Abstract
Background:
In the FLAURA trial, superiority of osimertinib over the standard of care (SOC) was not demonstrated in Asian patients; SOC seemed favorable among Japanese patients (hazard ratio 1.39, 95% confidence interval 0.82–2.33). Three reasons are suggested: since rechallenge with epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) is covered by health insurance in Japan, EGFR-TKI rechallenge rate was higher in SOC than in the osimertinib group, which resulted in a long-term sequential administration of EGFR-TKIs; treatment discontinuation rate was high in the osimertinib group due to adverse events such as interstitial pneumonia among Japanese patients. EGFR-TKIs enhance tumor antigen-specific cytotoxicity of T cells, especially first- and second-generation EGFR-TKIs, which are more active against various cells with wild-type EGFR, including regulatory T cells. Consequently, subsequent immune checkpoint inhibitor therapy seemed more promising in the SOC group. Therefore, optimal first-line EGFR-TKI for EGFR-mutant advanced lung cancer may not have been identified in Japanese patients.
Methods:
The Heat on Beat study is a randomized, open-label, multicenter, phase II study to compare OS between initial treatment with afatinib and osimertinib in treatment-naïve patients with advanced or recurrent EGFR-mutant NSCLC. Exploration of immunomonitoring through peripheral blood mononuclear cells will also be performed, before, during, and after treatment. Treatment-naïve EGFR mutation-positive non-small cell lung cancer (NSCLC) patients (N = 100) will be randomized to two groups in a 1:1 ratio. The co-primary endpoints are 3-year survival rate and characterization of immune environment associated with response to afatinib, osimertinib, or immune checkpoint inhibitors. Enrollment will start in May 2020 at 28 sites in Japan and continue for 1 year, with 3-year follow-up.
Discussion:
Because there is no clinical trial comparing second- with third-generation EGFR-TKI for advanced EGFR-mutant NSCLC, our study would provide a major impact on clinical practice.
Trial registration
Japan Registry of Clinical Trials, jRCTs031190221, registered date: 25 February 2020, https://jrct.niph.go.jp/en-latest-detail/jRCTs031190221
Keywords: afatinib, epidermal growth factor receptor-mutant lung cancer, osimertinib, peripheral blood mononuclear cells, translational research
Introduction
Epidermal growth factor receptor (EGFR) mutations are an important recent discovery for the treatment of lung cancer,1,2 and EGFR tyrosine kinase inhibitors (EGFR-TKIs) have been widely accepted as a first-line standard of care for EGFR mutation-positive advanced lung cancer based on higher response rates and longer progression-free survival than cytotoxic chemotherapy.3,4 EGFR-TKIs have evolved from first generation to second and third generation with gradual improvement in therapeutic efficacy.3–9
However, which EGFR-TKI is superior as a first-line treatment in terms of overall survival (OS), especially from the perspectives of EGFR mutation subtypes, race, and adverse events, remains controversial. Three phase III studies have been conducted with EGFR-TKIs.7–9 The LUX-Lung 7 trial was a phase IIB study in treatment-naïve patients with stage IIIB or IV non-small cell lung cancer (NSCLC), and EGFR mutation-positive (exon 19 deletion or exon 21 point mutation) patients were randomized to receive initial treatment with afatinib, a second-generation EGFR-TKI, or gefitinib, a first-generation EGFR-TKI.7 Progression-free survival (PFS) and time to treatment failure were significantly longer in the afatinib group than in the gefitinib group. While statistical analysis showed no significant difference in OS, Kaplan–Meier curves indicated a better prognosis in the afatinib group [hazard ratio (HR) 0.86, 95% confidence interval (CI) 0.66–1.12]. Likewise, the ARCHER 1050 trial was a phase III study comparing dacomitinib, which was developed as a second-generation EGFR-TKI, with gefitinib in first-line treatment of EGFR mutation-positive NSCLC.8 That study was designed to analyze PFS, objective response rate (ORR), and OS in a gatekeeping way. The PFS was significantly improved in the dacomitinib group (HR 0.59, 95% CI 0.47–0.74). In addition, the OS, which was not statistically analyzed due to the lack of significant difference in ORR, was better in the dacomitinib group than in the gefitinib group (HR 0.76, 95% CI 0.58–0.99).
The FLAURA trial, which was a phase III study of great interest in recent years, compared osimertinib, a third-generation EGFR-TKI, with gefitinib and with erlotinib, first-generation EGFR-TKIs established as the standard of care (SOC).9 The PFS was significantly prolonged in the osimertinib group (HR 0.46, 95% CI 0.37–0.57), whereas subgroup analysis showed that the OS was similar between the osimertinib group and the SOC group in the exon 21 point mutation and Asian patients (HR 0.996, 95% CI 0.708–1.404, and HR 0.995, 95% CI 0.752–1.319, respectively). On the contrary, the OS in Japanese patients seemed to be better in the SOC group than in the osimertinib group (HR 1.39, 95% CI; 0.82–2.33).10
On the other hand, OS data of second-generation EGFR-TKIs in Japanese patients are available from the following two studies: the LUX-Lung 3 trial, a phase III study comparing afatinib with a cytotoxic chemotherapy in first-line treatment, which revealed very long survival of Japanese patients with a median OS of 46.9 months,6–11 and the ARCHER 1050 trial, in which an analysis in Japanese patients showed that the median survival was not reached in the dacomitinib group, indicating very long OS.8 In addition, the OS curves of Japanese patients in the afatinib or dacomitinib group in these two studies were very close to the OS curve of Japanese patients in the SOC group in the FLAURA trial.8,10,11 From these results, the OS of Japanese patients who start first-line treatment with a second-generation EGFR-TKI may be reproducibly better than the OS in the osimertinib group in the FLAURA trial. On the other hand, osimertinib was hardly used as subsequent treatment in the LUX-Lung 3 or ARCHER 1050 trial. This suggests that first-line treatment with a second-generation EGFR-TKI followed by treatment with osimertinib in T790M-positive patients can be expected to result in longer OS than shown by the existing OS data of second-generation EGFR-TKIs in Japanese patients.
In addition to the speculation based on the findings obtained from past clinical trials, other reasons for anticipated longer OS after first-line treatment with afatinib than after first-line treatment with osimertinib in Japanese patients are explained in the Discussion below, especially in terms of EGFR-TKI rechallenge,10,12,13 and difference of adverse events in clinical practice.9–10,14
On the other hand, it is speculated that this study is extremely significant in terms of tumor and host immunological aspects. Recently, immune checkpoint inhibitor (ICI)-based combination therapy is expected to be a very effective treatment subsequent to afatinib, even if osimertinib cannot be used, because the efficacy may be higher after afatinib than after osimertinib, as described below. At present, more therapeutic options are available than were at the time the FLAURA trial was being conducted, and ICIs are now used for EGFR mutation-positive lung cancer. The IMpower150 trial has suggested that cytotoxic chemotherapies combined with an ICI and a vascular endothelial growth factor inhibitor (VEGFI) are promising for EGFR-mutant lung cancer.15
It was also reported that the tumor antigen-specific cytotoxicity of T lymphocytes cultured with tumor cells expressing the tumor antigen (OVA), to which the lymphocytes are specific, was enhanced by first- and second-generation EGFR-TKIs.16 In other words, it is suggested that EGFR-TKIs may be beneficial to antitumor immunity. In this regard, since EGFR is expressed in regulatory T cells, a subset of T cells, afatinib, which acts not only on lung cancer cells but also on various other cells with wild-type EGFR, may be more likely to stimulate CD8 T cells to damage lung cancer cells by inhibiting the activity of regulatory T cells than osimertinib, which acts exclusively on EGFR mutation-positive lung cancer cells.17 Indeed, afatinib has been demonstrated to activate CD8 T cells most strongly in all EGFR-TKIs, including osimertinib.16 Furthermore, DS8201a, an anti-HER2 agent, increased the expression of dendritic cell markers and major histocompatibility complex (MHC) class I in tumor cells, and induced acquired immunity to reject implanted tumor cells in an allograft model, suggesting that DS-8201a may enhance tumor recognition by T cells.18 Therefore, HER2 inhibition, like EGFR inhibition, may be beneficial to host immunity. Afatinib, an irreversible pan-ErbB inhibitor, strongly inhibits not only EGFR but also HER2.19 In contrast, osimertinib-induced inhibition of HER2 is very limited.20 Taken together, afatinib is highly expected to be immunologically more beneficial to subsequent ICI-based combination therapy than osimertinib. To verify the immunological dynamics of tumors and hosts, exploratory immunomonitoring through peripheral blood mononuclear cells (PBMCs) will also be performed, before, during, and after treatment in all patients to investigate differences in effects of afatinib versus osimertinib on the immune environment dynamically. This information is expected to characterize the immune environment associated with response to afatinib, osimertinib, or ICIs in EGFR mutation-positive patients.
Considering the aforementioned assumptions, it is clinically questionable whether first-line treatment with osimertinib is truly beneficial to Japanese patients for the purpose of prolonging OS. However, no head-to-head study of second-generation EGFR-TKI versus third-generation EGFR-TKI has been conducted. Therefore, we have planned a randomized comparative study to test the hypothesis that first-line treatment with afatinib is superior to that with osimertinib in terms of OS in Japanese patients.
Heat on Beat, the title of this study, is an abbreviation for Hypothesis generative head-to-head study comparing Efficacy of Afatinib and osimerTinib based On immuNological Biomarkers in Japanese NSCLC patients with EGFR mutATions.
Methods
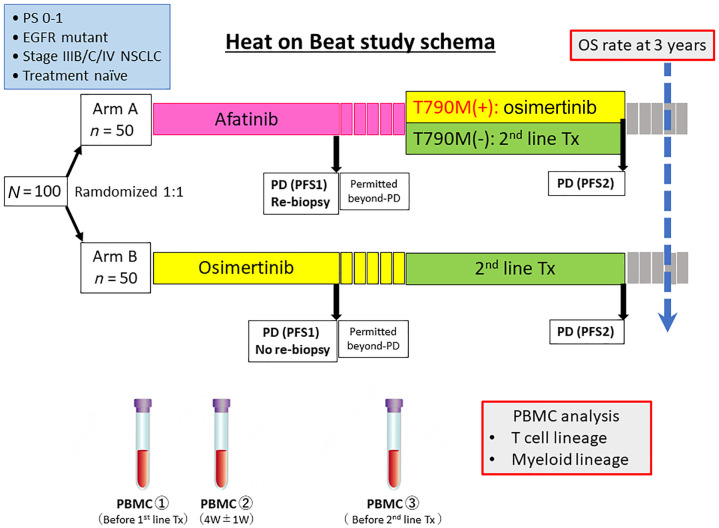
The Heat on Beat trial is a randomized, open-label, multicenter, phase II study to compare OS between initial treatment with afatinib and osimertinib in treatment-naïve patients with advanced or recurrent EGFR mutation-positive NSCLC (Figure 1). We have planned this study based on the hypothesis that first-line treatment with afatinib followed by osimertinib or ICI-based combination therapy is more likely to contribute to prolonged OS than first-line treatment with osimertinib.
Figure 1.
Study schema of the hypothesis generative head-to-head study comparing the efficacy of afatinib and osimertinib based on immunological biomarkers in Japanese NSCLC patients with EGFR mutations (Heat on Beat) study.
EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer; OS, overall survival; PBMC, peripheral blood mononuclear cells; PD, progressive disease; PFS, progression-free survival; PS, performance status; Tx, treatment; W, week.
After confirming cytological or pathological NSCLC, genetic analysis will be performed using a companion diagnostic kit (Cobas® EGFR Mutation Test v.2, Roche Molecular Diagnostics; or Oncomine™ Dx Target Test multi CDx system, NeoGenomics Laboratories). EGFR mutation-positive patients aged 20 years or older with a performance status (PS) of 0–1 will be randomized to the following two groups:
Group A: first-line treatment with afatinib at a daily dose of 40 mg until response evaluation criteria in solid tumors (RECIST) or clinical progressive disease (PD);
Group B: first-line treatment with osimertinib at a daily dose of 80 mg until RECIST or clinical PD.
In both groups, the dose will be interrupted or reduced according to toxicity at the discretion of the treating physician. Blood for PBMC sampling will be collected from all patients before first-line treatment, 1 month after the start of treatment, and before second-line treatment following PD.
In Group A, re-biopsy for detection of T790M mutation is mandatory to determine whether to use osimertinib for second-line treatment. Tissue biopsy is recommended because of its high sensitivity, but liquid biopsy is acceptable if direct biopsy is risky due to lesion site or size. In this study, tissue or plasma T790M mutation can be determined by ultrasensitive digital polymerase chain reaction, in addition to the Cobas assay used in clinical practice.
T790M-positive patients are required to receive protocol treatment with osimertinib until RECIST or clinical PD. The regimen is the same as that for initial treatment in Group B. On the other hand, T790M-negative patients are not required, but recommended, to receive ICI-based combination therapy as carboplatin, paclitaxel, bevacizumab and atezolizumab in the IMpower150 trial.
The co-primary endpoints are 3-year survival rate and characterization of immune environment associated with response to afatinib, osimertinib, or ICIs. The secondary endpoints are PFS1, PFS2, ORR, OS, time to discontinuation of treatment or death 1 (TDT1), TDT2, and time to first subsequent therapy or death. Stratification factors include PS, sex, presence or absence of brain metastases, and EGFR mutation type. A total of 100 treatment-naïve patients with EGFR mutation-positive advanced NSCLC will be randomized to receive afatinib or osimertinib in a 1:1 ratio. Enrollment in the study will start in May 2020 at 30 sites in Japan and continue for 1 year, with 3 years of follow-up (jRCT: s031190221).
Eligibility criteria
Enrollment eligibility criteria for the study are as follows: age ⩾20 years; histologically or cytologically confirmed metastatic or locally advanced NSCLC (Stage IIIB/C, IV, or postoperative recurrence); an EGFR mutation (common or uncommon, except for de novo T790M expression); Eastern Cooperative Oncology Group PS of 0 or 1; adequate bone marrow, renal, and hepatic functions; and life expectancy of at least 3 months. No systemic treatment. For example, irradiation of brain metastases is acceptable for local treatment. In Group A, re-biopsy is mandatory before second-line treatment. Tissue biopsy is preferable, in principle, but liquid biopsy is acceptable if re-biopsy of the relevant lesion is difficult.
Randomization
Patients will be randomized to Group A or Group B offered on-site and issued with a participant identification number.
Statistical considerations
The primary purpose of this phase II study is to evaluate the superiority of first-line afatinib to first-line osimertinib in terms of OS. This study is based on the results of the Japanese subset in the LUX-Lung 3 trial, which showed a 3-year OS of 62% for the afatinib arm, and of the Asian subset in the FLAURA trial, which showed a 3-year OS of 50% for the osimertinib arm. With a statistical power of 70% and a one-sided α error of 30%, we estimated that a total of 100 patients would be required for this study, with 50 patients in each arm. The data analysis will be conducted on an intention-to-treat basis. There will be no interim analysis.
Study assessments
For objective tumor assessment, imaging will be performed every 8 weeks until 24 weeks from initial drug administration, and also required every 12 weeks after 24 weeks until RECIST PD or clinical PD using RECIST v.1.1 for reference. In cases of radiological progression (according to RECIST v.1.1) without clinical worsening, ongoing treatment will be permitted as beyond PD.
Analytical methods
Blood sample analysis
Samples will be collected into heparinized CPT Vacutainer tubes (Becton Dickinson Vacutainer Systems, NJ, USA) and will be centrifuged at 1500 × g for 20 min at room temperature to separate PBMCs from erythrocytes and granulocytes over a Ficoll gradient. PBMCs will be frozen at –80°C in Cellbanker2 (Nippon Zenyaku Kogyo Co., Ltd., Fukushima, Japan) and the frozen cells will be transferred into a liquid nitrogen tank within 1 week. For T cell subset analyses, cells will be incubated for 32–48 h in culture medium consisting of RPMI1640 and 10% FCS before cell staining.
Cells will be stained with the following mAbs using a FACSCalibur flow cytometer (Becton Dickinson, NJ, USA): fluorescein isothiocyanate (FITC)-conjugated anti-CD3 (HIT3a) and anti-CD4 (RPA-T4), phycoerythrin (PE)-conjugated anti-CD8 (RPAT8) and anti-CD25 (M-A251), PE-Cy7-conjugated anti-CD25 (MA251), PE-Cy5-conjugated anti-CD62L (Dreg 56; all from BD Pharmingen, NJ, USA), and FITC-conjugated anti-CD62L (Dreg 56; eBioscience, CA, USA). Cell-surface phenotypes will be analyzed by direct immunofluorescence staining of 1 × 106 cells with fluorophore-conjugated mAbs. In brief, cells will be stained with fluorophore-conjugated mAbs in 100 mL of FACS buffer, PBS supplemented with 5% FCS, for 30 min at 4°C. Samples will be immediately washed twice with 1.0 mL FACS buffer. Samples will be prepared for intracellular staining using a FoxP3 fix and permeabilization kit in accordance with the manufacturer’s instructions (eBioscience, CA, USA) and will be stained at 4°C. After washing twice with FACS buffer, samples will be fixed using 0.5% paraformaldehyde in PBS. The gating strategy is shown in Supplemental Material Figure S1 online. From each sample, 10,000 cells will be analyzed using a FACSCalibur, LSR Fortessa flow microfluorometer, and FlowJo software (Becton Dickinson, NJ, USA).
Cell purification
Blood for PBMC sampling will be collected from all patients before first-line treatment, 1 month after the start of treatment, and before second-line treatment following PD. CD4+ T cells will be purified through negative selection using a human CD4+ T cell isolation kit (Dynabeads Untouched Human CD4+ T Cells Kit, 11346D) in accordance with the manufacturer’s instructions (Dynal Biotech, WI, USA). CD4+ T cells will be further separated into CD62Lhigh and CD62Llow cells using anti-CD62L mAb-coated microbeads and a MACS system (Miltenyi Biotec, Gladbach, Germany), following the manufacturer’s suggested procedure. Cell purities will all be >90% by FCM analysis.
Mass cytometry
The mAbs to be used for Helios™ mass cytometry (Fluidigm, CA, USA) analysis, as of June 2020, are listed in Supplemental Table S1. Up to 2.5 × 106 cells will be stained with mass cytometry antibodies in accordance with the manufacturer’s instructions. In brief, a 50-mL volume of 198Pt monoisotopic cisplatin (Fluidigm, CA, USA) in PBS will be added directly for a final concentration of 2.5 mmol/L for 5 min. Samples will be immediately washed twice with Maxpar Cell Staining Buffer (Fluidigm, CA, USA). Cells will be stained with mass cytometry antibodies for 30 min at room temperature. For intracellular staining, samples will be prepared using a FoxP3 fix and permeabilization kit as described above before staining. After washing twice with Maxpar Cell Staining Buffer, samples will be fixed using 1.6% paraformaldehyde in PBS supplemented with 100 nmol/L iridium nucleic acid intercalator (Fluidigm, CA, USA). Following fixation, cells will be washed twice with 0.5% BSA PBS and 0.1% BSA water and will be resuspended in 0.1% BSA water. Twenty thousand cells will be subjected to viSNE analysis and heatmap analysis using a Helios mass cytometer (Fluidigm, CA, USA) and Cytobank software (Cytobank Inc., CA, USA).
Statistical analysis
SAS 9.4 (SAS Institute Inc., NC, USA) and Prism 8 (GraphPad, CA, USA) will be used to conduct statistical analyses. Data will be expressed as means ± SEM, unless otherwise indicated. Tests for differences between two populations will be performed using Student’s t-test. Multiple-group comparison will be performed using one-way analysis of variance with Tukey post hoc analysis. The prediction formula will be developed using the discovery cohort data with a logistic regression model. The performance of the prediction formula will be evaluated using the independent validation cohort data. Survival curves will be estimated using the Kaplan–Meier method. All p-values will be two-sided, and p < 0.05 will be considered statistically significant.
Discussion
This is the world’s first phase II trial comparing second- with third-generation EGFR-TKI for advanced EGFR-mutant NSCLC. Especially in Japanese patients, anticipated longer OS after first-line treatment with afatinib than after first-line treatment with osimertinib may be explained in terms of the following reasons: first, since rechallenge with an EGFR-TKI is covered by health insurance in Japan, rechallenge with an effective EGFR-TKI can be repeated without limit. In the FLAURA trial, rechallenge with an EGFR-TKI may have contributed to prolonged OS in the SOC group in Japanese patients.10 The appropriateness of rechallenge with an EGFR-TKI in the SOC group may be supported by the following facts: it is known that treatment with osimertinib is followed by expression of many resistance genes that confer insensitivity to other EGFR-TKIs, including afatinib, whereas treatment with a first- or second-generation EGFR-TKI is followed by frequent expression of resistance genes, including T790M mutation, that confer sensitivity to other EGFR-TKIs.12,13 Indeed, the proportion of patients rechallenged with an EGFR-TKI in Japanese patients in the FLAURA trial was 30% in the osimertinib group and 80% in the SOC group. Second, in the FLAURA trial, the incidence of osimertinib-related interstitial pneumonia was higher in Japanese patients (12%) than in the overall population (4%), and the rate of treatment interruption due to all adverse events was also higher in Japanese patients (29%) than in the overall population (13%).9,10 It is already known that the risk of EGFR-TKI-related interstitial pneumonia is higher in Asians than in non-Asians,14 and osimertinib is found to be associated with a higher risk of interstitial pneumonia than conventional EGFR-TKIs.
We have also planned to examine the differential effects of afatinib and osimertinib on systemic immunity by serial PBMC collection and subsequent immune monitoring for each case to identify the patients who seems to be effective for ICI and cytotoxic chemotherapy combination treatment for NSCLC patients with EGFR mutations. Consequently, our study would provide a major impact on clinical practice.
Supplemental Material
Supplemental material, Fig._S1 for Hypothesis generative head-to-head study comparing efficacy of afatinib and osimertinib based on immunological biomarkers in Japanese NSCLC patients with EGFR mutations (Heat on Beat study) by Kei Morikawa, Hisashi Tanaka, Hidetoshi Itani, Saori Takata, Satoshi Watanabe, Kazuma Kishi, Kenzo Soejima, Kyoichi Kaira, Hiroshi Kagamu, Kenichi Yoshimura, Noriyuki Matsutani and Nobuhiko Seki in Therapeutic Advances in Medical Oncology
Supplemental material, Tab._S1 for Hypothesis generative head-to-head study comparing efficacy of afatinib and osimertinib based on immunological biomarkers in Japanese NSCLC patients with EGFR mutations (Heat on Beat study) by Kei Morikawa, Hisashi Tanaka, Hidetoshi Itani, Saori Takata, Satoshi Watanabe, Kazuma Kishi, Kenzo Soejima, Kyoichi Kaira, Hiroshi Kagamu, Kenichi Yoshimura, Noriyuki Matsutani and Nobuhiko Seki in Therapeutic Advances in Medical Oncology
Acknowledgments
This study will be sponsored by Nippon Boehringer-Ingelheim Co., Ltd. as collaborative research of the Heat on Beat study group. We also acknowledge the support of ASCA Corporation (http://www.asca-co.com/english_site/) for proofreading and editing a draft of this manuscript.
Footnotes
Author contributions: KM, HT, HI, ST, SW, KK, KS, KK, HK, KY, NM, and NS were involved in study conception and design. KM, HK, KY, NM, and NS will be involved in the analysis and interpretation of the data; KM, NM, and NS were involved in drafting the manuscript; and KM, NM, and NS were involved in revising the manuscript. All authors have read and approved the final manuscript.
Availability of data and material: All data generated or analyzed during this study are included in this published article.
Conflict of interest statement: KM received personal fees as honoraria from AstraZeneca K.K., Chugai Pharmaceutical, and Nippon Boehringer Ingelheim Co., Ltd, and research funding from DNA Chip Research Inc., and Nippon Boehringer Ingelheim Co., Ltd. SW received personal fees as honoraria from AstraZeneca K.K., Chugai Pharmaceutical. KS received personal fees as honoraria from AstraZeneca K.K., and research funding from AstraZeneca K.K., Nippon Boehringer Ingelheim Co., Ltd, and Taiho Pharmaceutical. KK received personal fees as honoraria from AstraZeneca K.K., and Nippon Boehringer Ingelheim Co, Ltd. NS received personal fees as honoraria from Lily Japan, AstraZeneca K.K., MSD Oncology, Chugai Pharmaceutical, Taiho Pharmaceutical, Pfizer Japan Inc., Ono Pharmaceutical, Nippon Boehringer Ingelheim Co., Ltd, and Bristol-Myers Squibb Japan, and research funding from AstraZeneca K.K. and Nippon Boehringer Ingelheim Co., Ltd.
Consent for publication: Consent for publication must be obtained from all patients.
Ethics approval and consent to participate: This study was reviewed and approved by Yokohama City University Center for Novel and Exploratory Clinical Trials (Y-NEXT), the central institutional review board, and then approved by the ethics committees of all participating study sites on 17 February 2020. This clinical trial is registered in the Japan Registry of Clinical Trials, jRCTs031190221, registered date: 25 February 2020. Written informed consent must be obtained from all patients.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study will be sponsored by Nippon Boehringer-Ingelheim Co., Ltd as collaborative research of the Heat on Beat study group.
ORCID iD: Kei Morikawa  https://orcid.org/0000-0002-0745-5118
https://orcid.org/0000-0002-0745-5118
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Kei Morikawa, Division of Respiratory Medicine, Department of Internal Medicine, St. Marianna University School of Medicine, Kanagawa, Japan.
Hisashi Tanaka, Department of Respiratory Medicine, Hirosaki University Graduate School of Medicine, Aomori, Japan.
Hidetoshi Itani, Department of Respiratory Medicine, Japanese Red Cross Ise Hospital, Mie, Japan.
Saori Takata, Department of Respiratory Medicine, Kyorin University School of Medicine, Tokyo, Japan.
Satoshi Watanabe, Department of Respiratory Medicine and Infectious Diseases, Niigata University Graduate School of Medical and Dental Sciences, Niigata, Japan.
Kazuma Kishi, Department of Respiratory Medicine, Toho University Omori Medical Center, Tokyo, Japan.
Kenzo Soejima, Clinical and Translational Research Center, Keio University Hospital, Tokyo, Japan.
Kyoichi Kaira, Department of Respiratory Medicine, Saitama Medical University International Medical Center, Saitama, Japan.
Hiroshi Kagamu, Department of Respiratory Medicine, Saitama Medical University International Medical Center, Saitama, Japan.
Kenichi Yoshimura, Medical Center for Translational and Clinical Research, Hiroshima University Hospital, Hiroshima, Japan.
Noriyuki Matsutani, Department of Surgery, Teikyo University Hospital, Mizonokuchi, Kanagawa, Japan.
Nobuhiko Seki, Division of Medical Oncology, Department of Internal Medicine, Teikyo University School of Medicine, 2-11-1, Kaga, Itabashi-ku, Tokyo 173-8606, Japan.
References
- 1. Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004; 350: 2129–2139. [DOI] [PubMed] [Google Scholar]
- 2. Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004; 304: 1497–1500. [DOI] [PubMed] [Google Scholar]
- 3. Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010; 362: 2380–2388. [DOI] [PubMed] [Google Scholar]
- 4. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010; 11: 121–128. [DOI] [PubMed] [Google Scholar]
- 5. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as 1st line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomized phase 3 trial. Lancet Oncol 2012; 13: 239–246. [DOI] [PubMed] [Google Scholar]
- 6. Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013; 31: 3327–3334. [DOI] [PubMed] [Google Scholar]
- 7. Paz-Ares L, Tan EH, O’Byrne K, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol 2017; 28: 270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mok TS, Cheng Y, Zhou X, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non-small-cell lung cancer and EGFR-activating mutations. J Clin Oncol 2018; 36: 2244–2250. [DOI] [PubMed] [Google Scholar]
- 9. Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med 2020; 382: 41–50. [DOI] [PubMed] [Google Scholar]
- 10. Nogami N, Ramalingam SS, Imamura F, et al. Osimertinib as first-line therapy for EGFRm advanced NSCLC (FLAURA): final OS in Japanese subset. In: The 60th annual meeting of the Japan lung cancer society https://www.haigan.gr.jp/journal/am/2019a/19a_pdsy0000PS-1.html (2019, accessed 13 October 2020) [Google Scholar]
- 11. Kato T, Yoshioka H, Okamoto I, et al. Afatinib versus cisplatin plus pemetrexed in Japanese patients with advanced non-small cell lung cancer harboring activating EGFR mutations: subgroup analysis of LUX-Lung 3. Cancer Sci 2015; 106: 1202–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yu H, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 2013; 19: 2240–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gelatti ACZ, Drilon A, Santini FC. Optimizing the sequencing of tyrosine kinase inhibitors (TKIs) in epidermal growth factor receptor (EGFR) mutation-positive non-small cell lung cancer (NSCLC). Lung Cancer 2019; 137: 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kudoh S, Kato H, Nishiwaki Y, et al. Interstitial lung disease in Japanese patients with lung cancer: a cohort and nested case-control study. Am J Respir Crit Care Med 2008; 177: 1348–1357. [DOI] [PubMed] [Google Scholar]
- 15. Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018; 378: 2288–2301. [DOI] [PubMed] [Google Scholar]
- 16. Lizotte PH, Hong RL, Luster TA, et al. A high-throughput immune-oncology screen identifies EGFR inhibitors as potent enhancers of antigen-specific cytotoxic T-lymphocyte tumor cell killing. Cancer Immunol Res 2018; 6: 1511–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zaiss DM, van Loosdregt J, Gorlani A, et al. Amphiregulin enhances regulatory T cell-suppressive function via the epidermal growth factor receptor. Immunity 2013; 38: 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iwata TN, Ishii C, Ishida S, et al. A HER2-targeting antibody-drug conjugate, trastuzumab deruxtecan (DS-8201a), enhances antitumor immunity in a mouse model. Mol Cancer Ther 2018; 17: 1494–1503. [DOI] [PubMed] [Google Scholar]
- 19. Evaluation and Licensing Division, Pharmaceutical Safety and Environmental Health Bureau, Ministry of Health, Labour and Welfare. Report on the deliberation results, https://www.pmda.go.jp/files/000210320.pdf (2013, 13 October 2020).
- 20. Evaluation and Licensing Division, Pharmaceutical Safety and Environmental Health Bureau, Ministry of Health, Labour and Welfare. Report on the deliberation results, https://www.pmda.go.jp/files/000220615.pdf (2016, 13 October 2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Fig._S1 for Hypothesis generative head-to-head study comparing efficacy of afatinib and osimertinib based on immunological biomarkers in Japanese NSCLC patients with EGFR mutations (Heat on Beat study) by Kei Morikawa, Hisashi Tanaka, Hidetoshi Itani, Saori Takata, Satoshi Watanabe, Kazuma Kishi, Kenzo Soejima, Kyoichi Kaira, Hiroshi Kagamu, Kenichi Yoshimura, Noriyuki Matsutani and Nobuhiko Seki in Therapeutic Advances in Medical Oncology
Supplemental material, Tab._S1 for Hypothesis generative head-to-head study comparing efficacy of afatinib and osimertinib based on immunological biomarkers in Japanese NSCLC patients with EGFR mutations (Heat on Beat study) by Kei Morikawa, Hisashi Tanaka, Hidetoshi Itani, Saori Takata, Satoshi Watanabe, Kazuma Kishi, Kenzo Soejima, Kyoichi Kaira, Hiroshi Kagamu, Kenichi Yoshimura, Noriyuki Matsutani and Nobuhiko Seki in Therapeutic Advances in Medical Oncology