Abstract
Objectives
Preoperative models, based on patient and tumor characteristics, predict risk for adverse outcomes after nephrectomy. Changes in renal tumor characteristics over the last decades, warrant further evaluation using contemporary cohorts.
We aimed to validate a previously published preoperative nomogram predicting 12-year metastasis-free probability after nephrectomy for localized renal tumors in a contemporary cohort.
Patients and Methods
After obtaining institutional review board approval, data of 1,760 patients who underwent nephrectomy for a localized renal mass between 2005 - 2011 were reviewed. Preoperative images were evaluated for the presence of tumor necrosis, lymphadenopathy and tumor size.
The study outcome was metastatic-free probability. Model discrimination was assessed with Gonen and Heller’s concordance probability estimate, and calibration was evaluated.
Results
The cohort included 1,102 male and 658 female patients with a median age of 60 years. Most patients presented incidentally (84%). On imaging, 3% had evidence of lymphadenopathy, 55% had necrosis and median tumor diameter was 3.7cm (IQR:2.5, 5.5).
Median follow-up in non-metastatic patients was 7.7 years (IQR 5.3, 9.7). Estimated 12-year metastatic-free probability was 88% (86% - 90%). The model showed strong discrimination (CPE: 0.77), and fair calibration. The time-dependent ROC curves showed strong discrimination at all time points and the AUC for year 12 was 0.83 (95% CI:0.78-0.89).
Conclusions
We validated the preoperative nomogram of 12-year metastasis-free probability in a contemporary cohort despite different tumor characteristics.
Future studies should evaluate the role of preoperative risk stratification in patient selection for neoadjuvant treatment.
Keywords: Metastases, Nephrectomy, Nomogram, Outcome, Preoperative, Renal cell carcinoma
Introduction
Over 70% of renal tumors are diagnosed at an early stage.[1] Despite curative surgical treatment, approximately one third of patients will develop metastatic disease at a follow-up of 10 years.[2] Neoadjuvant immunotherapy has an evolving role as a mean to improve outcome in solid malignancies and is currently evaluated for renal cell carcinoma (RCC).[3, 4] However, the use of immunotherapy, especially when combining PD-1 and CTLA-4 blockade, is associated with substantial toxicity necessitating careful patient selection prior to treatment.[3] Preoperative risk stratification can identify high-risk patients who are suitable candidates for neoadjuvant trials.[5]
Current preoperative predictive models rely on patient and tumor characteristics on imaging to predict the risk of metastases and cancer related mortality after nephrectomy.[6-13] In a previous publication from the Mayo Clinic and our center, Raj et. al. reported a preoperative nomogram for predicting the 12-year metastatic free probability (MFP) for patients with localized renal tumors. The nomogram included gender, mode of presentation, evidence of lymphadenopathy, evidence of necrosis and tumor size based on preoperative imaging.[8] Despite demonstrating an improved predictive capability over clinical stage alone, this nomogram has not been validated.
Over the last decades there has been a change in the characteristics of renal tumors at time of diagnosis including a higher rate of incidental findings and a decrease in tumor size and stage.[1, 14, 15] In light of these changes, it is unknown whether preoperative nomograms, published over a decade ago, are still accurate in the contemporary setting.
In the current study we aimed to validate our previously reported nomogram, published by Raj et. al. [8], using a contemporary cohort of patient with localized renal masses.
Patients and Methods
After obtaining institutional review board approval we reviewed our prospectively collected nephrectomy database and identified 2,048 consecutive patients who underwent a partial or radical nephrectomy at Memorial Sloan Kettering Cancer Center for a localized renal mass between the years 2005 and 2011. Patients treated for bilateral renal tumors (n=56), with metastatic disease from a source other than kidney (n=27), previous procedures on their ipsilateral kidney (n=6) and without preoperative imaging (n=199) were excluded, leaving a total of 1,760 patients for analysis (Supplementary Figure 1).
Pre-operative patient data including age, gender, race and mode of presentation were collected. Mode of presentation was classified as incidental (no symptoms), local (hematuria, flank pain, palpable mass), or systemic (weight loss, anorexia, asthenia, fever), based on a validated classification system.[16] Preoperative cross-sectional imaging was reviewed by radiologists (CD, OA) for the presence of lymphadenopathy (yes/no), necrosis (yes/no), and tumor size (cm). Lymphadenopathy was identified when the short axis diameter of the node was greater than 1 cm, the node had irregular shape and/or enhancement. Necrosis was defined as the presence of ill defined, irregular areas within the tumor that did not demonstrate contrast enhancement during the nephrographic and delayed phases. After nephrectomy, patients were followed with a history, physical exam, basic laboratory testing and serial imaging of the chest and abdomen every six to 12 months for at least three years based on the tumor risk group. The presence and site of metastases during follow-up were collected.
MFP was calculated from nephrectomy until diagnosis of metastases. Patients who died or were metastases free at last follow-up were censored, consistent with Raj et al. nomogram methodology. Overall survival (OS) was calculated from time of nephrectomy until death; patients alive at last follow-up were censored. Disease specific survival (DSS) was calculated from time of nephrectomy until death from disease. Patients who died of other or unknown causes, or who were alive at last follow-up were censored. MFP, OS, and DSS were estimated with Kaplan-Meier methods and plots.
Univariable Cox regression models assessed the relationship between factors included within the Raj et al. nomogram and metastasis-free probability. The linear predictor and 12-year predicted MFP from the nomogram was calculated, as visualized in Figure 1, and put into a univariable Cox model. Model discrimination was assessed with Gonen and Heller's CPE. We also assessed discrimination with time-dependent ROC curves plotted at available years using the inverse probability of censoring weighting approach.[17] Predicted 12-year MFP was stratified into tertiles (12-year MFP = 0-33%, 33-67%, 67%-100%), and plotted with Kaplan-Meier methods to further visualize model performance. Calibration of the model was visualized with a calibration plot. For the calibration plot, patients were placed into ranked tertiles (each 1/3 of patients) of predicted 12-year MFP and the mean predicted 12-year MFP of each group was plotted against the observed Kaplan-Meier estimate.
Figure 1 –
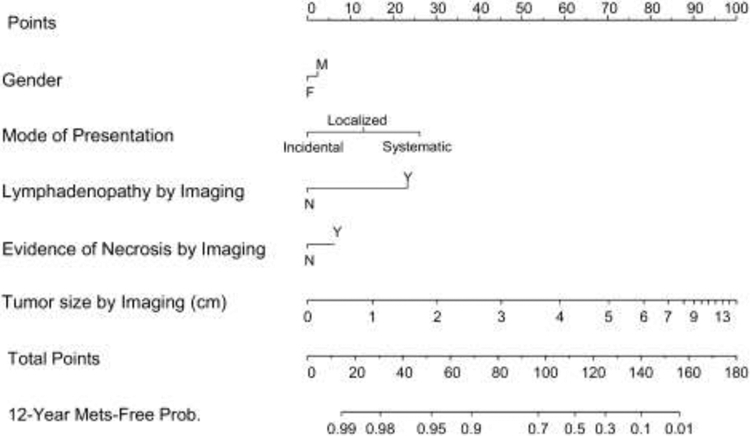
Preoperative nomogram predicting freedom from metastatic recurrence at 12 years following definitive surgical management. Obtained with permission from: Raj GV, Thompson RH, Leibovich BC, Blute ML, Russo P, Kattan MW. Preoperative nomogram predicting 12-year probability of metastatic renal cancer. The Journal of Urology. 2008; 179:2146-51; https://www.auajournals.org/doi/10.1016/j.juro.2008.01.101
As a sensitivity analysis, we examined the association between the linear predictor and MFP when death from other/unknown causes was treated as a competing risk. Two-sided p-values less than 0.05 were considered statistically significant. We used SAS 9.4 (The SAS Institute, Cary, NC) and the 'CPE' package[18] within Cran R Version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria) for all analyses.
Results
Clinical Characteristics of the Study Cohort
The cohort included 1,760 patients with a median age of 60 years (IQR:52-69), 658 of whom (37%) were female. Most patients presented incidentally (83%, 1469/1760), 274/1760 (16%) presented with localized symptoms due to their tumor, and 17/1760 (1%) presented with systemic symptoms. On imaging, lymphadenopathy was uncommon (3%, 46/1760) and 55% of patients had necrosis (966/1760). Median tumor diameter was 3.7cm (IQR: 2.5-5.5), (Table 1).
Table 1.
Clinical characteristics of the cohort (n=1,760); numbers represent frequency with percent of total in parentheses unless otherwise specified
| N (%) | ||
|---|---|---|
| Age at surgery, years | Median (IQR) | 60 (52-69) |
| Gender | Male | 1102 (63) |
| Female | 658 (37) | |
| Race | White | 1560 (89) |
| Black | 93 (5) | |
| Asian | 63 (4) | |
| Other | 26 (2) | |
| Unknown | 18 (1) | |
| Presentation | Incidental | 1469 (84) |
| Local | 274 (16) | |
| Systemic | 17 (1) | |
| Lymphadenopathy | Yes | 46 (3) |
| No | 1714 (97) | |
| Necrosis | Yes | 966 (55) |
| No | 794 (45) | |
| Maximum Dimension, cm | Median (IQR) | 3.7 (2.5-5.5) |
| ASA Score | I - II | 921 (52) |
| III - IV | 835 (47) | |
| Unknown | 4 (0) | |
| Operation type | Partial Nephrectomy | 1031 (59) |
| Radical Nephrectomy | 341 (19) | |
| Laparoscopic Partial Nephrectomy | 193 (11) | |
| Laparoscopic Radical Nephrectomy | 55 (3) | |
| Robotic Partial Nephrectomy | 133 (8) | |
| Robotic Radical Nephrectomy | 7 (0) | |
IQR = interquartile range; ASA = American Society of Anesthesiologists. Percentages of total may not add exactly to 100% due to rounding
Metastases Free Survival and Nomogram Validation
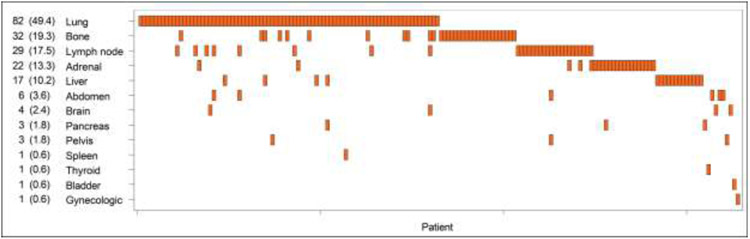
Median follow-up in non-metastatic patients (N=1594) was 7.7 years (IQR:5.3-9.7). By the end of follow-up, 166 patients had metastatic disease. Six-year MFP was 91% (89-92%) and 12-year MFP was 88% (86-90%), (Supplementary Figure 2). The most common sites of metastases included lung (49%, 82/166), bone (19%, 32/166) and lymph nodes (17%, 29/166), (Figure 2).
Figure 2 -.
Heatmap of metastatic sites with associated frequency among the cohort (n=1,760)
Age at surgery, mode of presentation, lymphadenopathy, necrosis and tumor dimension were all significant predictors of MFP on univariate analysis (Table 2). Male gender was associated with decreased MFP; however, the association did not reach statistical significance (HR:1.37, 95% CI:0.99-1.91, p=0.06).
Table 2.
Univariable Cox regression for preoperative predictors of metastasis-free probability included in the Raj et al. nomogram (n=1760)
| Univariable | |||||
|---|---|---|---|---|---|
| N(E) | HR | [95% CI] | p-value | ||
| Age at Surgery, years | 1760 (166) | 1.02 | [1.00 – 1.03] | 0.010 | |
| Gender | Male | 1102 (115) | 1.37 | [0.99 – 1.91] | 0.06 |
| Female | 658 (51) | REF | |||
| Presentation | Systemic | 17 (11) | 19.73 | [10.54 – 36.94] | <.001 |
| Local | 274 (60) | 3.75 | [2.71 – 5.18] | <.001 | |
| Incidental | 1469 (95) | REF | |||
| Lymphadenopathy | Yes | 46 (20) | 6.82 | [4.27 - 10.90] | <.001 |
| No | 1714 (146) | REF | |||
| Necrosis | Yes | 966 (144) | 5.94 | [3.79 - 9.30] | <.001 |
| No | 794 (22) | REF | |||
| Maximum Dimension, cm | 1760 (166) | 1.25 | [1.22 - 1.29] | <.001 | |
N=Total # patients for level; #E = # events for level; HR = hazard ratio; 95%CI = 95% confidence interval
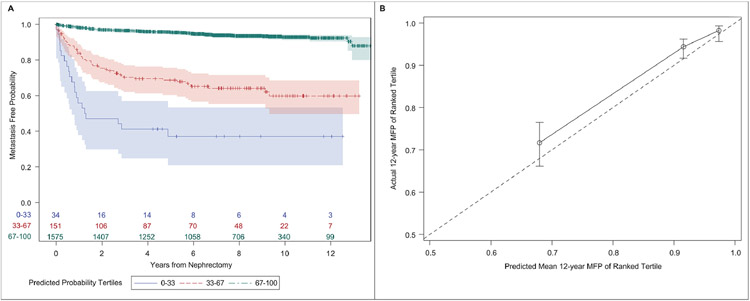
The linear predictor from Raj et al. showed good discrimination (CPE:0.77+/− 0.01). The three probability tertiles had clearly separated MFP curves. At 12 years, MFP was 37% (21-53%) in the 1st tertile, 60% (50-69%) in the 2nd tertile, and 92% (90-94%) in the 3rd tertile (Figure 3A). While discrimination was high, calibration of the model was fair. Predicted mean survival was consistently lower than actual survival. While the highest tertile overlapped with the 45-degree reference line, the last two tertiles had minimal overlap, suggesting overestimation (Figure 3B).
Figure 3 –
(A) Kaplan-Meier plot of metastasis-free probability stratified by tertiles of predicted 12-year metastasis-free probability in the cohort; (B) Calibration plot of the nomogram fit in the cohort of patients who underwent nephrectomy for localized renal tumor (n=1,760). The circles represent the predicted mean and actual 12-year MFS with the bars representing confidence limits around the estimate. The dashed line represents the 45-degree line
The time-dependent ROC curves showed strong discrimination at all time points, however, the performance was strongest in earlier years, where the AUC for years 2 and 4 were 0.87 (95% CI:0.84-0.91) and 0.87 (95% CI:0.84-90), and decreased to 0.83 (95% CI:0.79-0.87) and 0.83 (95% CI:0.78-0.89) by years 10 and 12 (Figure 4).
Figure 4 –
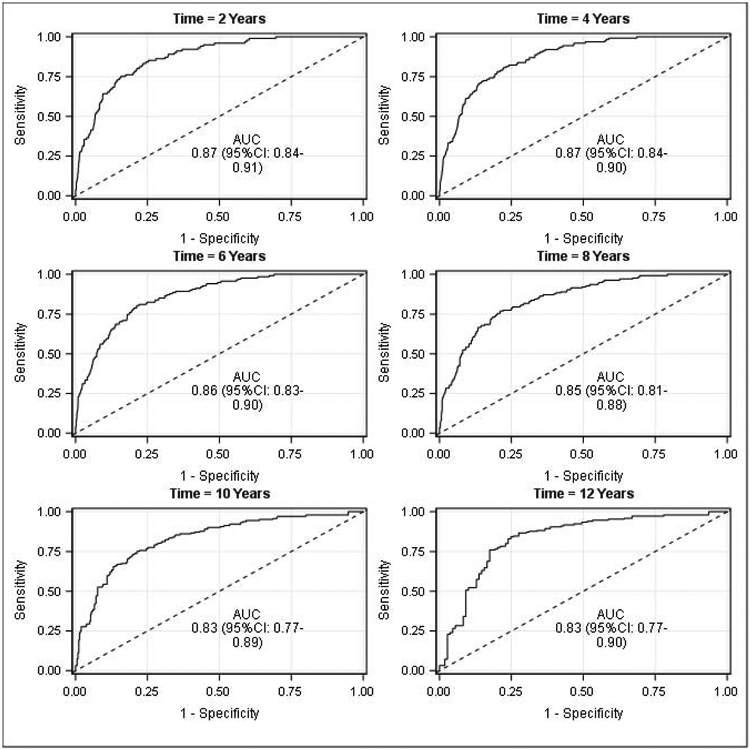
Time-dependent ROC curves for metastasis-free probability for all patients (n=1760)
Overall and Disease Specific Survival
By the end of follow-up, 277 patients had died. Six- and 12-year OS were 89% (87-91%) and 74% (71-77%), respectively. Six- and 12-year DSS estimates were 96% (95-97%) and 95% (94-97%), respectively (Supplementary Figure 3).
MFP Sensitivity Analyses
With death as a competing risk, 6- and 12-year cumulative incidence of metastases was 2.3% (1.7-3.1%) and 3.9% (3.1-4.9%), respectively (Supplementary Figure 4). The nomogram linear predictor was significantly associated with MFP using competing risks methodology (HR:3.14, 95% CI:2.72-3.63, p<0.001).
Discussion
Several preoperative models, based on patient and tumor characteristics on imaging, were developed to predict recurrence free and DSS in patients undergoing nephrectomy.[6-11] Model accuracy at external validation ranged from 65% - 67% for models evaluating patients with localized RCC and 78% - 88% for those evaluating patients at all stages.[9, 12, 13] However, since most of these models were published, there has been a change in the clinical characteristics of localized renal masses. The extensive use of abdominal imaging for the evaluation of other causes increased the rate of incidentally detected renal masses with a decrease in the rate of patients diagnosed with local or systemic symptoms.[15] Similarly, tumor size and stage at diagnosis decreased over time.[1, 14, 15] These changes in the landscape of renal masses question the validity of previous preoperative nomograms that were built based on non-contemporary cohorts.
Raj et al. used pooled data from the institutional databases of the Mayo Clinic and Memorial Sloan Kettering Cancer Center and developed a preoperative model to predict 12-year likelihood of metastatic recurrence among patients with localized renal masses treated with nephrectomy. In their publication, the median follow-up was 4.7 years with a MFP rate of 70% (95% CI: 68% - 72%), the concordance index of the nomogram was 0.8 and the model had good calibration.[8] The current study is the first to validate the nomogram proposed by Raj et al. Compared to the original cohort, and consistent with the reported changes in tumor characteristics at diagnosis in recent years, the current contemporary cohort had a higher rate of incidental findings (84% vs. 51%), lower rate of lymphadenopathy (2% vs. 6%) and smaller median tumor size (3.7cm vs. 5.3cm). We observed a longer follow-up (7.7 years) and a higher estimated 12-year MFP (88%) than previously reported, possibly due to a higher rate of low stage disease in the current cohort. We noted a high rate of necrosis likely related to the use of central radiology review rather than relying on imaging reports, which may have reduced the calibration of the model together with the relatively low rate of metastatic events we observed. Despite these changes in the characteristics of the cohort, the individual predictors of the nomogram remained associated with outcome and the model demonstrated strong discrimination with a concordance index of 0.77 and a time-dependent AUC at 12 years of 0.83. Thus, the current study, performed on a large contemporary cohort with long follow-up, demonstrates the applicability of the preoperative nomogram despite the change in the clinicopathologic characteristic of RCC over time.
Neoadjuvant treatment for RCC may be beneficial by treating micro-metastatic disease and reducing tumor size making it amendable to surgery, and is expected to be better tolerated than treatment after surgery.[19] Furthermore, it has been suggested that pro-angiogenic and pro-immunogenic factors present in the primary tumor may enhance the effect of targeted therapy and immunotherapy given in the neoadjuvant setting.[20] Phase II trials evaluating the use of neoadjuvant tyrosine kinase inhibitors in patients with localized and locally advanced renal tumor have shown substantial tumor shrinkage and a decrease in nephrometry scores following treatment.[21-23] Recent phase II trials in non-small-cell lung cancer and melanoma have demonstrated the feasibility and potential benefit of neoadjuvant immunotherapy.[3, 4] Current ongoing trials are evaluating the safety and benefit of neoadjuvant immunotherapy and combination treatments of tyrosine kinase inhibitors and immune checkpoint blockade for RCC.[20] Due to the substantial rate of high-grade toxicities, especially when combining ipilimumab and nivolumab,[3] accurate preoperative risk stratification to identify high-risk patients who may be trial candidates is warranted. The current nomogram, validated on a contemporary cohort, may be suitable for this purpose. The highest risk group of patients had a metastasis free probability of 37% at years 6 and 12, most of whom recurred early. This high-risk group of patients may be most suitable for inclusion in future neoadjuvant trials while limiting the exposure of lower risk patients to neoadjuvant treatment thus avoiding the associated toxicities.
Recent studies have shown an improvement in the diagnostic accuracy of renal mass biopsy which is currently above 90%.[24] Furthermore, recurrent genomic alterations, which may be identified on renal biopsy, have been associated with pathological and clinical outcome in RCC; however, only few studies evaluated the added benefit of these alterations to commonly used prognostic models.[25-27] While an initial report did not show an increase in the predictive accuracy when adding genomic markers to a preoperative model including tumor size and age and the postoperative Mayo Clinic stage, size, grade, and necrosis (SSIGN) prognostic scoring system,[25] subsequent studies suggest TP53, SETD2 and BAP1 may have an independent prognostic role after adjusting for various postoperative models. [26, 27] Since renal mass biopsies are routinely obtained prior to inclusion in neoadjuvant trials, future studies should evaluate whether histologic and genomic data obtained from these biopsies may enhance preoperative risk stratification among patients with high risk renal tumors.
The limitations of our study include its retrospective nature which is associated with an inconsistent follow-up schedule for all patients. Although we had 8 years of follow up and over 1700 patients, due to the rarity of metastases, it would be ideal to have had a longer follow-up period. Furthermore, the relatively small number of metastatic events, most likely due to the low stage of patients included within contemporary cohorts, limit the robustness of the results and their applicability to higher risk patients; thus, additional studies are required to validate these findings in higher risk cohorts. The focus of the current study was to validate the pre-operative model and assess whether the model performs well on an external contemporary dataset rather than assess whether additional pre-operative factors may improve prediction of outcome in patients with renal masses. Furthermore, we were unable to compare the predictive abilities of the validated nomogram to those of other pre-operative models which predict cancer-specific and recurrence-free survival rather than metastatic-free probability.
Conclusions
The current study validates our previously published preoperative nomogram in a contemporary cohort of patients, demonstrating its relevance despite the changing population of patient diagnosed with RCC. Future studies should evaluate the role of preoperative risk stratification in patient selection for neoadjuvant treatment. In addition, studies should explore whether inclusion of information obtained from pretreatment renal biopsy can improve preoperative risk prediction in this group of patients.
Supplementary Material
Highlights.
Contemporary RCC patients have more incidental findings, less lymphadenopathy and smaller tumors
Estimated 12-year metastatic-free probability was 88% for localized RCC
Preoperative nomogram for metastatic-free probability showed strong discrimination in contemporary patients
Preoperative nomograms may aid in patient selection for neoadjuvant treatment
Acknowledgments
Funding
This work was supported by The Sidney Kimmel Center for Prostate and Urologic Cancers.
This work was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Conflict of Interest and Disclosure Statement
Oguz Akin holds stock options and serves as a scientific advisor for Ezra AI, Inc., which is developing Artificial Intelligence algorithms and software unrelated to the research being reported.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Patel HD, Gupta M, Joice GA, Srivastava A, Alam R, Allaf ME, et al. Clinical Stage Migration and Survival for Renal Cell Carcinoma in the United States. European Urology Oncology. 2018. [DOI] [PubMed]
- [2].Leibovich BC, Lohse CM, Cheville JC, Zaid HB, Boorjian SA, Frank I, et al. Predicting Oncologic Outcomes in Renal Cell Carcinoma After Surgery. European urology. 2018;73:772–80. [DOI] [PubMed] [Google Scholar]
- [3].Amaria RN, Reddy SM, Tawbi HA, Davies MA, Ross MI, Glitza IC, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nature medicine. 2018;24:1649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Forde PM, Chaft JE, Pardoll DM. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. The New England journal of medicine. 2018;379:e14. [DOI] [PubMed] [Google Scholar]
- [5].Dey S, Peabody HN, Noyes SL, Lane BR. Neoadjuvant targeted molecular therapy before renal surgery. Urologic Clinics. 2017;44:289–303. [DOI] [PubMed] [Google Scholar]
- [6].Yaycioglu O, Roberts WW, Chan T, Epstein JI, Marshall FF, Kavoussi LR. Prognostic assessment of nonmetastatic renal cell carcinoma: a clinically based model. Urology. 2001;58:141–5. [DOI] [PubMed] [Google Scholar]
- [7].Cindolo L, de la Taille A, Messina G, Romis L, Abbou CC, Altieri V, et al. A preoperative clinical prognostic model for non-metastatic renal cell carcinoma. BJU international. 2003;92:901–5. [DOI] [PubMed] [Google Scholar]
- [8].Raj GV, Thompson RH, Leibovich BC, Blute ML, Russo P, Kattan MW. Preoperative nomogram predicting 12-year probability of metastatic renal cancer. The Journal of urology. 2008;179:2146–51; discussion 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Karakiewicz PI, Suardi N, Capitanio U, Jeldres C, Ficarra V, Cindolo L, et al. A preoperative prognostic model for patients treated with nephrectomy for renal cell carcinoma. European urology. 2009;55:287–95. [DOI] [PubMed] [Google Scholar]
- [10].Kanao K, Mizuno R, Kikuchi E, Miyajima A, Nakagawa K, Ohigashi T, et al. Preoperative prognostic nomogram (probability table) for renal cell carcinoma based on TNM classification. The Journal of urology. 2009;181:480–5; discussion 5. [DOI] [PubMed] [Google Scholar]
- [11].Yaycioglu O, Eskicorapci S, Karabulut E, Soyupak B, Gogus C, Divrik T, et al. A preoperative prognostic model predicting recurrence-free survival for patients with kidney cancer. Japanese journal of clinical oncology. 2013;43:63–8. [DOI] [PubMed] [Google Scholar]
- [12].Utsumi T, Ueda T, Fukasawa S, Komaru A, Kobayashi M, Sazuka T, et al. External validation of a pre-operative prognostic nomogram for renal cell carcinoma in two patient populations: a retrospective cohort study. Japanese journal of clinical oncology. 2011;41:1147–51. [DOI] [PubMed] [Google Scholar]
- [13].Gontero P, Sun M, Antonelli A, Bertini R, Carini M, Carmignani G, et al. External validation of the preoperative Karakiewicz nomogram in a large multicentre series of patients with renal cell carcinoma. World journal of urology. 2013;31:1285–90. [DOI] [PubMed] [Google Scholar]
- [14].Cooperberg MR, Mallin K, Ritchey J, Villalta JD, Carroll PR, Kane CJ. Decreasing size at diagnosis of stage 1 renal cell carcinoma: analysis from the National Cancer Data Base, 1993 to 2004. The Journal of urology. 2008;179:2131–5. [DOI] [PubMed] [Google Scholar]
- [15].Thorstenson A, Bergman M, Scherman-Plogell AH, Hosseinnia S, Ljungberg B, Adolfsson J, et al. Tumour characteristics and surgical treatment of renal cell carcinoma in Sweden 2005-2010: a population-based study from the national Swedish kidney cancer register. Scandinavian journal of urology. 2014;48:231–8. [DOI] [PubMed] [Google Scholar]
- [16].Patard JJ, Leray E, Cindolo L, Ficarra V, Rodriguez A, De La Taille A, et al. Multi-institutional validation of a symptom based classification for renal cell carcinoma. The Journal of urology. 2004;172:858–62. [DOI] [PubMed] [Google Scholar]
- [17].Uno H, Cai T, Tian L, Wei L. Evaluating prediction rules for t-year survivors with censored regression models. Journal of the American Statistical Association. 2007;102:527–37. [Google Scholar]
- [18].Mo Q, Gonen M, Heller G. CPE: concordance probability estimates in survival analysis. R package version. 2012;1. [Google Scholar]
- [19].Grivas NK. Neoadjuvant targeted therapy for advanced renal cell carcinoma: Where do we stand? Urology annals. 2019;11:115–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gleeson JP, Motzer RJ, Lee CH. The current role for adjuvant and neoadjuvant therapy in renal cell cancer. Current opinion in urology. 2019;29:636–42. [DOI] [PubMed] [Google Scholar]
- [21].Karam JA, Devine CE, Urbauer DL, Lozano M, Maity T, Ahrar K, et al. Phase 2 trial of neoadjuvant axitinib in patients with locally advanced nonmetastatic clear cell renal cell carcinoma. European urology. 2014;66:874–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rini BI, Plimack ER, Takagi T, Elson P, Wood LS, Dreicer R, et al. A Phase II Study of Pazopanib in Patients with Localized Renal Cell Carcinoma to Optimize Preservation of Renal Parenchyma. The Journal of urology. 2015;194:297–303. [DOI] [PubMed] [Google Scholar]
- [23].Lane BR, Derweesh IH, Kim HL, O'Malley R, Klink J, Ercole CE, et al. Presurgical sunitinib reduces tumor size and may facilitate partial nephrectomy in patients with renal cell carcinoma. Urologic oncology. 2015;33:112 e15–21. [DOI] [PubMed] [Google Scholar]
- [24].Marconi L, Dabestani S, Lam TB, Hofmann F, Stewart F, Norrie J, et al. Systematic Review and Meta-analysis of Diagnostic Accuracy of Percutaneous Renal Tumour Biopsy. European urology. 2016;69:660–73. [DOI] [PubMed] [Google Scholar]
- [25].Hakimi AA, Mano R, Ciriello G, Gonen M, Mikkilineni N, Sfakianos JP, et al. Impact of recurrent copy number alterations and cancer gene mutations on the predictive accuracy of prognostic models in clear cell renal cell carcinoma. The Journal of urology. 2014;192:24–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Joseph RW, Kapur P, Serie DJ, Eckel-Passow JE, Parasramka M, Ho T, et al. Loss of BAP1 protein expression is an independent marker of poor prognosis in patients with low-risk clear cell renal cell carcinoma. Cancer. 2014;120:1059–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Manley BJ, Zabor EC, Casuscelli J, Tennenbaum DM, Redzematovic A, Becerra MF, et al. Integration of Recurrent Somatic Mutations with Clinical Outcomes: A Pooled Analysis of 1049 Patients with Clear Cell Renal Cell Carcinoma. European urology focus. 2017;3:421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.