mechanism of this potential function remains unknown.
Vitamin B2 is an essential precursor for flavocoenzymes, involved in numerous physiological processes such as the circadian clock [25, 49–51], or acting as chromophores in blue light photoreceptors of plants and fungi [52–54]. Flavocoenzymes can catalyze redox processes involving one- and two-electron transitions as well as a variety of reactions such as photorepair of thymidine dimers in photodamaged DNA [55, 56].
Vitamin B3 is required for assimilatory nitrate reductase (NR) activity in photoautrotrophs [57, 58], beside its physiological role under the form of NADH [58]. Niacin vitamers—nicotinic acid, nicotinamide, NAD, and NADP—contribute to the antioxidant cell machinery. The antioxidant enzyme monodehydroascorbate reductase can use either NADH or NADPH [59], reduced by the plant mitochondrial electron transport chain [60].
Vitamin B5 is synthesized de novo by plants and micro-organisms. It is involved in many secondary metabolite biosynthetic pathways [61] and acts as precursor of the 40-phosphopantetheine moiety of coenzyme A (CoA) and acyl carrier protein. Due to the crucial role of the CoA in central carbohydrate and lipid metabolism, vitamin B5, synthesized in the cytosol, is subsequently transported into mitochondria and plastids [61].
Vitamin B6 is a cofactor for numerous metabolic enzymes [62] and acts as a potent antioxidant [4, 62] quenching efficiently reactive oxygen species [36, 63] with an efficacy comparable to ascorbic acid and α-tocopherol [64, 65]. Recently, a role of vitamin B6 in UV-B leaf acclimation has been demonstrated in plants, showing that vitamin B6 deficient Arabidopsis thaliana rsr4-1 mutant cannot cope with supplementary UV-B radiation [66].
Vitamin B7 is a cofactor for some carboxylases, decarboxylases and transcarboxylases involved in metabolic processes such as fatty acid and carbohydrate metabolism [67].
Vitamin B9 is an essential cofactor for one-carbon metabolism and primarily for the synthesis of the purine ring [68]. In algae, that are able to accumulate high concentrations of folates [69], folate biosynthetic route has recently been elucidated, showing that algae possess single isoforms of the genes in the pathway, while plant species tend to have multiple isoforms regulating the same steps in folate metabolism [70].
Vitamin B12 is involved in two core enzymatic reactions in algae, the DNA synthesis—being the cofactor of a form of the enzyme methionine synthase, and the inorganic carbon assimilation being required as a cofactor by the enzyme methylmalonyl CoA mutase [71].
Content and modulation of vitamins in microalgae
Vitamin A content
Vitamin A in microalgae varied from 0.01 mg per gram of dry weight (mg/g DW) as reported in the genera Chlorella and Isochrysis to 4.28 mg/g DW reported in the genus Tetraselmis (Table 1).
Table 1.
Vitamin A content in microalgae
| Phylum/class | Genus | Vitamin A | Refs |
|---|---|---|---|
| Cyanobacteria | Anabaena | 0.28 | [72] |
| Arthrospira | 0.65 | [73] | |
| Synechococcus | 0.18 | [73] | |
| Chlorophyta | Chlamydomonas | 0.11–0.13 | [72] |
| Chlorella | 0.01–0.65 | [72, 74, 75] | |
| Dunaliella | 0.01–0.63 | [74, 76] | |
| Stichococcus | 0.06 | [77] | |
| Tetraselmis | 0.05–4.28 | [73, 74, 77, 78] | |
| Rhodophyta | Porphyridium | 0.75 | [73] |
| Bacillariophyceae | Chaetoceros | 0.52–0.97 | [73, 78] |
| Skeletonema | 0.14 | [78] | |
| Haptophyta | Isochrysis | 0.01–0.27 | [74, 78] |
| Pavlova | 0.10 -0.26 | [77, 78] | |
| Eustigmatophyceae | Nannochloropsis | 0.05–0.08 | [73, 77] |
| Euglenozoa | Euglena | 0.30 | [79] |
Values are expressed as mg/g DW of retinol equivalents
Low vitamin A content was recorded in Nannochloropsis (ranging between 0.05 and 0.08 mg/g DW), while the latter displays high content of vitamins C and E, and in the euglenoid Euglena (0.30 mg/g DW) and in Pavlova (0.27 mg/g DW). Vitamin A content generally ranged between 0.50–0.80 mg/g DW (Table 1), such as in the cyanophyte Arthrospira, or in the green microalgae Chlorella and Dunaliella (Table 1). High vitamin A concentration in diatoms were reported in the genus Chaetoceros (from 0.52 to 0.97 mg/g DW), as well as in the red microalga Porphyridium (0.75 mg/g DW, Table 1). Vitamin A content strongly varied among and inside algal classes hypothesizing that no link between vitamin A concentration and microalgal divisions do exist (Table 1). Using a conversion factor between dry and fresh weight for microalgae of around 10% [80], it can be estimated a content of 0.42 and 0.1 mg of retinol equivalents per g of fresh weight (mg RE/g FW) in Tetraselmis and Chaetoceros. These values are much higher than those reported in edible carrot (circa 0.011 mg RE/ g FW) or in orange (0.0003 mg RE/g FW) [81].
Vitamin C content
Vitamin C content in microalgae varied between 0.06 and 18.79 mg/g DW (Table 2), displaying a great inter and intra specific variability. Indeed, in green microalga Chlorella, ascorbic acid content ranged from 0.10 to 15 mg/g DW (Table 2). Yet, a variation from 0.16 to 2.20 mg/g DW ascorbic acid was reported in the genus Dunaliella (Table 2). Diatoms displayed a great variability in vitamin C content, ranging from 0.06 to 6.7 mg/g DW in Skeletonema, or from 0.12 to 18.79 mg/g DW in Chaetoceros (Table 2). Large variability was also reported in haptophytes (Table 2), while ascorbic acid content was high in Nannochloropsis, with values ranging from 2.50 to 6.04 mg/g DW (Table 2). Values around 2 mg/g DW of ascorbic acid were recorded in the green microalga Scenedesmus, the cyanophyte Anabaena, the cryptophyte Chroomonas and the euglenoid Euglena (Table 2). Using the 10% conversion factor between DW and FW [80], microalgal vitamin C can reach concentrations of 1.88 mg/g FW (Chaetoceros), 1.5 mg/g FW (Chlorella) or 0.6 mg/g FW (Nannochloropsis) in the range or higher than some vitamin C-rich fruits, as strawberries, kiwis or lemons (0.54, 0.52 and 0.42 mg/g FW, respectively [82]).
Table 2.
Vitamin C content in microalgae
| Phylum/class | Genus | Vitamin C | Refs |
|---|---|---|---|
| Cyanobacteria | Anabaena | 2.00 | [72] |
| Chlorophyta | Chlamydomonas | 2.00 | [72] |
| Chlorella | 0.10–15 | [72, 74, 75] | |
| Dunaliella | 0.16–2.2 | [74, 76, 83] | |
| Nannochloris | 5.24 | [83] | |
| Scenedesmus | 2.00 | [72] | |
| Stichococcus | 2.50 | [77] | |
| Tetraselmis | 0.19–3 | [74, 77, 78] | |
| Bacillariophyceae | Chaetoceros | 0.12–18.79 | [78, 83] |
| Skeletonema | 0.06–6.7 | [13, 78, 83] | |
| Thalassiosira | 1.79 | [83] | |
| Haptophyta | Isochrysis galbana | 0.12–4.45 | [74, 78, 83] |
| Pavlova lutheri | 0.84–1.3 | [77, 78, 83] | |
| Eustigmatophyceae | Nannochloropsis | 2.50–6.04 | [77, 83] |
| Ochromonadaceae | Chroomonas | 2.13 | [83] |
| Euglenozoa | Euglena gracilis | 1.82 | [79] |
Values are expressed as mg/g DW of ascorbic acid
Vitamin E content
Vitamin E concentration in microalgae ranged between 0.01 and 6.32 mg/g DW (Table 3). High vitamin E content was found within the genera Tetraselmis (6.32 mg/g DW), Chlamydomonas (4 mg/g DW), Chlorella (2 mg/g DW) and Dunaliella (1.90 mg/g DW). Among cyanobacteria, high values were reported for the genera Anabaena (4 mg/g DW), and Arthrospira (2.50 mg/g DW) or Synechococcus (1.40 mg/g DW).
Table 3.
Vitamin E content in microalgae
| Phylum/class | Genus | Vitamin E | Refs |
|---|---|---|---|
| Cyanobacteria | Anabaena | 4 | [72] |
| Aphanizomenon | 0.10–0.14 | [84] | |
| Arthrospira | 0.11–2.50 | [73, 84] | |
| Oscillatoria | 0.09–0.10 | [84] | |
| Synechococcus | 1.40 | [73] | |
| Synechocystis | 0.17 | [84] | |
| Chlorophyta | Asterochloris | 0.09 | [84] |
| Botrydiopsis | 0.06–0.17 | [84] | |
| Botryococcus | 0.16–0.26 | [84] | |
| Bracteacoccus | 0.17 | [84] | |
| Chlamydomonas | 0.34–4 | [72, 84] | |
| Chlorella | 0.01–2 | [72, 74, 84–86] | |
| Chlorellidium | 0.69 | [84] | |
| Chloridella | 0.04 | [84] | |
| Chlorococcum | 0.79 | [84] | |
| Chloroidium | 0.32 | [84] | |
| Chloromonas | 0.41–0.7 | [84] | |
| Chorycystis | 0.26 | [84] | |
| Chromochloris | 0.18 | [84] | |
| Coccomyxa | 0.66 | [84] | |
| Coelastrella | 0.42–0.51 | [84] | |
| Coelastrum | 0.07 | [84] | |
| Coenochloris | 0.74 | [84] | |
| Coenocystis | 0.36 | [84] | |
| Coleochlamys | 0.37 | [84] | |
| Desmodesmus | 0.19–0.39 | [84, 85] | |
| Dunaliella | 0.12–1.9 | [74, 76, 85, 87] | |
| Edaphochlorella | 0.24 | [84] | |
| Enallax | 0.02 | [84] | |
| Fottea | 0.48 | [84] | |
| Geminella | 0.01–0.08 | [84] | |
| Haematococcus | 0.27–0.88 | [84] | |
| Heterochlorella | 0.01 | [84] | |
| Interfilum | 0.05 | [84] | |
| Klebsormidium | 0.06–0.09 | [84] | |
| Lobosphaeropsis | 0.10 | [84] | |
| Monodopsis | 0.46 | [84] | |
| Monodus | 0.32–0.5 | [84] | |
| Muriella | 0.62 | [84] | |
| Neocystis | 0.38 | [84] | |
| Neospongiococcum | 0.06 | [84] | |
| Pabia | 0.36 | [84] | |
| Pectinodesmus | 0.03 | [84] | |
| Pseudobumilleriopsis | 0.18 | [84] | |
| Pseudochlorella | 0.05–0.15 | [84] | |
| Pseudomuriella | 0.24 | [84] | |
| Scenedesmus | 0.08–1 | [72, 84] | |
| Scotiellopsis | 0.44 | [84] | |
| Stichococcus | 0.13–0.44 | [84] | |
| Tetradesmus | 0.05–0.13 | [84] | |
| Tetraedron | 0.12–0.22 | [73, 74, 77, 84, 87, 88] | |
| Tetraselmis | 0.04–6.32 | [78] | |
| Trebouxia | 0.07–0.14 | [84] | |
| Trentepohlia | 0.28 | [84] | |
| Rhodophyta | Porphyridium | 0.02–1.30 | [73, 84] |
| Rhodella | 0.03–0.07 | [84] | |
| Bacillariophyceae | Chaetoceros | 0.89–1.63 | [73, 78] |
| Phaeodactylum | 0.01 | [85] | |
| Skeletonema | 0.11 | [78] | |
| Haptophyta | Diacronema | 0.40 | [89] |
| Isochrysis | 0.06–0.12 | [74, 78] | |
| Pavlova | 0.14–0.35 | [78] | |
| Eustigmatophyceae | Microchloropsis | 0.23–0.67 | [84] |
| Nannochloropsis | 0.02–4.72 | [73, 77, 84, 85, 88, 90] | |
| Xanthophyceae | Heterococcus | 0.09–0.22 | [84] |
| Xanthonema | 0.16–0.39 | [84] | |
| Vischeria | 0.04–0.05 | [84] | |
| Euglenozoa | Euglena | 0.28–1.2 | [79, 91] |
Values are expressed as mg/g DW of α-tocopherol
High α-tocopherol concentration variability was described in Nannochloropsis (0.02–4.72 mg/g DW) and Porphyridium (0.02–1.30 mg/g DW). Low values were reported in the xanthophyceans Heterococcus, Xanthonema and Vischeria (0.04–0.39 mg/g DW), and in the red microalga Rhodella (0.03–0.07 mg/g DW) as well as in the haptophytes Diacronema, Isochrysis and Pavlova or in diatoms, with the exception of Chaetoceros (1.63 mg/g DW). Using the 10% conversion factor between DW and FW [80], Tetraselmis, Nannochloropsis and Anabaena reached 0.63, 0.40 and 0.48 mg/g FW of α-tocopherol, respectively. These values are notably higher than the vitamin E content of common dietary food, e.g. 0.03 mg/g FW for green olives, 0.02 mg/g FW for raw spinaches and 0.01 mg/g FW for blackberries and cranberries [92].
Vitamins B content
Among the microalgal vitamins B data, vitamins B1 and B12 were the most studied (Table 4).
Table 4.
Vitamins B content in microalgae (μg/g DW except for vitamins B7 and B12 in ng/g DW)
| Phylum/class | Genus | Vitamin B1 | Vitamin B2 | Vitamin B3 | Vitamin B5 | Vitamin B6 | Vitamin B7 | Vitamin B9 | Vitamin B12 | Refs |
|---|---|---|---|---|---|---|---|---|---|---|
| Cyanobacteria | Anabaena | 5.8 | 55 | 78 | 88 | 7 | 0.18 | 15 | 1.5 | [72, 94] |
| Aphanizomenon | 40 | 6 | 130 | 8 | 13 | 1 | 6 | [95] | ||
| Arthrospira | 10–23.8 | 33–45 | 0.13–149 | 13 | 9.6 | 0.27–4.8 | 0.50–6.6 | [6, 94, 95] | ||
| Chlorophyta | Chlamydomonas | 0.26 | 9 | [72] | ||||||
| Chlorella | 18–23 | 20–68 | 0.15–250 | 21.4–190 | 1.9–25 | 0.45–1.1 | 3.1–34 | 0.08–2.5 | [6, 69, 72, 75, 95] | |
| Dunaliella | 9–29 | 9–31.2 | 10 | 5–13.2 | 2.2–4 | 0.9 | 0.4–53.7 | 0.04–0.7 | [69, 74, 95] | |
| Haematococcus | 4.7 | 17 | 66 | 14 | 3.6 | 2.9 | 1.2 | [95] | ||
| Picochlorum | 64.7 | [69] | ||||||||
| Stichococcus | 29 | 25 | 17 | 1.3 | 24 | 1.95 | [77] | |||
| Tetradesmus | 25.9 | [69] | ||||||||
| Tetraselmis | 32.3–627 | 19.1–42 | 1410 | 37.7 | 2.8–155 | 0.8–1.3 | 3–20 | 1.95–9 | [74, 77, 78] | |
| Scenedesmus | 46 | 6 | [72] | |||||||
| Rhodophyta | Porphyridium | 5.39 | [69] | |||||||
| Bacillariophyceae | Chaetoceros | 655 | 12 | 25 | 4 | 8 | [78] | |||
| Skeletonema | 710 | 37 | 511 | 134 | 117 | [78] | ||||
| Haptophyta | Isochrysis | 14–462 | 14–30 | 2690 | 9.1 | 1.8–183 | 1 | 3 | 0.6–89 | [74, 78] |
| Pavlova | 36–290 | 6–50 | 955 | 4–8.4 | 1.9 | 23 | 1.7–1162 | [77, 78] | ||
| Eustigmatophyceae | Microchloropsis | 43.6 | [69] | |||||||
| Nannochloropsis | 70 | 22–25 | 0.12 | 3.6 | 1.1 | 17–22 | 0.3–1.7 | [6, 77] | ||
| Ochromonadaceae | Poteriochromonas | 27.57 | 4.86 | [96] | ||||||
| Euglenozoa | Euglena | 55.71 | 14.71 | 0.22 | [96] |
Diatoms and Haptophytes displayed higher average concentrations of vitamins B1 and B12 compared to Chlorophyta and Cyanobacteria (Table 4), with the highest B12 concentration (1.17 ng/g DW) reported in the haptophyte Pavlova (Table 4). High B12 contents were also revealed in the cyanobacteria Aphanizomenon and Arthrospira as well as in the green alga Chlorella (Table 4). Notably, microalgae can reach high concentration of vitamins B2, B3 and B6, as in the cyanobacterium Aphanizomenon or in the haptophyte Pavlova (Table 4). Vitamin B6 content was reported to be high in several macroalgae [93], as well as in microalgae (Table 4). A wide range of vitamin B6 concentrations has been reported for the green microalga Tetraselmis and for the haptophyte Isochrysis, with values from 2.8 to 155 μg/g DW and from 1.8 to 183 μg/g DW, respectively.
Very few studies reported microalgal vitamin B5 concentration, with a maximum of 190 μg/g DW measured in Chlorella (Table 4). In the same species, high vitamin B9 concentration was also revealed (from 3.1 to 34 μg/g DW). High vitamin B9 content was described in Picochlorum sp. (64.7 μg/g DW) and Michrochloropsis (43.6 μg/g DW). The range of variability of vitamin B9 content was greater in green algae (from 0.4 to 64.7 μg/g DW) than in cyanobacteria (0.27 to 15 μg/g DW). Vitamin B7 content ranged between 0.18 and 1.9 ng/g DW (Table 4) with the highest values reported in Stichococcus and Tetraselmis (1.3 ng/g DW), Nannochloropsis (1.1 ng/g DW) and in the haptophycean Pavlova (1.9 ng/g DW).
Vitamins D and K content
Microalgae can contain a high concentration of the two forms of vitamin D (D2 and D3, [97], Table 5) and represent the main source of these vitamins for fish, which is one of the major providers of vitamin D for humans [10].
Table 5.
Vitamin D content in microalgae
| Phylum/class | Species | Vitamin D | References |
|---|---|---|---|
| Cyanobacteria | Arthrospira maxima | 0.004 | [22] |
| Chlorophyta | Chlorella minutissima | 0.004 | [22] |
| Tetraselmis sp. CS-362 | 0.35 | [77] | |
| Tetraselmis suecica | 14 | [78] | |
| Stichococcus sp. CS-92 | 0.35 | [77] | |
| Rhodophyta | Rhodomonas salina | 0.004 | [22] |
| Bacillariophyceae | Skeletonema costatum | 11 | [78] |
| Haptophyta | Isochrysis galbana | 5 | [78] |
| Pavlova lutheri | 39 | [78] | |
| Pavlova pinguis | 0.35 | [77] | |
| Eustigmatophyceae | Nannochloropsis sp. CS-246 | 0.35 | [77] |
| Nannochloropsis oceanica | 0.48 | [22] |
Values are expressed as µg/g DW
Very high concentration of vitamin D was reported in Pavlova lutheri (39 µg/g DW), Tetraselmis suecica (14 µg/g DW) and Skeletonema costatum (11 µg/g DW).
Conversely, vitamin D concentration was very low (0.004 µg/g DW) in other species, such as Rhodomonas salina, Arthrospira maxima or Chlorella minutissima (Table 5). Also, ergosterol, precursor of vitamin D2, was found in various species of microalgae, e.g., Dunaliella tertiolecta [98], Chlamydomonas reinhardtii [35, 99], Chlorella vulgaris [37], Cyanidium caldarium [38] and account up to 0.1% of the dry weight in the coccolitophore Emiliania huxleyi [21].
Vitamin K was also higher in marine photosynthetic organisms than in terrestrial plants [7]. Vitamin K1 and vitamin K2 are unevenly distributed among algal divisions. Vitamin K1 concentration in microalgae ranged from 0.1 µg/g DW in the green microalga Dunaliella salina to 200.25 µg/g DW in the cyanobacterium Anabaena cylindrica (Table 6). High value (28 µg/g DW) was also found in the green microalga Tetraselmis suecica. Conversely, low values were reported in the haptophyceans Pavlova lutheri, Isochrysis galbana and in the cyanobacterium Arthrospira (6.5, 8 and 12.7 µg/g DW, respectively; Table 6). Although vitamin K1 was reported in Skeletonema costatum (5.5 µg/g DW), its presence was not revealed in other diatoms such as Phaeodactylum and Chaetoceros [78, 94]. To date, vitamin K2 has been reported in the red microalgae Porphyridium purpureum and Cyanidium caldarium [100], in the diatom Chaetoceros gracilis [101], as well as in the cyanobacteria Gloeobacter violaceus [102] and Synechococcus sp. [103]. Vitamin K2 content was generally reported as unit per photosystem; in the red microalga Cyanidium and in the cyanobacterium Gloeobacter two molecules of menaquinone per one molecule of chlorophyll have been found [100].
Table 6.
Vitamin K1 content in microalgae
| Phylum/class | Species | Vitamin K1 | References |
|---|---|---|---|
| Cyanobacteria | Anabaena cylindrica | 200.25 | [7] |
| Arthrospira | 12.7 | [7] | |
| Chlorophyta | Chlorella vulgaris | 0.73 | [7] |
| Desmodesmus asymmetricus | 0.46 | [7] | |
| Dunaliella salina | 0.1 | [7] | |
| Tetraselmis suecica | 28 | [78] | |
| Bacillariophyceae | Skeletonema costatum | 5.5 | [78] |
| Haptophyta | Isochrysis galbana | 8 | [78] |
| Pavlova lutheri | 6.5 | [78] | |
| Eustigmatophyceae | Nannochloropsis oculata | 0.17 | [78] |
Values are expressed as µg/g DW
Vitamins’ content modulation in microalgae
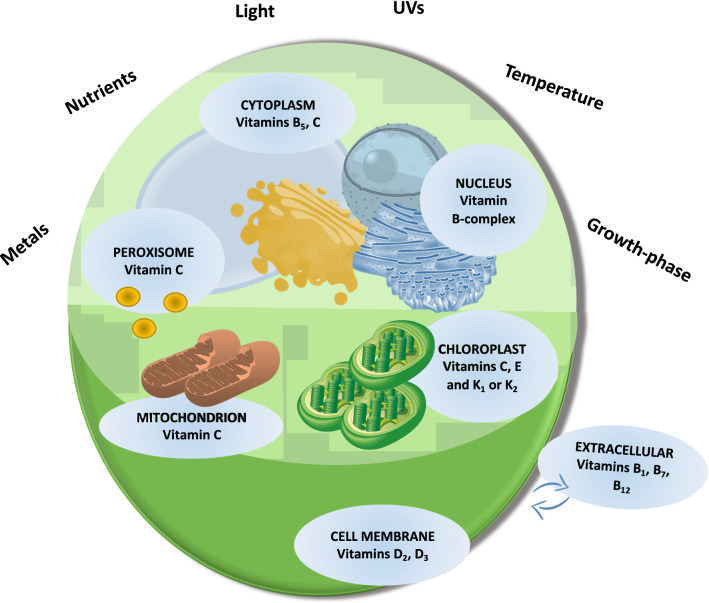
Since vitamins are often used by photosynthetic organisms to regulate vital functions, their modulation in response to environmental changes is noteworthy; and this knowledge might be an important key for increasing vitamins production in microalgae. Many external parameters can affect vitamin synthesis and/or use in microalgae, namely light, temperature, salinity, nutrient or metal concentrations (Fig. 2), as well as cell density and growth stage. However, some vitamins are little investigated compared to others, e.g., vitamins C, A (pro-vitamin A = β-carotene) and E (α-tocopherol). Yet, less information is available on the vitamins’ content modulation in microalgae, compared to macroalgae. For instance, the brown macroalga Eisenia arborea modulates its vitamin pool content along with the seasonality, with the highest amount of vitamins A, B1, B2 and C revealed in spring in parallel with the lowest content of vitamin E [104]. Also, in the red macroalga Palmaria palmata, the provitamin A (β-carotene) increased during summer and lowered during winter [105]. Although seasonal variability of vitamins’ content in microalgae was not reported, their modulation by environmental changes were investigated in different studies. Light is known as strongly triggering bioactive compounds variations in microalgae [106]. For instance, vitamin E enhanced with increasing light intensity [79, 87, 107]. Similarly, in the cyanobacterium Synechocystis sp.PCC 6803, high light intensity increased the concentration of α-tocopherol [108]. Light intensity and spectral properties have been shown to significantly modulate ascorbic acid production and/or use in the coastal diatom Skeletonema marinoi [12, 13]. Vitamins content in microalgae is also affected by UV radiations. For instance, in the green alga Chlorella vulgaris vitamin E increased in presence of UV-B [109] while ascorbic acid did not [110]. Also, Nannochloropsis oceanica enhanced vitamin D3 in presence of UV-B, in a dose–response dependent manner, whereas no UV-B modulation of D3 concentration was recorded in other microalgae such as Rhodomonas salina, Chlorella minutissima or Arthrospira maxima [22].
Fig. 2.
Intracellular location of vitamins in microalgae, and the environmental factors mainly modulating their content
The effect of temperature on vitamins production was poorly investigated. A seven-fold increase of α-tocopherol production was reported in Euglena gracilis under low temperature acclimation and oxygen stress [111].
Salinity variations induced vitamin B1 accumulation in microalgae such as Nodularia spumigena (cyanobacteria), Phaeodactylum tricornutum, Skeletonema costatum (diatoms), Dunaliella tertiolecta (chlorophyta), Prorocentrum minimum (dinoflagellate) and Rhodomonas salina (cryptophyte) [47].
Chemical variations of the cultivation environment also do affect vitamins production. Nutrient depletion enhanced the production of vitamins C and E in Chlorella vulgaris, Tetraselmis suecica and in the diatom Phaeodactylum tricornutum [26]. Similarly, α-tocopherol accumulated along with nitrogen concentration decrease in Nannochloropsis oculata [90], or with the addition of nitrate and phosphate in Tetraselmis suecica [87].
Pollutants such as heavy metals might also have effects on vitamins production or utilization in microalgae. For instance, the green alga Scenedesmus quadricauda lowered ascorbic acid to increasing heavy metals concentration [28]. Conversely, increased availability of cobalt chloride increased vitamin B12 concentration in Chlorella vulgaris [112]. Biological modulation of vitamins content in microalgae was also related to the growth phase (actively growing vs. stationary phase). The content of vitamin B2 increased by 2- to 3-folds in the stationary phase compared to the exponential phase in many microalgae (e.g., Chaetoceros gracilis, Thalassiosira pseudonana, Isochrysis sp., Pavlova lutheri, Nannochloris atomus or Nannochloropsis oculate [83]. Also, vitamin B1 enhanced during stationary phase in Nannochloris atomus, Nannochloropsis oculata, Isochrysis sp. and Pavlova lutheri [77], as well as in the diatoms Chaetoceros muelleri, Thalassiosira pseudonana [77]. and Nitzschia microcephala [113]. In Chlorella ellipsoidea, the vitamins B1, B2, B6 and B9 were more produced during the stationary phase of growth, while vitamins C, B3 and B7 were mainly synthetized during the active growth phase [114]. However, vitamin C modulation by growth phase is highly variable within microalgae [13, 77], and probably related to the biochemical function of ascorbic acid in cells [13].
Vitamins B12, B7 and B1 auxotrophy in microalgae
As pointed out recently [115], microalgae can be auxotrophs for the vitamins B12, B7 and B1. Among 306 microalgal species surveyed [116], more than half required vitamin B12 (cobalamin), while 22% required B1 (thiamine) and 5% required B7 (biotin), revealing that auxotrophy is shared by many species from unrelated classes (e.g., dinophyceae, raphidophyceae, bacillariophyceae, cryptophyceae and prymnesiophyceae). For instance, Gymnodinium brevis requires all three vitamins whereas Gymnodinium spendens requires only vitamin B12 [1]. Auxotrophy for B12 is ubiquitous in the haptophyte lineage [117], as in the coccolithophore Emiliania huxleyi [118], while a high variability is noteworthy in other classes. Some species can overcome B12 limitation in the environment thanks to a B12-independent methionine synthesis enzyme (e.g., Chlorella sp. NC64A, Phaeodactylum tricornutum CCAP1055/1, Ectocarpus siliculosus Ec32; (Katherine E [119]). Some microalgae (e.g., cyanobacteria, (Katherine Emma [120, 121]) are able to synthetize pseudocobalamin, which can be transformed into vitamin B12, the latter being more bioavailable (Katherine Emma [120, 121].
Vitamin B1 auxotrophy is diffused in marine microalgae [122], e.g. 80% of prymnesiophytes [1, 123] although with a lower percentage in diatoms [123]. Interestingly, thiamine biosynthesis in some microalgae (e.g., Chlamydomonas reinhardtii) can be induced and even regulated thanks to a riboswitch process regarding the gene encoding for the enzymes involved in thiamine biosynthesis [124] activated by the presence of thiamine in the environment. Microalgae can therefore become performant producers of thiamine [47].
Although some algae are auxotrophs for biotin, the ability to produce this molecule is transversally present in diverse microalgal classes, as shown by a genome-wide analysis performed on 14 photosynthetic microalgae (10 Chlorophyta, 1 Rhodophyta; 1 Haptophyta and 2 Heterokontophyta) that revealed the presence of a bifunctional enzyme involved in vitamin B7 (biotin) production [125].
Vitamins and human health
Although vitamins are not structural components, and required by cells in low amount, they are essential for life, growth and development. Vitamins participate to cell homeostasis and to anabolic pathways as enzymatic cofactors. Humans are not able to endogenously synthesize adequate concentrations of vitamins for the normal physiological functions requiring their exogenous intake through foods and dietary supplements. Indexes such as Adequate Intakes (AI) and Recommended Dietary Allowances (RDA) were provided (Table 7; modified from [126]).
Table 7.
Recommended Dietary Allowances (RDA) and Adequate Intakes (AI, values with *)
| Vit. A (μg/day) | Vit.C (mg/day) | Vit.D (μg/day) | Vit.E (mg/day) | Vit.K (μg/day) | Vit.B1 (mg/day) | Vit.B2 (mg/day) | Vit.B3 (mg/day) | Vit.B5 (mg/day) | Vit.B6 (mg/day) | Vit.B7 (μg/day) | Vit.B9 (μg/day) | Vit.B12 (μg/day) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Infants (months) | |||||||||||||
| 0–6 | 400* | 40* | 10* | 4* | 2.0* | 0.2* | 0.3* | 2* | 1.7* | 0.1* | 5* | 65* | 0.4* |
| 6–12 m | 500* | 50* | 10* | 5* | 2.5* | 0.3* | 0.4* | 4* | 1.8* | 0.3* | 6* | 80* | 0.5* |
| Children (years) | |||||||||||||
| 1–3 | 300 | 15 | 15 | 6 | 30* | 0.5 | 0.5 | 6 | 2* | 0.5 | 8* | 150 | 0.9 |
| 4–8 | 400 | 25 | 15 | 7 | 55* | 0.6 | 0.6 | 8 | 3* | 0.6 | 12* | 200 | 1.2 |
| Males (years) | |||||||||||||
| 9–13 | 600 | 45 | 15 | 11 | 60* | 0.9 | 0.9 | 12 | 4* | 1.0 | 20* | 300 | 1.8 |
| 14–18 | 900 | 75 | 15 | 15 | 75* | 1.2 | 1.3 | 16 | 5* | 1.3 | 25* | 400 | 2.4 |
| 19–30 | 900 | 90 | 15 | 15 | 120* | 1.2 | 1.3 | 16 | 5* | 1.3 | 30* | 400 | 2.4 |
| 31–50 | 900 | 90 | 15 | 15 | 120* | 1.2 | 1.3 | 16 | 5* | 1.3 | 30* | 400 | 2.4 |
| 51–70y | 900 | 90 | 15 | 15 | 120* | 1.2 | 1.3 | 16 | 5* | 1.7 | 30* | 400 | 2.4 |
| > 70 | 900 | 90 | 20 | 15 | 120* | 1.2 | 1.3 | 16 | 5* | 1.7 | 30* | 400 | 2.4 |
| Females | |||||||||||||
| 9–13 | 600 | 45 | 15 | 11 | 60* | 0.9 | 0.9 | 12 | 4* | 1.0 | 20* | 300 | 1.8 |
| 14–18 | 700 | 65 | 15 | 15 | 75* | 1.0 | 1.0 | 14 | 5* | 1.2 | 25* | 400 | 2.4 |
| 19–30 | 700 | 75 | 15 | 15 | 90* | 1.1 | 1.1 | 14 | 5* | 1.3 | 30* | 400 | 2.4 |
| 31–50 | 700 | 75 | 15 | 15 | 90* | 1.1 | 1.1 | 14 | 5* | 1.3 | 30* | 400 | 2.4 |
| 51–70 | 700 | 75 | 15 | 15 | 90* | 1.1 | 1.1 | 14 | 5* | 1.5 | 30* | 400 | 2.4 |
| > 70 | 700 | 75 | 20 | 15 | 90* | 1.1 | 1.1 | 14 | 5* | 1.5 | 30* | 400 | 2.4 |
RDA represents the average daily dietary intake level sufficient to meet the nutrient requirements of nearly all healthy individuals. It is calculated from an Estimated Average Requirement (EAR). If sufficient scientific evidence is not available to establish an EAR, an AI is usually developed.
Fat-soluble vitamins are absorbed through the intestinal tract with the help of lipids (fats), and can be retained for long periods of time in the body while if consumed in excess can pose a greater risk for toxicity than water-soluble vitamins.
Water-soluble vitamins dissolve easily in water, so consistent daily intake is often required, being easily excreted and not stored in the body. In addition, water soluble vitamins are difficult to preserve during food storage and preparation because readily destroyed or washed out.
Vitamin A is essential for embryonic development, tissues differentiation, growth, epithelial integrity, red blood cell production, reproduction, immune function, and the visual system [127]. Retinol functions as an electron carrier in mitochondria [128] and is the precursor of bioactive retinaldehyde and retinoic acid. Vitamin A derivatives have dual functions in physiology: 11-cis-Retinal serves as the universal chromophore of the visual pigments in the eye, whereas retinoic acid regulates the expression of target genes via activation of two classes of nuclear receptors, the retinoic acid receptors and the retinoid X receptors [129]. Deficiency in vitamin A is one of the major factors implicated in the pathogenesis of anaemia. During pregnancy, an additional intake of vitamin A is recommended by the World Health Organization (WHO) in developing countries, for the prevention of night blindness, without exceeding in consumption for its teratogenic side effect and for the increased risk of vomiting and fontanel bulging observed in trials testing therapeutic doses among infants. Many studies conducted among populations deficient in vitamin A, revealed that vitamin A reduces diarrhoea-related mortality (28%) and new episodes of diarrhoea (15%). Concerning chemoprevention strategy for cardiovascular and cancer diseases, some epidemiological and clinical trial studies [130] revealed an increase in lung cancer incidence for patients (mainly smokers) that have supplemented their diet with vitamin A in combination with β-carotene in last five years before diagnosis.
Vitamin D regulates calcium and phosphate metabolism, so it is responsible for the formation and maintenance of bones. It is related to the postmenopausal women health, with particular attention to the fracture prevention in the case of osteoporosis disease [131]. Another important role of vitamin D in good health status maintenance regards the correct intake of it during pregnancy for the prevention of low birth-weight and preterm delivery [132]. Vitamin D and its analogues may be effective in preventing many types of human cancer diseases including breast cancer, prostate cancer, colorectal cancer, and some hematological malignances [133]. Most recent finding about vitamin D bioactivity regards its role in the prevention of COVID19 infection and mortality [134]. A relationship between vitamin D presence and the reduction of complications in COVID19 patients attributed to downregulated inflammation and cytokine production has been highlighted [135].
Vitamin E role is mainly based on its antioxidant properties, especially in prevention [136]. of lipid peroxidation and oxidative stress related diseases especially in epithelial tissues [137]. These pathological conditions include cardiovascular diseases, cancers, cataracts, macular degeneration, and neurodegenerative diseases such as Alzheimer disease [138].
Vitamin K is a key regulator for the synthesis of blood clotting factors in the liver: It is associated with disorders mainly related to coagulation. In particular, vitamin K deficiency is also linked to other pathological conditions, such as malabsorption disorders, antibiotics and drug interactions, especially with coumarin-based anticoagulants [139].
Vitamin C is an essential dietary component for human nutrition, being a strong antioxidant and exerting an immunostimulant and chemopreventive function. Deficiency of vitamin C causes “scurvy” with severe symptoms such as impaired wound healing, hemorrhage and edema, commonly manifest as swollen bleeding gums [140]. Unfortunately, vitamin C is one of the most unstable nutrients in presence of oxygen, metal ions, increased pH, heat or light [141]. In fact, cooking processes and long-term storage determine a significant loss of vitamin C [142]. Another important bioactivity of vitamin C concerns its role against chronic and acute diseases mainly related to oxidative stress such as cancer, cardiovascular disease [143], hypertension, stroke [143], and neurodegenerative disorder [144, 145].
Vitamin B1—thiamine pyrophosphate is the metabolically functional form—is a nitrogen containing catalyst which plays a major role in glycolysis [115]. Vitamin B1 has a key role in the synthesis of neurotransmitters and in the correct function of the neural system [146]. Deficiency in vitamin B1 causes syndromes such as beriberi, polyneuritis, and Wernicke-Korsakoff. The primary symptoms of this vitamin lack include severe decreases in appetite, in growth, bradycardia, and muscular weakness.
Vitamin B2 (riboflavin) functions as a catalyst for redox reactions in numerous metabolic pathways and in energy production [140]. The active forms of vitamin B2 are cofactors for enzymatic reactions in the TCA cycle and in fatty acid oxidization [147]. Vitamin B2 has also role in chemoprevention of cancer and infective diseases due to its involvement in redox and photoreactions with nucleic acids for the inactivation and destruction of host cells [148, 149]. Another crucial role of vitamin B2 is the involvement in the metabolism of vitamins B6, B9 and B12 and its deficiency determines an insufficient recruitment of these other vitamins [150, 151]. Also, deficiency states in vitamin B2 generate various symptoms such as loss of appetite and depressed growth, cheilosis, angular stomatitis, and dermatitis, at neural level ataxia and paralysis, and vascular disorders.
Vitamin B3 (niacin) can be synthesized by mammals via an endogenous enzymatic pathway from tryptophan and is stored in the liver [152]. Vitamin B3 is also synthesized from tryptophan by intestinal bacteria [153, 154]. In the form of the coenzymes NAD and NADP, niacin functions in many biological redox reactions. Niacin deficiency affects many organs, such as skin inflammation with exposure to sunlight becoming pathology well known as pellagra. Pellagra includes other symptoms such as diarrhea, depression or dementia [155]. In some cases, it was observed that niacin deficiency is also associated with schizophrenia [156]. Niacin is metabolically synthetized from the amino acid tryptophan with a ratio of 1 mg of dietary niacin for 60 mg of tryptophan [157].
Vitamin B5 (pantothenic acid) has a potential cardioprotective role exerting anti-inflammatory effects through antioxidant properties [5]. Pantothenic acid deficiency although rare, causes dangerous effects on the liver (e.g., steatosis) and the nervous system (e.g., paralysis), together with a-specific symptoms such as decreased appetite and fatigue [140]. Pantethine, a disulphide form of panthothenic acid, is synthesized in the body and considered as the most active form of vitamin B5 due to its sulfhydryl-group [158].
Vitamin B6 is widely distributed in dietary sources and in addition synthetized by gut microflora [159]. Clinical deficiency of vitamin B6 generally occurs together with all vitamin B complex [160]. In particular there are cases of vitamin B6 deficiency, such as anemia post pancreaticoduodenectomy [161]. Vitamin B6 contributes to fatty acid biosynthesis, breakdown of certain storage compounds as well as in the biosynthesis of neurotransmitters [20, 162–167].
Vitamin B7 (biotin) is widely distributed in food items and synthesized in meaningful amounts by gut microflora in humans. Recently it was showed the role of biotin in immune-mediated intestinal inflammation [168].
Vitamin B9 is converted by intestinal bacteria into its active form tetrahydrofolate [169, 170] starting from folates, which are widely available in dietary sources of plant and animal origins [140]. Folates have important roles in various catabolic and biosynthetic routes through numerous reactions that involve, among the others DNA and purine synthesis [171]. Folates are also involved in amino acid and nucleotide metabolism and methylation reactions, thus having a fundamental role in normal embryogenesis by supporting cell division. For this reason, it is recommended to assume a correct dietary intake of folate during early pregnancy, in order to significantly reduce the risk of neural tube defects at birth [172]. Folate deficiency may cause impaired biosynthesis of DNA together with clinical symptoms of megaloblastic anemia, alopecia, achromotrichia, and neuropathy [173]. It is noteworthy that the bioavailability of naturally occurring folates is low if compared to synthetic folic acid, normally used in food fortification and supplements [174].
Vitamin B12 (cyanocobalamin) can be converted to either of the two important active forms: methylcobalamin and 5-deoxyadenosylcobalamin [175]. In humans, where it is required in trace amounts, B12 is a cofactor for two enzymes: methionine synthase and L-methylmalonyl-CoA mutase [71, 176]. These enzymes have crucial roles in amino acid and fatty acid metabolism, and DNA synthesis. Methionine synthase also requires folate for its action. Vitamin B12 is widely distributed in human food of animal or vegetable origin, such as edible algae and fermented soybean-based foods [177]. Deficiency in vitamin B12 might induce peripheral neuropathy and neurological dysfunction (e.g., cognition) [140] and, when associated with folate depletion, it becomes one of the main causes of megaloblastic anemia [178].
Microalgal vitamins and human health
Algal foods offer one of the few vegetarian alternatives for cobalamin in the diet. While some studies hypothesized that algal-derived vitamin B12 was not bioavailable to humans [179], other authors showed that increased consumption of Chlorella or nori by vegan people prevented B12 deficiency [177]. Also, feeding nori to vitamin B12-deficient rats yielded a 1.9-fold increase in hepatic levels of total B12 compared to those without nori supplementation [180]. Therefore, algal foods offer one of the few vegetarian alternatives for cobalamin in the diet [181].
Among microalgae, Spirulina is called “superfood” [182] thanks to its richness in vitamins (A, E, K, B1, B2, B3, B6 and B12) together with its macromolecular composition, in term of proteins and other bioactive compounds [5, 182]. One g of commercial Spirulina powder supplies up to half of the RDA for β-carotene and vitamin B12 [183], with a recommended consumption of less than 4 g per day for an average healthy adult to avoid any toxic effect [184]. Yet, Chlorella pyrenoidosa powder reduced the risk of anemia, proteinuria and edema in pregnant women [185] thanks to its high content in thiamine, riboflavin, folic acid, and biotin [186].
One of the main sources of vitamin D is represented indirectly by (micro)algae, that which ingested by seafood, allow them to provide vitamin D to humans. The direct use of micro(algae) in this context would increase the efficiency and meet with the vegetarian or vegan requirements.
The microalgal production related industry is currently increasing as the global nutraceutical market size is projected to reach USD 722.49 billion by 2027 [187]. Vitamins and minerals together accounted for over 40.71% share in 2019 while functional food accounted for the largest share in 2019 and generated revenue of USD 187.51 billion [187].
Algal species of Nannochloropsis and Chlorella vulgaris are primary ingredients used in the sport nutrition industry and are priced at about USD 18,000–36,000 t−1 [187]. For instance, Chlorella is one of the top-selling food supplements in Japan and it is produced by > 70 companies worldwide [188, 189]. Also, β-carotene from Dunaliella currently values USD 1500 per kilogram, and its use as a nontoxic vitamin A precursor has made it a mainstay in multivitamin and specialty formulations [190].
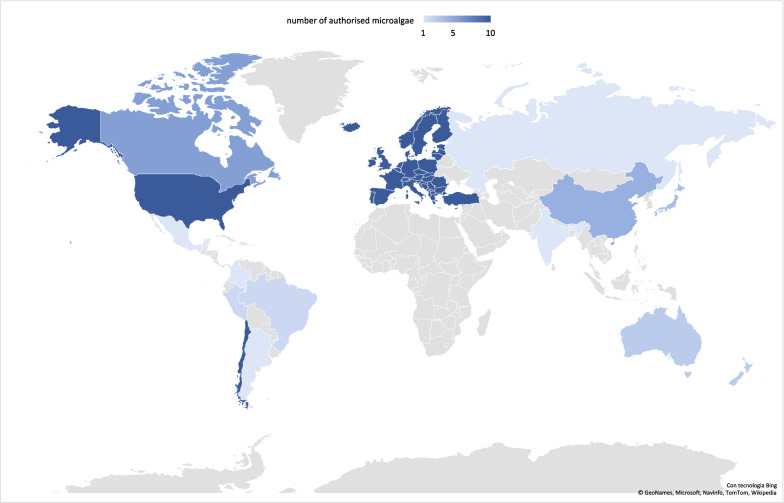
In EU, under the European Food Safety Authority (EFSA) (Regulation ECNo 2015/2283 [191] several microalgae are authorized as food products (Fig. 3), including Anabaena flos-aquae, Arthrospira platensis, Chlorella luteoviridis, Chlorella pyrenoidosa, Chlorella vulgaris, Odontella aurita, Tetraselmis chui and astaxanthin from Haematococcus pluvialis. In USA, the Food and Drug Administration [191, 192] currently recognizes few microalgae as safe for human consumption (Fig. 3), namely Arthrospira platensis, Chlamydomonas reinhardtii, Auxenochlorella protothecoides, Chlorella vulgaris, Dunaliella bardawil and Euglena gracilis [189]. While Arthrospira platensis is currently used as food worldwide (Canada, China, EU, India, and Japan), the other species vary with the geographical areas (Chlorella protothecoides in the U.S. and Japan, C. pyrenoidesa in EU and China, C. vulgaris in Canada, EU and Japan, etc. [189]). Also, it has to be noted that all these microalgae belong to cyanobacteria or green algae groups, except O. aurita which is the unique diatom in this regulated panorama.
Fig. 3.
Map of the number of authorised microalgae species worldwide for direct human consumption. In grey, absence of data. (Data sources: https://www.argentina.gob.ar/anmat/codigoalimentario (Argentina); https://www.foodstandards.gov.au/Pages/default.aspx (Australia and New Zealand); https://portal.anvisa.gov.br (Brasil); https://health-products.canada.ca/lnhpd-bdpsnh/index-eng.jsp (Canada); https://www.fia.cl/wp-content/uploads/2018/03/N-3-Revista-Mayo-2016.pdf (Chile); https://en.nhc.gov.cn/2018-10/22/c_74485.htm (China); https://www.invima.gov.co (Colombia); https://old.fssai.gov.in/GazettedNotifications.aspx (India); https://www.jetro.go.jp/ext_images/en/reports/regulations/pdf/foodext2010e.pdf (Japan); https://www.gob.mx/cofepris (Mexico); https://www.ins.gob.pe/insvirtual/images/otrpubs/pdf/Tabla%20de%20Alimentos.pdf (Peru); https://patents.google.com/patent/RU2137402C1/en (Russia); https://ec.europa.eu/food/safety/novel_food/catalogue/search/public/index.cfm (UE and observers); https://www.fda.gov/food/generally-recognized-safe-gras/gras-notice-inventory (USA))
Studies combining the analysis of vitamin concentrations together with testing algal product as food complements or functional food are needed to enhance the role of microalgae as food complements [180]. Also, the evaluation of the digestibility of microalgal biomass is required. In vitro models simulating human digestion are used to assess structural changes, digestibility and release of food components [193, 194], e.g. evaluating several seaweeds and microalgae food products, which highlighted class-related differences [192, 195–197]. Eukaryotic microalgae can present a robust multi-layered cell wall in which cellulose, hemicellulose, pectin compounds, glycoproteins and algaenan can limit the access of the digestive enzymes to the cell components. Conversely, cyanobacteria appear to be more easily digestible due to their peptidoglycan layer and the proteic and lipopolysaccharidic outer membrane [192, 198].
The relationship between vitamins content and human health or wellness is not direct. The bioaccessibility and bioavailability of vitamins are different amongst vitamins and foods. Also, they are not all absorbed/retained in the same way. Furthermore, synergy between different bioactive compounds might enhance their beneficial effects [199]. For instance, the effectiveness of carotenoids as antioxidants is dependent upon their interaction with other co-antioxidants, especially vitamins E and C [200, 201]. Vitamin C acts as a potent synergist in the presence of α-tocopherol enhancing its antioxidant activity [201, 202]. This effect can be further enhanced by phenolic compounds such as quercetin, forming a non-covalent association at the cytosol-membrane interface within the lipid bilayer in membranes, originating a complex in which antioxidant regeneration is significantly enhanced [203].
Microalgal challenges for vitamin production
Environmental manipulations can be a low-cost and effective way to modulate biosynthetic pathways and the natural production of vitamins enriched microalgal biomass, starting from the optimization of the resonance between growth, ecophysiological requirements and the environmental/cultivation climate (Fig. 4). Attempts regarding enhancing microalgal vitamins production were already carried out. Optimization of α-tocopherol production has been done with Euglena gracilis Z, also maximizing β-carotene yield with mixotrophic cultivation [204]. UV-B light administration (until 4.4 kJ m−2) improved the production of α-tocopherol and β-carotene in Chlorella vulgaris [109]. Also, high α-tocopherol productivity was achieved in Euglena by modifying culture conditions [111] or through a two-step cultivation strategy [79, 107]. Two-step cultivation strategy was also carried out for enhancing β-carotene production in Dunaliella [98, 205]. They reported increased β-carotene productivity to 450 mg m−2 day−1 in stage one and to 300 mg m−2 day−1 in stage two, instead of the 200 mg β-carotene m−2 day−1 yield obtained via the conventional cultivation.
Fig. 4.
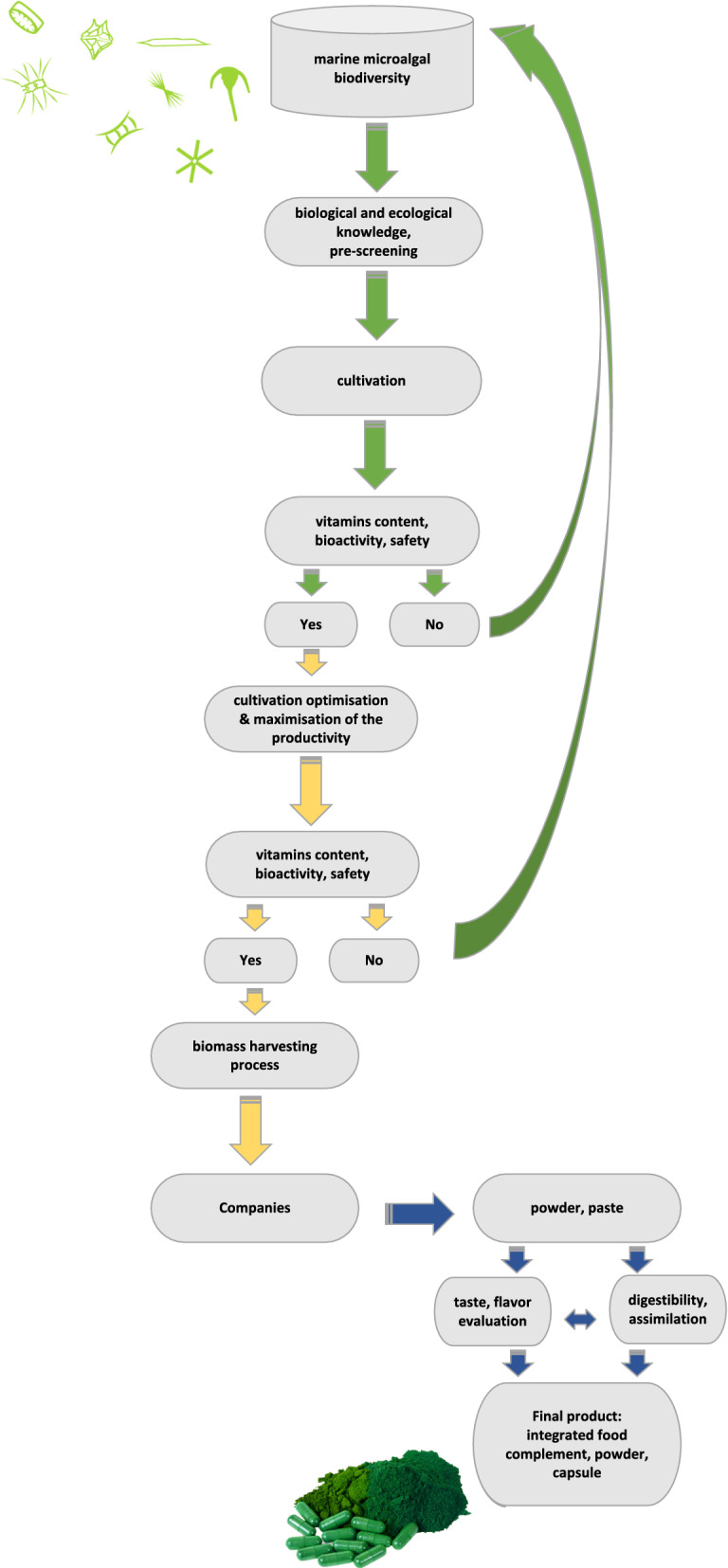
Pipeline design: Research and development strategies for improving microalgal vitamins uses for human food
A significant increase of ascorbic acid production (0.79 mg ascorbic acid g−1) has also been obtained in Tetraselmis sp. cultivated in 100 m3 photobioreactors [181]. Light climate—spectrum and intensity—variations tuned up the production of ascorbic acid in the diatom Skeletonema marinoi [12, 13]. Heterotrophy is also a way for increasing productive the yield of ascorbic acid, as shown in the red microalga Galdieria partita [206]. Heterotrophic synthesis of L-ascorbic acid has been also performed in the green microalga Chlorella pyrenoidosa [207]. Production of vitamin K1 of 40 μg L−1 day−1 was achieved by cultivating the cyanobacteria Anabaena cylindrical varying the medium composition and day length [94]. Vitamin D3 accumulation might be obtained thanks to interactions between microalga and UV-B light as revealed in the eustigmatophycean Nannochloropsis sp. [22]. The addition of cobalt chloride salt in Bold’s Basal Medium maximized vitamin B12 content in Chlorella vulgaris with a 7–12% higher content than control condition [112]. Also, for vitamins B1 and B2, the tuning of light could increase the production of vitamins B1 and B2 [77, 83].
Although physical or chemical manipulation of cultivation techniques is one way to improve the yield of vitamin production per microalgal biomass unit, biological manipulation might be undertaken. Some attempts of genetic manipulations of microalgae for enhancing bioactive compounds production (e.g., vitamins) are also on-going. However, this route does not ensure the maintenance of optimal growth of such organisms and poses the question of “genetically modified organisms” whose entrance into the food market could be extremely difficult. Nuclear transformants of the green model alga Chlamydomonas reinhardtii expressing protein intrinsic factors have been generated, suggesting that microalgae can represent a viable host for the production of a vegetarian protein intrinsic factor, source for B12 enrichment [208]. Also, the potential of riboswitches in microalgae [209] might be of interest for genetic manipulations aiming to enhanced thiamine production.
Another route of biological manipulation for vitamins’ productive yield increase is the co-cultivation between at least two different species (alga-bacteria or alga-alga; [15]). Bacteria-microalga co-cultivation might improve the yield of harvested microalgal biomass [101], and a way to protect microalgae against pathogens through the synthesis of antibiotics from bacteria as well as to enhance the synthesis of microalgal specific compounds. Vitamins can be a target for such strategy, especially concerning vitamins B supply since microalgae are mainly auxotrophs for some of them [1, 15, 115, 210]. Different studies showed the interests of the mutualistic relationships between microalgae and bacteria, the latter providing vitamin B12 [211]. Interests of co-cultivation for thiamine production in some microalgae, e.g. Chlamydomonas reinhardtii, are linked to the capacity of these microalgae to activate the biosynthetic pathway of vitamin B1 by sensing the presence of vitamin B1 from outside, e.g. produced by bacteria [212]. For biotin (vitamin B7), results on mutualistic relationships between algae and other organisms (bacteria or fungus) are few [115, 213].
Microalga-microalga co-cultivation might be a real alternative, aiming to improve the yield synergetic bioactive compounds production. This strategy requires knowledge on species/genera/classes of microalgae and the selection of species enhancing mutualism or commensalism, avoiding parasitism or competition for the same resources, e.g. light spectrum (e.g., blue: green ratio), nitrogen source (nitrates, ammonium, organic nitrogen sources) or silica. This route focusses on the final harvested microalgal product more than on the functional mutualistic relationships between microalgae. The complementarity of microalgae in terms of nutritional values paves the way to investigate their integration in a unique cultivation step. For instance, diatoms, rich in carotenoids, polyphenols, some vitamins (e.g., A and C) and lipids can be mixed with cyanophytes, rich in proteins, vitamins (e.g., B) and phycobiliproteins, to provide a “super synergetic microalgal product”. Yet, a co-cultivation of small and big species might be a choice, small species having a lower level of requirements from outside than bigger species. Also, the co-cultivation of vitamins B producer alga and a non-vitamin B producer (with greater ability to synthesize other bioactive compounds) is a way to finally produce and high bioactive quality biomass. Research activities in this sense are on-going and the results highly promising (Brunet et al., personal communication).
All the aforementioned strategies could increase the yield of both the biomass and the molecules of interest. Prior microalgal utilization as functional ingredients or nutraceuticals, further investigation must be undertaken. Certain types of manipulation could imbalance microalgal nutritional values or even compromise their safety. Therefore, downstream studies assessing the safety and quality of the final product are mandatory.
Conclusions
For humans, microalgae can be a source of vitamins, together with other compounds, which increase the bioactive and nutraceutical value of microalgal biomass. The biotechnological interest of microalgae relies on their small size, high growth rate, reduced space needed for cultivation, and richness in bioactive compounds [214–216]. Microalgae have the potential to fill many of the global demand regarding different fields (e.g., nutraceuticals, energy, animal feed) being considered as valuable biofactories [217]. Increasing literature assessed that microalgae cover antiviral, antitumor, antioxidant, anti-inflammatory, antiallergenic, antidiabetic, and antibacterial properties [218–220]. So far, the limitations of developing industrial microalgal biotechnology are mainly represented by the high production costs [221, 222]. Lowering costs require an optimization of all the steps from the microalgal species selection to the cultivation and biomass harvesting until the extraction and fractionation of products. Multidisciplinary integration of tools (bioinformatics, system biology, molecular biology; [223]) as well as artificial intelligence [224] might provide a synergy for a systems-level understanding of microalgal production, improving the output of industrially valuable strains. Moreover biological, physiological and ecological data need to be integrated to better develop the biotechnological pipeline (Fig. 4) from species chemo- or bio-diversity to its industrial up-scaling [14]. Indeed, the great biodiversity enhances the microalgal potential for the biotechnological production of high valuable molecules, such as vitamins. Thanks to the richness and diversity of vitamins present in microalgae, they are potentially one of the main targets for developing microalgal biotechnology.
Authors’ contributions
CB and CS conceived the study. All authors drafted, revised the final manuscript. All authors read and approved the final manuscript.
Funding
This research was funded by Stazione Zoologica Anton Dohrn and by “Antitumor Drugs and Vaccines from the Sea (ADViSE)” project (PG/2018/0494374).
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Croft MT, Warren MJ, Smith AG. Algae need their vitamins. Eukaryot Cell. 2006 doi: 10.1128/EC.00097-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vicente AR, Manganaris GA, Sozzi GO, Crisosto CH. Nutritional quality of fruits and vegetables. Postharvest Handling. 2009 doi: 10.1016/B978-0-12-374112-7.00005-6. [DOI] [Google Scholar]
- 3.Smith AG, Croft MT, Moulin M, Webb ME. Plants need their vitamins too. Curr Opin Plant Biol. 2007;10(3):266–275. doi: 10.1016/j.pbi.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Fitzpatrick TB, Basset GJC, Borel P, Carrari F, DellaPenna D, Fraser PD, Hellmann H, et al. Vitamin deficiencies in humans: can plant science help? Plant Cell. 2012;24(2):395–414. doi: 10.1105/tpc.111.093120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jung F, Krüger-Genge A, Waldeck P, Küpper J-H. Spirulina platensis, a super food? J Cell Biotechnol. 2019;5(1):43–54. doi: 10.3233/JCB-189012. [DOI] [Google Scholar]
- 6.Edelmann M, Aalto S, Chamlagain B, Kariluoto S, Piironen V. Riboflavin, niacin, folate and vitamin B12 in commercial microalgae powders. J Food Compos Anal. 2019 doi: 10.1016/j.jfca.2019.05.009. [DOI] [Google Scholar]
- 7.Tarento TDC, McClure DD, Vasiljevski E, Schindeler A, Dehghani F, Kavanagh JM. Microalgae as a source of vitamin K1. Algal Res. 2018;36:77–87. doi: 10.1016/j.algal.2018.10.008. [DOI] [Google Scholar]
- 8.Jäpelt RB, Jakobsen J. Vitamin D in plants: a review of occurrence, analysis, and biosynthesis. Front Plant Sci. 2013 doi: 10.3389/fpls.2013.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lehmann U, Riedel A, Hirche F, Brandsch C, Girndt M, Ulrich C, Seibert E, et al. Vitamin D 3 supplementation: response and predictors of vitamin D 3 metabolites—a randomized controlled trial. Clin Nutr. 2016;35(2):351–358. doi: 10.1016/j.clnu.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 10.Lock EJ, WaagbØ R, Wendelaar Bonga S, Flik G. The significance of vitamin D for fish: a review. Aquac Nutr. 2010 doi: 10.1111/j.1365-2095.2009.00722.x. [DOI] [Google Scholar]
- 11.Udayan A, Arumugam M, Pandey A. Nutraceuticals from algae and cyanobacteria. Algal Green Chem Recent Progress Biotechnol. 2017 doi: 10.1016/B978-0-444-63784-0.00004-7. [DOI] [Google Scholar]
- 12.Smerilli A, Balzano S, Maselli M, Blasio M, Orefice I, Galasso C, Sansone C, Brunet C. Antioxidant and photoprotection networking in the coastal diatom skeletonema marinoi. Antioxidants. 2019 doi: 10.3390/antiox8060154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smerilli A, Orefice I, Corato F, Olea AG, Ruban AV, Brunet C. Photoprotective and antioxidant responses to light spectrum and intensity variations in the coastal diatom skeletonema marinoi. Environ Microbiol. 2017 doi: 10.1111/1462-2920.13545. [DOI] [PubMed] [Google Scholar]
- 14.Butler T, Kapoore RV, Vaidyanathan S. Phaeodactylum tricornutum: a diatom cell factory. Trends Biotechnol. 2020 doi: 10.1016/j.tibtech.2019.12.023. [DOI] [PubMed] [Google Scholar]
- 15.Padmaperuma G, Kapoore RV, Gilmour DJ, Vaidyanathan S. Microbial consortia: a critical look at microalgae co-cultures for enhanced biomanufacturing. Crit Rev Biotechnol. 2018 doi: 10.1080/07388551.2017.1390728. [DOI] [PubMed] [Google Scholar]
- 16.Sarkar S, Dammer EB, Malovic E, Olsen AL, Raza SA, Gao T, Xiao H, et al. Molecular signatures of neuroinflammation induced by ΑSynuclein aggregates in microglial cells. Front Immunol. 2020 doi: 10.3389/fimmu.2020.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toti E, Oliver Chen CY, Palmery M, Valencia DV, Peluso I. Non-Provitamin A and provitamin A carotenoids as immunomodulators: recommended dietary allowance, therapeutic index, or personalized nutrition? Oxid Med Cell Longev. 2018 doi: 10.1155/2018/4637861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sies H, Stahl W, Sundquist AR. Antioxidant functions of vitamins. Ann NY Acad Sci. 1992 doi: 10.1111/j.1749-6632.1992.tb17085.x. [DOI] [PubMed] [Google Scholar]
- 19.Björn LO, Wang T. Is provitamin D a UV-B receptor in plants? Plant Ecol. 2001 doi: 10.1007/978-94-017-2892-8_1. [DOI] [Google Scholar]
- 20.Cuesta-Seijo JA, Ruzanski C, Krucewicz K, Meier S, Hägglund P, Svensson B, Palcic MM. Functional and structural characterization of plastidic starch phosphorylase during barley endosperm development. PLoS ONE. 2017 doi: 10.1371/journal.pone.0175488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Göring H. Vitamin D in nature: a product of synthesis and/or degradation of cell membrane components. Biochemistry. 2018;83(11):1350–1357. doi: 10.1134/S0006297918110056. [DOI] [PubMed] [Google Scholar]
- 22.Ljubic A, Jacobsen C, Holdt SL, Jakobsen J. Microalgae nannochloropsis oceanica as a future new natural source of vitamin D3. Food Chem. 2020 doi: 10.1016/j.foodchem.2020.126627. [DOI] [PubMed] [Google Scholar]
- 23.Havaux M, García-Plazaola JI. Beyond non-photochemical fluorescence quenching: the overlapping antioxidant functions of zeaxanthin and tocopherols. Dordrecht: Springer; 2014. [Google Scholar]
- 24.Krieger-Liszkay A, Trebst A. Tocopherol is the scavenger of singlet oxygen produced by the triplet states of chlorophyll in the PSII reaction centre. J Exp Bot. 2006 doi: 10.1093/jxb/erl002. [DOI] [PubMed] [Google Scholar]
- 25.Lee J. Lumazine protein and the excitation mechanism in bacterial bioluminescence. Biophys Chem. 1993 doi: 10.1016/0301-4622(93)85006-4. [DOI] [PubMed] [Google Scholar]
- 26.Goiris K, Van Colen W, Wilches I, León-Tamariz F, De Cooman L, Muylaert K. Impact of nutrient stress on antioxidant production in three species of microalgae. Algal Res. 2015 doi: 10.1016/j.algal.2014.12.002. [DOI] [Google Scholar]
- 27.Hamed SM, Selim S, Klöck G, AbdElgawad H. Sensitivity of Two Green Microalgae to Copper Stress: Growth, Oxidative and Antioxidants Analyses. Ecotoxicol Environ Saf. 2017 doi: 10.1016/j.ecoenv.2017.05.048. [DOI] [PubMed] [Google Scholar]
- 28.Strejckova A, Dvorak M, Klejdus B, Krystofova O, Hedbavny J, Adam V, Huska D. The strong reaction of simple phenolic acids during oxidative stress caused by nickel, cadmium and copper in the microalga Scenedesmus quadricauda. New Biotechnol. 2019 doi: 10.1016/j.nbt.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Collins MD, Jones D. Distribution of isoprenoid quinone structural types in bacteria and their taxonomic implications. Microbiol Rev. 1981 doi: 10.1128/mmbr.45.2.316-354.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lefebvre-Legendre L, Rappaport F, Finazzi G, Ceol M, Grivet C, Hopfgartner G, Rochaix JD. Loss of phylloquinone in chlamydomonas affects plastoquinone pool size and photosystem II synthesis. J Biol Chem. 2007 doi: 10.1074/jbc.M610249200. [DOI] [PubMed] [Google Scholar]
- 31.van Oostende C, Widhalm JR, Basset GJC. Detection and quantification of vitamin K1 quinol in leaf tissues. Phytochemistry. 2008 doi: 10.1016/j.phytochem.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 32.Sigfridsson K, Hansson O, Brzezinski P. Electrogenic light reactions in photosystem I: resolution of electron-transfer rates between the iron-sulfur centers. Proc Natl Acad Sci USA. 1995 doi: 10.1073/pnas.92.8.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gross J, Won KC, Lezhneva L, Falk J, Krupinska K, Shinozaki K, Seki M, Herrmann RG, Meurer J. A plant locus essential for phylloquinone (vitamin K1) biosynthesis originated from a fusion of four eubacterial genes. J Biol Chem. 2006 doi: 10.1074/jbc.M601754200. [DOI] [PubMed] [Google Scholar]
- 34.Lohmann A, Schöttler MA, Bréhélin C, Kessler F, Bock R, Cahoon EB, Dörmann P. Deficiency in phylloquinone (vitamin K1) methylation affects prenyl quinone distribution, photosystem I abundance, and anthocyanin accumulation in the arabidopsis AtmenG mutant. J Biol Chem. 2006 doi: 10.1074/jbc.M609412200. [DOI] [PubMed] [Google Scholar]
- 35.Brumfield KM, Laborde SM, Moroney JV. A model for the ergosterol biosynthetic pathway in Chlamydomonas reinhardtii. Eur J Phycol. 2017 doi: 10.1080/09670262.2016.1225318. [DOI] [Google Scholar]
- 36.Osmani AH, May GS, Osmani SA. The extremely conserved PyroA gene of Aspergillus nidulans is required for pyridoxine synthesis and Is required indirectly for resistance to photosensitizers. J Biol Chem. 1999 doi: 10.1074/jbc.274.33.23565. [DOI] [PubMed] [Google Scholar]
- 37.Patterson GW. Sterols of Chlorella-III. Species containing ergosterol. Comp Biochem Physiol. 1969 doi: 10.1016/0010-406X(69)90019-X. [DOI] [Google Scholar]
- 38.Seckbach J, Ikan R. Sterols and chloroplast structure of cyanidium caldarium. Plant Physiol. 1972 doi: 10.1104/pp.49.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gallie DR. The role of L-ascorbic acid recycling in responding to environmental stress and in promoting plant growth. J Exp Bot. 2013 doi: 10.1093/jxb/ers330. [DOI] [PubMed] [Google Scholar]
- 40.Lisko KA, Aboobucker SI, Torres R, Lorence A. Engineering elevated vitamin C in plants to improve their nutritional content, growth, and tolerance to abiotic stress. Phytochem Biosynthesis Func Appl. 2014 doi: 10.1007/978-3-319-04045-5_6. [DOI] [Google Scholar]
- 41.Neubauer C, Yamamoto HY. Mehler-peroxidase reaction mediates zeaxanthin formation and zeaxanthin-related fluorescence quenching in intact chloroplasts. Plant Physiol. 1992 doi: 10.1104/pp.99.4.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smirnoff N. The function and metabolism of ascorbic acid in plants. Ann Bot. 1996 doi: 10.1006/anbo.1996.0175. [DOI] [Google Scholar]
- 43.Akram NA, Shafiq F, Ashraf M. Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front Plant Sci. 2017 doi: 10.3389/fpls.2017.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vidal-Meireles A, Neupert J, Zsigmond L, Rosado-Souza L, Kovács L, Nagy V, Galambos A, Fernie AR, Bock R, Tóth SZ. Regulation of ascorbate biosynthesis in green algae has evolved to enable rapid stress-induced response via the VTC2 gene encoding GDP-l-galactose phosphorylase. New Phytol. 2017 doi: 10.1111/nph.14425. [DOI] [PubMed] [Google Scholar]
- 45.Liso R, Calabrese G. Research on ascorbic acid physiology in red algae I. A method for the determination of dehydroascorbic acid. Phycologia. 1974 doi: 10.2216/i0031-8884-13-1-1.1. [DOI] [Google Scholar]
- 46.Rapala-Kozik M. Vitamin B 1 (Thiamine). A cofactor for enzymes involved in the main metabolic pathways and an environmental stress protectant. Adv Bot Res. 2011 doi: 10.1016/B978-0-12-386479-6.00004-4. [DOI] [Google Scholar]
- 47.Sylvander P, Häubner N, Snoeijs P. The thiamine content of phytoplankton cells is affected by abiotic stress and growth rate. Microb Ecol. 2013 doi: 10.1007/s00248-012-0156-1. [DOI] [PubMed] [Google Scholar]
- 48.Lukienko PI, Mel’nichenko NG, Zverinskii IV, Zabrodskaya SV. Antioxidant properties of thiamine. Bull Exp Biol Med. 2000 doi: 10.1023/A:1015318413076. [DOI] [PubMed] [Google Scholar]
- 49.Meighen EA. Bacterial bioluminescence: organization, regulation, and application of the lux genes. FASEB J. 1993 doi: 10.1096/fasebj.7.11.8370470. [DOI] [PubMed] [Google Scholar]
- 50.Sancar A. Cryptochrome: the second photoactive pigment in the eye and its role in circadian photoreception. Annu Rev Biochem. 2000 doi: 10.1146/annurev.biochem.69.1.31. [DOI] [PubMed] [Google Scholar]
- 51.Sancar A. Regulation of the mammalian circadian clock by cryptochrome. J Biol Chem. 2004 doi: 10.1074/jbc.R400016200. [DOI] [PubMed] [Google Scholar]
- 52.Briggs WR, Beck CF, Cashmore AR, Christie JM, Hughes J, Jarillo JA, Kagawa T, et al. The phototropin family of photoreceptors. Plant Cell. 2001 doi: 10.1105/tpc.13.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Christie JM, Salomon M, Nozue K, Wada M, Briggs WR. LOV (Light, Oxygen, or Voltage) domains of the blue-light photoreceptor phototropin (Nph1): binding sites for the chromophore flavin mononucleotide. Proc Natl Acad Sci. 1999;96(15):8779–8783. doi: 10.1073/pnas.96.15.8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin C, Robertson DE, Ahmad M, Raibekas AA, Jorns MS, Leslie Dutton P, Cashmore AR. Association of flavin adenine dinucleotide with the arabidopsis blue light receptor CRY1. Science. 1995 doi: 10.1126/science.7638620. [DOI] [PubMed] [Google Scholar]
- 55.Imada Y, Iida H, Ono S, Murahashi SI. Flavin catalyzed oxidations of sulfides and amines with molecular oxygen. J Am Chem Soc. 2003 doi: 10.1021/ja028276p. [DOI] [PubMed] [Google Scholar]
- 56.Sancar A. Structure and function of DNA photolyase. Biochemistry. 1994 doi: 10.1021/bi00167a001. [DOI] [PubMed] [Google Scholar]
- 57.Kaiser WM, Kandlbinder A, Stoimenova M, Glaab J. Discrepancy between nitrate reduction rates in intact leaves and nitrate reductase activity in leaf extracts: what limits nitrate reduction in situ? Planta. 2000 doi: 10.1007/s004250050682. [DOI] [PubMed] [Google Scholar]
- 58.Kaiser WM, Stoimenova M, Man H-M. What limits nitrate reduction in leaves? In: Foyer CH, editor. Photosynthetic nitrogen assimilation and associated carbon and respiratory metabolism. Berlin: Springer; 2002. pp. 63–70. [Google Scholar]
- 59.Hossain MA, Asada K. Monodehydroascorbate reductase from cucumber is a flavin adenine dinucleotide enzyme. J Biol Chem. 1985;260:12920. [PubMed] [Google Scholar]
- 60.Rasmusson AG, Soole KL, Elthon TE. Alternative NAD(P)H dehydrogenases of plant mitochondria. Annu Rev Plant Biol. 2004 doi: 10.1146/annurev.arplant.55.031903.141720. [DOI] [PubMed] [Google Scholar]
- 61.Webb ME, Smith AG. Pantothenate biosynthesis in higher plants. Biochem Soc Trans. 2011 doi: 10.1016/B978-0-12-386479-6.00001-9. [DOI] [PubMed] [Google Scholar]
- 62.Wondrak GT, Jacobson EL. Vitamin B6: beyond coenzyme functions. Sub Cell Biochem. 2012 doi: 10.1007/978-94-007-2199-9_15. [DOI] [PubMed] [Google Scholar]
- 63.Bilski P, Li MY, Ehrenshaft M, Daub ME, Chignell CF. Vitamin B6 (pyridoxine) and its derivatives are efficient singlet oxygen quenchers and potential fungal antioxidants. Photochem Photobiol. 2007 doi: 10.1562/0031-8655(2000)0710129sipvbp2.0.co2. [DOI] [PubMed] [Google Scholar]
- 64.Gliszczyńska-Świgło A, Ciska E, Pawlak-Lemańska K, Chmielewski J, Borkowski T, Tyrakowska B. Changes in the content of health-promoting compounds and antioxidant activity of broccoli after domestic processing. Food Addit Contam. 2006 doi: 10.1080/02652030600887594. [DOI] [PubMed] [Google Scholar]
- 65.Ramis R, Ortega-Castro J, Caballero C, Casasnovas R, Cerrillo A, Vilanova B, Adrover M, Frau J. How does pyridoxamine inhibit the formation of advanced glycation end products? The role of its primary antioxidant activity. Antioxidants. 2019 doi: 10.3390/antiox8090344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Czégény G, Kőrösi L, Strid Å, Hideg É. Multiple roles for vitamin B6 in plant acclimation to UV-B. Sci Rep. 2019 doi: 10.1038/s41598-018-38053-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alban C, Job D, Douce R. Biotin metabolism in plants. Ann Rev Plant Biol. 2000;51:17–47. doi: 10.1146/annurev.arplant.51.1.17. [DOI] [PubMed] [Google Scholar]
- 68.Ravanel S, Douce R, Rébeillé F. Metabolism of folates in plants. Adv Bot Res. 2011 doi: 10.1016/B978-0-12-385853-5.00004-0. [DOI] [Google Scholar]
- 69.Woortman DV, Fuchs T, Striegel L, Fuchs M, Weber N, Brück TB, Rychlik M. Microalgae a superior source of folates: quantification of folates in halophile microalgae by stable isotope dilution assay. Front Bioeng Biotechnol. 2020 doi: 10.3389/fbioe.2019.00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gorelova V, Bastien O, De Clerck O, Lespinats S, Rébeillé F, Van Der Straeten D. Evolution of folate biosynthesis and metabolism across algae and land plant lineages. Sci Rep. 2019 doi: 10.1038/s41598-019-42146-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Panzeca C, Tovar-Sanchez A, Agustí S, Reche I, Duarte CM, Taylor GT, Sañudo-Wilhelmy SA. B Vitamins as regulators of phytoplankton dynamics. Eos Trans Am Geophys Union. 2006;87(52):593. doi: 10.1029/2006EO520001. [DOI] [Google Scholar]
- 72.Aaronson S, Dhawale SW, Patni NJ, Deangelis B, Frank O, Baker H. The cell content and secretion of water-soluble vitamins by several freshwater algae. Arch Microbiol. 1977;112(1):57–59. doi: 10.1007/BF00446654. [DOI] [PubMed] [Google Scholar]
- 73.Santiago-Morales IS, Trujillo-Valle L, Márquez-Rocha FJ, López Hernández JF. Tocopherols, phycocyanin and superoxide dismutase from microalgae: as potential food antioxidants. Appl Food Biotechnol. 2018;5(1):19–27. doi: 10.22037/afb.v5i1.17884. [DOI] [Google Scholar]
- 74.Fabregas J, Herrero C. Vitamin content of four marine microalgae. Potential use as source of vitamins in nutrition. J Ind Microbiol. 1990;5(4):259–263. doi: 10.1007/BF01569683. [DOI] [Google Scholar]
- 75.Siegel BZ, Siegel SM. The chemical composition of algal cell walls. Crit Rev Microbiol. 1973 doi: 10.3109/10408417309108743. [DOI] [PubMed] [Google Scholar]
- 76.Abalde J, Fabregas J, Herrero C. β-carotene, vitamin C and vitamin E content of the marine microalga dunaliella tertiolecta cultured with different nitrogen sources. Biores Technol. 1991;38(2–3):121–125. doi: 10.1016/0960-8524(91)90142-7. [DOI] [Google Scholar]
- 77.Brown MR, Mular M, Miller I, Farmer C, Trenerry C. The vitamin content of microalgae used in aquaculture. J Appl Phycol. 1999;11(3):247–255. doi: 10.1023/A:1008075903578. [DOI] [Google Scholar]
- 78.De Roeck-Holtzhauer Y, Quere I, Claire C. Vitamin analysis of five planktonic microalgae and one macroalga. J Appl Phycol. 1991;3(3):259–264. doi: 10.1007/BF00003584. [DOI] [Google Scholar]
- 79.Takeyama H, Kanamaru A, Yoshino Y, Kakuta H, Kawamura Y, Matsunaga T. Production of antioxidant vitamins, β-carotene, vitamin C, and vitamin E, by two-step culture of Euglena gracilis Z. Biotechnol Bioeng. 1997 doi: 10.1002/(SICI)1097-0290(19970120)53:2<185::AID-BIT8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 80.Xiao R, Chen R, Zhang HY, Li H. Microalgae scenedesmus quadricauda grown in digested wastewater for simultaneous CO2 fixation and nutrient removal. J Biobased Mater Bioenerg. 2011 doi: 10.1166/jbmb.2011.1146. [DOI] [Google Scholar]
- 81.Beltrán-de-Miguel B, Estévez-Santiago R, Olmedilla-Alonso B. Assessment of dietary vitamin A intake (retinol, α-carotene, β-carotene, β-cryptoxanthin) and its sources in the national survey of dietary intake in Spain (2009–2010) Int J Food Sci Nutr. 2015 doi: 10.3109/09637486.2015.1077787. [DOI] [PubMed] [Google Scholar]
- 82.Szeto YT, Tomlinson B, Benzie IFF. Total antioxidant and ascorbic acid content of fresh fruits and vegetables: implications for dietary planning and food preservation. Br J Nutr. 2002 doi: 10.1079/bjn2001483. [DOI] [PubMed] [Google Scholar]
- 83.Brown MR, Farmer CL. Riboflavin content of six species of microalgae used in mariculture. J Appl Phycol. 1994 doi: 10.1007/BF02185905. [DOI] [Google Scholar]
- 84.Mudimu O, Koopmann IK, Rybalka N, Friedl T, Schulz R, Bilger W. Screening of microalgae and cyanobacteria strains for α-tocopherol content at different growth phases and the influence of nitrate reduction on α-tocopherol production. J Appl Phycol. 2017 doi: 10.1007/s10811-017-1188-1. [DOI] [Google Scholar]
- 85.Safafar H, Van Wagenen J, Møller P, Jacobsen C. Carotenoids, phenolic compounds and tocopherols contribute to the antioxidative properties of some microalgae species grown on industrial wastewater. Marine Drugs. 2015 doi: 10.3390/md13127069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Skinner WA, Sturm PA. Investigation of algae and yeast for α-tocopherol and α-tocopherolquinone content. Phytochemistry. 1968;7(10):1893–1896. doi: 10.1016/S0031-9422(00)86670-4. [DOI] [Google Scholar]
- 87.Carballo-Cárdenas EC, Tuan PM, Janssen M, Wijffels RH. Vitamin E (α-tocopherol) production by the marine microalgae Dunaliella tertiolecta and Tetraselmis suecica in batch cultivation. Biomol Eng. 2003 doi: 10.1016/S1389-0344(03)00040-6. [DOI] [PubMed] [Google Scholar]
- 88.Bong, S. C., and S. P. Loh. 2013. A study of fatty acid composition and tocopherol content of lipid extracted from marine microalgae, nannochloropsis oculata and Tetraselmis suecica, using solvent extraction and supercritical fluid extraction.” Int Food Re J.
- 89.Donato M, Helena Vilela M, Bandarra NM. Fatty acids, sterols, α-tocopherol and total carotenoids composition of Diacronema vlkianum. J Food Lipids. 2003 doi: 10.1111/j.1745-4522.2003.tb00020.x. [DOI] [Google Scholar]
- 90.Durmaz Y. Vitamin E (α-tocopherol) production by the marine microalgae nannochloropsis oculata (Eustigmatophyceae) in nitrogen limitation. Aquaculture. 2007 doi: 10.1016/j.aquaculture.2007.07.213. [DOI] [Google Scholar]
- 91.Fujita T, Aoyagi H, Ogbonna JC, Tanaka H. Effect of mixed organic substrate on α-tocopherol production by Euglena gracilis in photoheterotrophic culture. Appl Microbiol Biotechnol. 2008 doi: 10.1007/s00253-008-1443-0. [DOI] [PubMed] [Google Scholar]
- 92.Chun J, Lee J, Ye L, Exler J, Eitenmiller RR. Tocopherol and tocotrienol contents of raw and processed fruits and vegetables in the United States Diet. J Food Compos Anal. 2006 doi: 10.1016/j.jfca.2005.08.001. [DOI] [Google Scholar]
- 93.Lalitha N, Dhandapani R. Estimation of vitamin components in selected green algal sea weeds collected from Gulf of Mannar Islands, Tamil Nadu State South India. 2018; 8: 426–33. www.ijpbs.comorwww.ijpbsonline.com.
- 94.Tarento TDC, McClure DD, Talbot AM, Regtop HL, Biffin JR, Valtchev P, Dehghani F, Kavanagh JM. A potential biotechnological process for the sustainable production of vitamin K1. Crit Rev Biotechnol. 2019 doi: 10.1080/07388551.2018.1474168. [DOI] [PubMed] [Google Scholar]
- 95.Bishop M, Zubeck HM. Evaluation of microalgae for use as nutraceuticals and nutritional supplements. J Nutr Food Sci. 2012 doi: 10.4172/2155-9600.1000147. [DOI] [Google Scholar]
- 96.Baker ER, McLaughlin JJA, Hutner SH, DeAngelis B, Feingold S, Frank O, Baker H. Water-soluble vitamins in cells and spent culture supernatants of Poteriochromonas stipitata, Euglena gracilis, and Tetrahymena thermophila. Arch Microbiol. 1981 doi: 10.1007/BF00414703. [DOI] [Google Scholar]
- 97.Mattila P, Piironen V, Haapala R, Hirvi T, Uusi-Rauva E. Possible factors responsible for the high variation in the cholecalciferol contents of fish. J Agric Food Chem. 1997 doi: 10.1021/jf970243j. [DOI] [Google Scholar]
- 98.Ben-Amotz A, Avron M. The biotechnology of cultivating the halotolerant alga dunaliella. Trends Biotechnol. 1990 doi: 10.1016/0167-7799(90)90152-N. [DOI] [Google Scholar]
- 99.Miller MB, Haubrich BA, Wang Q, Snell WJ, David Nes W. Ergosterol biosynthesis pathway in the alga Chlamydomonas reinhardtii. J Lipid Res. 2012 doi: 10.1194/jlr.M027482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yoshida E, Nakamura A, Watanabe T. Reversed-phase HPLC determination of chlorophyll a’ and naphthoquinones in photosystem I of red algae: existence of two menaquinone-4 molecules in photosystem I of cyanidium caldarium. Anal Sci. 2003;19(7):1001–1005. doi: 10.2116/analsci.19.1001. [DOI] [PubMed] [Google Scholar]
- 101.Ikeda Y, Komura M, Watanabe M, Minami C, Koike H, Itoh S, Kashino Y, Satoh K. Photosystem I complexes associated with fucoxanthin-chlorophyll-binding proteins from a marine centric diatom, Chaetoceros gracilis. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbabio.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 102.Mimuro M, Tsuchiya T, Inoue H, Sakuragi Y, Itoh Y, Gotoh T, Miyashita H, Bryant DA, Kobayashi M. The secondary electron acceptor of photosystem I in gloeobacter violaceus PCC 7421 is menaquinone-4 that is synthesized by a unique but unknown pathway. FEBS Lett. 2005 doi: 10.1016/j.febslet.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 103.Sakuragi Y, Zybailov B, Shen G, Bryant DA, Golbeck JH, Diner BA, Karygina I, Pushkar Y, Stehlik D. Recruitment of a foreign quinone into the A1 site of photosystem I: characterization of a MenB RubA double deletion mutant in Synechococcus Sp. PCC 7002 Devoid of FX, FA, and FB and containing plastoquinone or exchanged 9,10-anthraquinone. J Biol. 2005 doi: 10.1074/jbc.M412943200. [DOI] [PubMed] [Google Scholar]
- 104.Hernández-Carmona G, Carrillo-Domínguez S, Arvizu-Higuera DL, Rodríguez-Montesinos YE, Murillo-Álvarez JI, Muñoz-Ochoa M, Castillo-Domínguez RM. Monthly variation in the chemical composition of Eisenia Arborea J.E. Areschoug. J Appl Phycol. 2009 doi: 10.1007/s10811-009-9454-5. [DOI] [Google Scholar]
- 105.Holdt SL, Kraan S. Bioactive compounds in seaweed: functional food applications and legislation. J Appl Phycol. 2011 doi: 10.1007/s10811-010-9632-5. [DOI] [Google Scholar]
- 106.Barra L, Chandrasekaran R, Corato F, Brunet C. The challenge of ecophysiological biodiversity for biotechnological applications of marine microalgae. Marine Drugs. 2014 doi: 10.3390/md12031641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ogbonna JC, Tomiyama S, Tanaka H. Production of α-tocopherol by sequential heterotrophic-photoautotrophic cultivation of Euglena gracilis. Prog Ind Microbiol. 1999 doi: 10.1016/S0079-6352(99)80115-5. [DOI] [Google Scholar]
- 108.Backasch N, Schulz-Friedrich R, Appel J. Influences on tocopherol biosynthesis in the Cyanobacterium synechocystis sp. PCC 6803. J Plant Physiol. 2005 doi: 10.1016/j.jplph.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 109.Malanga G, Puntarulo S. Oxidative stress and antioxidant content in Chlorella vulgaris after exposure to ultraviolet-B radiation. Physiol Plant. 1995 doi: 10.1111/j.1399-3054.1995.tb00983.x. [DOI] [Google Scholar]
- 110.Malanga G, Calmanovici G, Puntarulo S. Oxidative damage to chloroplasts from Chlorella vulgaris exposed to ultraviolet-B radiation. Physiol Plant. 1997 doi: 10.1034/j.1399-3054.1997.1010301.x. [DOI] [Google Scholar]
- 111.Ruggeri BA, Gray RJH, Watkins TR, Tomlins RI. Effects of low-temperature acclimation and oxygen stress on tocopherol production in Euglena gracilis Z. Appl Environ Microbiol. 1985 doi: 10.1128/aem.50.6.1404-1408.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jalilian N, Najafpour GD, Khajouei M. Enhanced vitamin B12 production using chlorella vulgaris. Int J Eng Trans A Basics. 2019 doi: 10.5829/ije.2019.32.01a.01. [DOI] [Google Scholar]
- 113.Pintoa E, Van Nieuwerburgha L, Barros MPD, Pedersén M, Colepicolo P, Snoeijs P. Density-dependent patterns of thiamine and pigment production in the diatom nitzschia microcephala. Phytochemistry. 2003 doi: 10.1016/S0031-9422(03)00048-7. [DOI] [PubMed] [Google Scholar]
- 114.Morimura Y. Synchronous culture of Chlorella II Changes in content of various vitamins during the course of the algal life cycle. Plant Cell Physiol. 1959 doi: 10.1093/oxfordjournals.pcp.a075751. [DOI] [Google Scholar]
- 115.Tandon P, Jin Q, Huang L. A promising approach to enhance microalgae productivity by exogenous supply of vitamins. Microb Cell Fact. 2017 doi: 10.1186/s12934-017-0834-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tang YZ, Koch F, Gobler CJ. Most harmful algal bloom species are vitamin B1 and B 12 auxotrophs. Proc Natl Acad Sci USA. 2010 doi: 10.1073/pnas.1009566107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nef C, Jung S, Mairet F, Kaas R, Grizeau D, Garnier M. How haptophytes microalgae mitigate vitamin B12 limitation. Sci Rep. 2019 doi: 10.1038/s41598-019-44797-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sañudo-Wilhelmy SA, Gómez-Consarnau L, Suffridge C, Webb EA. The role of B vitamins in marine biogeochemistry. Annu Rev Mar Sci. 2014 doi: 10.1146/annurev-marine-120710-100912. [DOI] [PubMed] [Google Scholar]
- 119.Helliwell KE, Wheeler GL, Leptos KC, Goldstein RE, Smith AG. Insights into the evolution of vitamin B 12 auxotrophy from sequenced algal genomes. Mol Biol Evol. 2011 doi: 10.1093/molbev/msr124. [DOI] [PubMed] [Google Scholar]
- 120.Heal KR, Qin W, Ribalet F, Bertagnolli AD, Coyote-Maestas W, Hmelo LR, Moffett JW, et al. Two distinct pools of B12 analogs reveal community interdependencies in the ocean. Proc Natl Acad Sci USA. 2017 doi: 10.1073/pnas.1608462114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Helliwell KE, Lawrence AD, Holzer A, Kudahl UJ, Sasso S, Kräutler B, Scanlan DJ, Warren MJ, Smith AG. Cyanobacteria and eukaryotic algae use different chemical variants of vitamin B12. Curr Biol. 2016 doi: 10.1016/j.cub.2016.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Paerl RW, Bertrand EM, Allen AE, Palenik B, Azam F. Vitamin B1 ecophysiology of marine picoeukaryotic algae: strain-specific differences and a new role for bacteria in vitamin cycling. Limnol Oceanogr. 2015 doi: 10.1002/lno.10009. [DOI] [Google Scholar]
- 123.Provasoli L. Nutrition and ecology of protozoa and algae. Annu Rev Microbiol. 1958 doi: 10.1146/annurev.mi.12.100158.001431. [DOI] [PubMed] [Google Scholar]
- 124.Croft MT, Moulin M, Webb ME, Smith AG. Thiamine biosynthesis in algae is regulated by riboswitches. Proc Natl Acad Sci USA. 2007 doi: 10.1073/pnas.0705786105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cui C, Pengfei X, Li G, Qiao Y, Han W, Geng C, Liao D, Yang M, Chen D, Jiang P. Vitamin D receptor activation regulates microglia polarization and oxidative stress in spontaneously hypertensive rats and angiotensin II-exposed microglial cells: role of renin-angiotensin system. Redox Biol. 2019 doi: 10.1016/j.redox.2019.101295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Price MY, Preedy VR. Vitamin k status in nutritionally compromised circumstances. Handb Famine Starvation Nutr Deprivation Biol Policy. 2019 doi: 10.1007/978-3-319-55387-0_119. [DOI] [Google Scholar]
- 127.Imdad A, Mayo-Wilson E, Herzer K, Bhutta ZA. Vitamin A supplementation for preventing morbidity and mortality in children from six months to five years of age. Cochrane Database Syst Rev. 2017 doi: 10.1002/14651858.CD008524.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hammerling U. Retinol as electron carrier in redox signaling, a new frontier in vitamin A research. Hepatobiliary Surg Nutr. 2016 doi: 10.3978/j.issn.2304-3881.2016.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mangelsdorf DJ. Vitamin A receptors. Nutr Rev. 2009;52(2):S32–44. doi: 10.1111/j.1753-4887.1994.tb01385.x. [DOI] [PubMed] [Google Scholar]
- 130.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334(18):1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 131.Malabanan AO, Holick MF. Vitamin D and bone health in postmenopausal women. J Womens Health. 2003 doi: 10.1089/154099903321576547. [DOI] [PubMed] [Google Scholar]
- 132.Mannion CA, Gray-Donald K, Koski KG. Association of low intake of milk and vitamin D during pregnancy with decreased birth weight. CMAJ. 2006 doi: 10.1503/cmaj.1041388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Giammanco M, Di Majo D, La Guardia M, Aiello S, Crescimannno M, Flandina C, Tumminello FM, Leto G. Vitamin D in cancer chemoprevention. Pharm Biol. 2015 doi: 10.3109/13880209.2014.988274. [DOI] [PubMed] [Google Scholar]
- 134.Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res. 2020 doi: 10.1007/s40520-020-01570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.“The possible role of vitamin D in suppressing cytokine storm and associated mortality in COVID-19 patients.” n.d. doi:10.1101/2020.04.08.20058578.
- 136.Lee KW, Lee HJ, Surh Y-J, Lee CY. Vitamin C and cancer chemoprevention: reappraisal. Am J Clin Nutr. 2003;78(6):1074–1078. doi: 10.1093/ajcn/78.6.1074. [DOI] [PubMed] [Google Scholar]
- 137.Kurutas EB. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr J. 2015;15(1):71. doi: 10.1186/s12937-016-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Browne D, McGuinness B, Woodside JV, McKay GJ. Vitamin E and Alzheimer’s disease: what do we know so far? Clin Interv Aging. 2019 doi: 10.2147/CIA.S186760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Halder M, Petsophonsakul P, Akbulut AC, Pavlic A, Bohan F, Anderson E, Maresz K, Kramann R, Schurgers L. Vitamin K: double bonds beyond coagulation insights into differences between vitamin K1 and K2 in health and disease. Int J Mol Sci. 2019 doi: 10.3390/ijms20040896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.McCormick DB. The vitamins: fundamental aspects in nutrition and health. Am J Clin Nutr. 1999 doi: 10.1093/ajcn/70.3.426. [DOI] [Google Scholar]
- 141.Boon CS, Julian McClements D, Weiss J, Decker EA. Factors influencing the chemical stability of carotenoids in foods. Crit Rev Food Sci Nutr. 2010 doi: 10.1080/10408390802565889. [DOI] [PubMed] [Google Scholar]
- 142.Sun DW. Handbook of frozen food processing and packaging. Handb Frozen Food Process Packag. 2005 doi: 10.1016/j.tifs.2012.07.001. [DOI] [Google Scholar]
- 143.Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, et al. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018 doi: 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lin MT, Flint Beal M. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006 doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 145.Traber MG, Stevens JF. Vitamins C and E: beneficial effects from a mechanistic perspective. Free Radical Biol Med. 2011;51(5):1000–1013. doi: 10.1016/j.freeradbiomed.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kerns JC, Arundel C, Chawla LS. Thiamin deficiency in people with obesity. Adv Nutr. 2015 doi: 10.3945/an.114.007526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Huskisson E, Maggini S, Ruf M. The role of vitamins and minerals in energy metabolism and well-being. J Int Med Res. 2007 doi: 10.1177/147323000703500301. [DOI] [PubMed] [Google Scholar]
- 148.Insińska-Rak M, Sikorski M. Riboflavin interactions with oxygen—a survey from the photochemical perspective. Chem Eur J. 2014 doi: 10.1002/chem.201403895. [DOI] [PubMed] [Google Scholar]
- 149.Reddy HL, Dayan AD, Cavagnaro J, Gad S, Li J, Goodrich RP. Toxicity testing of a novel riboflavin-based technology for pathogen reduction and white blood cell inactivation. Transfus Med Rev. 2008 doi: 10.1016/j.tmrv.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 150.Thakur K, Tomar SK, Singh AK, Mandal S, Arora S. Riboflavin and health: a review of recent human research. Crit Rev Food Sci Nutr. 2017 doi: 10.1080/10408398.2016.1145104. [DOI] [PubMed] [Google Scholar]
- 151.Thakur K, Zhang JG, Wei ZJ, Kumar N, Tomar SK, and Pophaly SD. n.d. Cross talk between functional foods and gut health. 195–216. doi:10.4018/978-1-5225-2970-5.ch009.
- 152.Yoshii K, Hosomi K, Sawane K, Kunisawa J. Metabolism of dietary and microbial vitamin b family in the regulation of host immunity. Front Nutr. 2019 doi: 10.3389/fnut.2019.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gazzaniga F, Stebbins R, Chang SZ, McPeek MA, Brenner C. Microbial NAD metabolism: lessons from comparative genomics. Microbiol Mol Biol Rev. 2009 doi: 10.1128/mmbr.00042-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kurnasov O, Goral V, Colabroy K, Gerdes S, Anantha S, Osterman A, Begley TP. NAD biosynthesis: identification of the tryptophan to quinolinate pathway in bacteria. Chem Biol. 2003 doi: 10.1016/j.chembiol.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 155.Hegyi J, Schwartz RA, Hegyi V. Pellagra: dermatitis, dementia, and diarrhea. Int J Dermatol. 2004 doi: 10.1111/j.1365-4632.2004.01959.x. [DOI] [PubMed] [Google Scholar]
- 156.Xu XJ, Jiang GS. Niacin-respondent subset of schizophrenia—a therapeutic review. Eur Rev Med Pharmacol Sci. 2015;19:988. [PubMed] [Google Scholar]
- 157.Yamada T, Alpers DH, Kalloo AN, Kaplowitz N, Owyang C, Powell DW. Textbook of gastroenterology. 5. New York: Wiley; 2009. [Google Scholar]
- 158.Espinós C, Galindo MI, García-Gimeno MA, Ibáñez-Cabellos JS, Martínez-Rubio D, Millán JM, Rodrigo R, et al. Oxidative stress, a crossroad between rare diseases and neurodegeneration. Antioxidants. 2020;9(4):313. doi: 10.3390/antiox9040313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Magnúsdóttir S, Ravcheev D, De Crécy-Lagard V, Thiele I. Systematic genome assessment of B-vitamin biosynthesis suggests cooperation among gut microbes. Front Genet. 2015 doi: 10.3389/fgene.2015.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Brown MJ, Beier K. Vitamin B6 Deficiency (Pyridoxine). StatPearls. 2018. [PubMed]
- 161.Yasuda H, Fujiwara N, Ishizaki Y, Komatsu N. Anemia attributed to vitamin B6 deficiency in post-pancreaticoduodenectomy patients. Pancreatology. 2015 doi: 10.1016/j.pan.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 162.Adams JB, George F, Audhya T. Abnormally high plasma levels of vitamin B6 in children with autism not taking supplements compared to controls not taking supplements. J Altern Complement Med. 2006 doi: 10.1089/acm.2006.12.59. [DOI] [PubMed] [Google Scholar]
- 163.Choudhury SR, Singh SK, Roy S, Sengupta DN. An insight into the sequential, structural and phylogenetic properties of banana 1-aminocyclopropane-1-carboxylate synthase 1 and study of its interaction with pyridoxal-5’-phosphate and aminoethoxyvinylglycine. J Biosci. 2010 doi: 10.1007/s12038-010-0032-4. [DOI] [PubMed] [Google Scholar]
- 164.Ercan-Fang N, Taylor MR, Treadway JL, Levy CB, Genereux PE, Michael Gibbs E, Rath VL, Kwon Y, Cannon MC, Nuttall FQ. Endogenous effectors of human liver glycogen phosphorylase modulate effects of indole-site inhibitors. Am J Physiol Endocrinol Metab. 2005 doi: 10.1152/ajpendo.00264.2004. [DOI] [PubMed] [Google Scholar]
- 165.Geng MY, Saito H, Katsuki H. Effects of vitamin B6 and its related compounds on survival of cultured brain neurons. Neurosci Res. 1995 doi: 10.1016/0168-0102(96)81279-X. [DOI] [PubMed] [Google Scholar]
- 166.Plecko B, Stöckler S. Vitamin B6 dependent seizures. Can J Neurol Sciences. 2009 [PubMed]
- 167.Tsang EWT, Zhiyuan Hu, Qing Chang D, McGregor I, Keller WA. Expression of a brassic napus glutamate 1-semialdehyde aminotransferase in Escherichia coli and characterization of the recombinant protein. Protein Expr Purif. 2003 doi: 10.1016/S1046-5928(03)00010-X. [DOI] [PubMed] [Google Scholar]
- 168.Elahi A, Sabui S, Narasappa NN, Agrawal S, Lambrecht NW, Agrawal A, Said HM. Biotin deficiency induces Th1- and Th17-mediated proinflammatory responses in human CD4+ T lymphocytes via activation of the MTOR signaling pathway. J Immunol. 2018 doi: 10.4049/jimmunol.1701200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Maguire F, Henriquez FL, Leonard G, Dacks JB, Brown MW, Richards TA. Complex patterns of gene fission in the eukaryotic folate biosynthesis pathway. Genome Biol Evol. 2014 doi: 10.1093/gbe/evu213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rossi M, Amaretti A, Raimondi S. Folate production by probiotic bacteria. Nutrients. 2011 doi: 10.3390/nu3010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Baggott JE, Tamura T. Folate-dependent purine nucleotide biosynthesis in humans. Adv Nutr. 2015 doi: 10.3945/an.115.008300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Imbard A, Benoist JF, Blom HJ. Neural tube defects, folic acid and methylation. Int J Environ Res Public Health. 2013 doi: 10.3390/ijerph10094352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Laird EJ, O’Halloran AM, Carey D, O’Connor D, Kenny RA, Molloy AM. Voluntary fortification is ineffective to maintain the vitamin B12 and Folate Status of Older Irish adults: evidence from the Irish Longitudinal Study on Ageing (TILDA) Br J Nutr. 2018 doi: 10.1017/S0007114518001356. [DOI] [PubMed] [Google Scholar]
- 174.Ohrvik VE, Witthoft CM. Human folate bioavailability. Nutrients. 2011 doi: 10.3390/nu3040475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gruber K, Puffer B, Kräutler B. Vitamin B12-derivatives—Enzyme cofactors and ligands of proteins and nucleic acids. Chem Soc Rev. 2011 doi: 10.1039/c1cs15118e. [DOI] [PubMed] [Google Scholar]
- 176.Green R, Allen LH, Bjørke-Monsen A-L, Brito A, Guéant J-L, Miller JW, Molloy AM, et al. Vitamin B12 deficiency. Nat Rev Dis Prim. 2017;3(1):17040. doi: 10.1038/nrdp.2017.40. [DOI] [PubMed] [Google Scholar]
- 177.Watanabe F, Yabuta Y, Bito T, Teng F. Vitamin B12-containing plant food sources for vegetarians. Nutrients. 2014 doi: 10.3390/nu6051861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wolffenbuttel BHR, Wouters HJCM, Rebecca Heiner-Fokkema M, van der Klauw MM. The many faces of cobalamin (Vitamin B12) deficiency. Mayo Clinic Proc Innovations Quality Outcomes. 2019;3(2):200–214. doi: 10.1016/j.mayocpiqo.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dagnelie PC, van Staveren WA, van den Berg H. Vitamin B-12 from algae appears not to be bioavailable. Am J Clin Nutr. 1991;53(3):695–697. doi: 10.1093/ajcn/53.3.695. [DOI] [PubMed] [Google Scholar]
- 180.Takenaka S, Sugiyama S, Ebara S, Miyamoto E, Abe K, Tamura Y, Watanabe F, Tsuyama S, Nakano Y. Feeding dried purple laver (Nori) to vitamin B 12—deficient rats significantly improves vitamin B 12 status. Br J Nutr. 2001 doi: 10.1079/bjn2001352. [DOI] [PubMed] [Google Scholar]
- 181.Pereira H, Silva J, Santos T, Gangadhar KN, Raposo A, Nunes C, Coimbra MA, Gouveia L, Barreira L, Varela J. Nutritional potential and toxicological evaluation of Tetraselmis Sp. CtP4 microalgal biomass produced in industrial photobioreactors. Molecules. 2019 doi: 10.3390/molecules24173192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Farag MR, Alagawany M, El-Hack MEA, Dhama K. Nutritional and healthical aspects of spirulina (Arthrospira) for poultry, animals and human. Int J Pharmacol. 2016 doi: 10.3923/ijp.2016.36.51. [DOI] [Google Scholar]
- 183.Vonshak A, Guy R. Photoadaptation, photoinhibition and productivity in the blue-green alga, spirulina platensis grown outdoors. Plant Cell Environ. 1992 doi: 10.1111/j.1365-3040.1992.tb01496.x. [DOI] [Google Scholar]
- 184.Siva Kiran RR, Madhu GM, Satyanarayana SV. Spirulina in combating protein energy malnutrition (PEM) and protein energy wasting (PEW)—a review. J Nutr Res. 2015 doi: 10.13140/RG.2.1.3149.0325. [DOI] [Google Scholar]
- 185.Nakano S, Takekoshi H, Nakano M. Chlorella pyrenoidosa supplementation reduces the risk of anemia, proteinuria and edema in pregnant women. Plant Foods Hum Nutr. 2010 doi: 10.1007/s11130-009-0145-9. [DOI] [PubMed] [Google Scholar]
- 186.de Araújo FO, Giudici R, de Sousa JJMS. Cultivation of the microalgae chlorella pyrenoidosa using the processes of biotechnology. Revista Eletrônica Acervo Científico. 2019;2:121. doi: 10.25248/reac.e121.2019. [DOI] [Google Scholar]
- 187.Global Nutraceutical Market Growth Analysis Report, 2020–2027. n.d. https://www.grandviewresearch.com/industry-analysis/nutraceuticals-market. Accessed 19 June 2020
- 188.Milledge JJ. Commercial application of microalgae other than as biofuels: a brief review. Rev Environ Sci Biotechnol. 2011 doi: 10.1007/s11157-010-9214-7. [DOI] [Google Scholar]
- 189.Torres-Tiji Y, Fields FJ, Mayfield SP. Microalgae as a future food source. Biotechnol Adv. 2020 doi: 10.1016/j.biotechadv.2020.107536. [DOI] [PubMed] [Google Scholar]
- 190.Gellenbeck KW. Utilization of algal materials for nutraceutical and cosmeceutical applications-what do manufacturers need to know? J Appl Phycol. 2012 doi: 10.1007/s10811-011-9722-z. [DOI] [Google Scholar]
- 191.FDA Advisory No. 2018-208. Public health warning against the purchase and use of unregistered medical device product ‘Bang-Ze Abacterial Flexible Fabric Bandage’—Food and Drug Administration of the Philippines. n.d. https://www.fda.gov.ph/fda-advisory-no-2018-208-public-health-warning-against-the-purchase-and-use-of-unregistered-medical-device-product-bang-ze-abacterial-flexible-fabric-bandage/. Accessed 19 June 2020.
- 192.Niccolai A, Zittelli GC, Rodolfi L, Biondi N, Tredici MR. Microalgae of interest as food source: biochemical composition and digestibility. Algal Res. 2019 doi: 10.1016/j.algal.2019.101617. [DOI] [Google Scholar]
- 193.Hur SJ, Lim BO, Decker EA, Julian McClements D. In vitro human digestion models for food applications. Food Chem. 2011 doi: 10.1016/j.foodchem.2010.08.036. [DOI] [Google Scholar]
- 194.Minekus M, Alminger M, Alvito P, Ballance S, Bohn T, Bourlieu C, Carrière F, et al. A standardised static in vitro digestion method suitable for food-an international consensus. Food Function. 2014 doi: 10.1039/c3fo60702j. [DOI] [PubMed] [Google Scholar]
- 195.Machů L, Mišurcová L, Samek D, Hrabě J, Fišera M. In vitro digestibility of different commercial edible algae products. J Aquat Food Prod Technol. 2014 doi: 10.1080/10498850.2012.721873. [DOI] [Google Scholar]
- 196.Mišurcová L, Kráčmar S, Klejdus B, Vacek J. Nitrogen content, dietary fiber, and digestibility in algal food products. Czech J Food Sci. 2010 doi: 10.17221/111/2009-cjfs. [DOI] [Google Scholar]
- 197.Wong KH, Cheung PCK. Nutritional evaluation of some subtropical red and green seaweeds part II. In vitro protein digestibility and amino acid profiles of protein concentrates. Food Chem. 2001 doi: 10.1016/S0308-8146(00)00176-X. [DOI] [Google Scholar]
- 198.Janczyk P. Evaluation of nutritional value and activity of green microalgae Chlorella vulgaris in rats and mice. Berlin: Freie Univ Berlin; 2005. [Google Scholar]
- 199.Phan MA, Thu JP, Bucknall M, Arcot J. Interactions between phytochemicals from fruits and vegetables: effects on bioactivities and bioavailability. Crit Rev Food Sci Nutr. 2018 doi: 10.1080/10408398.2016.1254595. [DOI] [PubMed] [Google Scholar]
- 200.Cardozo KHM, Guaratini T, Barros MP, Falcão VR, Tonon AP, Lopes NP, Campos S, et al. Metabolites from algae with economical impact. Comp Biochem Physiol C Toxicol Pharmacol. 2007 doi: 10.1016/j.cbpc.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 201.Le Tutour B, Guedon D. Antioxidative activities of olea europaea leaves and related phenolic compounds. Phytochemistry. 1992 doi: 10.1016/0031-9422(92)80255-D. [DOI] [Google Scholar]
- 202.Sánchez-Machado DI, López-Cervantes J, López-Hernández J, Paseiro-Losada P. Fatty acids, total lipid, protein and ash contents of processed edible seaweeds. Food Chem. 2004 doi: 10.1016/j.foodchem.2003.08.001. [DOI] [Google Scholar]
- 203.Fabre G, Bayach I, Berka K, Paloncýová M, Starok M, Rossi C, Duroux JL, Otyepka M, Trouillas P. Synergism of antioxidant action of vitamins E, C and quercetin is related to formation of molecular associations in biomembranes. Chem Commun. 2015 doi: 10.1039/c5cc00636h. [DOI] [PubMed] [Google Scholar]
- 204.Mokrosnop VM, Polishchuk AV, Zolotareva EK. Accumulation of α-tocopherol and β-carotene in euglena gracilis cells under autotrophic and mixotrophic culture conditions. Appl Biochem Microbiol. 2016 doi: 10.1134/S0003683816020101. [DOI] [PubMed] [Google Scholar]
- 205.Ben-Amotz A. Production of β-carotene and vitamins by the halotolerant alga dunaliella. Pharm Bioactive Natural Prod. 1993 doi: 10.1007/978-1-4899-2391-2_11. [DOI] [Google Scholar]
- 206.Fu HY, Liu SL, Chiang YR. Biosynthesis of ascorbic acid as a glucose-induced photoprotective process in the extremophilic red alga galdieria partita. Front Microbiol. 2020 doi: 10.3389/fmicb.2019.03005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wen ZY, Chen F. Heterotrophic production of eicosapentaenoic acid by microalgae. Biotechnol Adv. 2003 doi: 10.1016/S0734-9750(03)00051-X. [DOI] [PubMed] [Google Scholar]
- 208.Lima S, Webb C, Deery E, Robinson C, Zedler J. Human intrinsic factor expression for bioavailable vitamin B12 enrichment in microalgae. Biology. 2018 doi: 10.3390/biology7010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Anh Nguyen M, Hoang AL. n.d. A review on microalgae and cyanobacteria in biofuel production. Econ Financ. 2016. https://hal-enpc.archives-ouvertes.fr/hal-01383026.
- 210.Grant MAA, Kazamia E, Cicuta P, Smith AG. Direct exchange of vitamin B 12 is demonstrated by modelling the growth dynamics of algal-bacterial cocultures. ISME J. 2014 doi: 10.1038/ismej.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kazamia E, Czesnick H, Nguyen TTV, Croft MT, Sherwood E, Sasso S, Hodson SJ, Warren MJ, Smith AG. Mutualistic interactions between vitamin B12-dependent algae and heterotrophic bacteria exhibit regulation. Environ Microbiol. 2012 doi: 10.1111/j.1462-2920.2012.02733.x. [DOI] [PubMed] [Google Scholar]
- 212.Men Y, Seth EC, Yi S, Crofts TS, Allen RH, Taga ME, Alvarez-Cohen L. Identification of specific corrinoids reveals corrinoid modification in dechlorinating microbial communities. Environ Microbiol. 2015 doi: 10.1111/1462-2920.12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Mandal S, Mallick N. Microalga Scenedesmus obliquus as a potential source for biodiesel production. Appl Microbiol Biotechnol. 2009 doi: 10.1007/s00253-009-1935-6. [DOI] [PubMed] [Google Scholar]
- 214.Angelo MD, Smerilli A, Ambrosino L, Albini A, Noonan DM, Sansone CBC. n.d. Insights in phenolic compounds from microalgae: structural variety and complex beneficial activities from health to nutraceutics. Crit Rev Biotechnol. [DOI] [PubMed]
- 215.Galasso C, Gentile A, Orefice I, Ianora A, Bruno A, Noonan DM, Sansone C, Albini A, Brunet C. Microalgal derivatives as potential nutraceutical and food supplements for human health: a focus on cancer prevention and interception. Nutrients. 2019 doi: 10.3390/nu11061226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sansone C, Brunet C. Marine algal antioxidants. Antioxidants. 2020 doi: 10.3390/antiox9030206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Benedetti M, Vecchi V, Barera S, Dall’Osto L. Biomass from microalgae: the potential of domestication towards sustainable biofactories. Microb Cell Fact. 2018 doi: 10.1186/s12934-018-1019-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Sannino F, Sansone C, Galasso C, Kildgaard S, Tedesco P, Fani R, Marino G, et al. Pseudoalteromonas haloplanktis TAC125 produces 4-hydroxybenzoic acid that induces pyroptosis in human A459 lung adenocarcinoma cells. Sci Rep. 2018 doi: 10.1038/s41598-018-19536-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sansone C, Brunet C. Promises and challenges of microalgal antioxidant production. Antioxidants. 2019 doi: 10.3390/antiox8070199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Sansone C, Brunet C, Noonan DM, Albini A. Marine algal antioxidants as potential vectors for controlling viral diseases. Antioxidants. 2020 doi: 10.3390/antiox9050392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kroumov AD, Scheufele FB, Trigueros DEG, Modenes AN, Zaharieva M, Najdenski H. Modeling and technoeconomic analysis of algae for bioenergy and coproducts. In: Rastogi RP, Madamwar D, Pandey A, editors. Algal green chemistry: recent progress in biotechnology. Amsterdam: Elsevier; 2017. [Google Scholar]
- 222.Williams PJ, Le B, Laurens LML. Microalgae as biodiesel & biomass feedstocks: review & analysis of the biochemistry, energetics & economics. Energy Environ Sci. 2010 doi: 10.1039/b924978h. [DOI] [Google Scholar]
- 223.Reijnders MJMF, van Heck RGA, Lam CMC, Scaife MA, Vitor AP, dos Santos M, Smith AG, Schaap PJ. Green genes: bioinformatics and systems-biology innovations drive algal biotechnology. Trends Biotechnol. 2014 doi: 10.1016/j.tibtech.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 224.Lala Behari S. Artificial intelligence and virtual environment for microalgal source for production of nutraceuticals. Biomed J Sci Tech Res. 2019 doi: 10.26717/bjstr.2019.13.002459. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.