Abstract
Plant homeodomain finger protein 8 (PHF8) has been reported to participate in cancer development and metastasis of various types of tumors. However, little is known about the functional mechanism of PHF8 in gastric cancer (GC). This study aimed to explore the PHF8 expression pattern and function, and the role of the MYC/miRNA/PHF8 axis in GC. PHF8 expression was upregulated in GC tissues and cells as measured using quantitative reverse transcription polymerase chain reaction and western blotting. PHF8 knockdown suppressed the proliferation, migration, and invasion of GC cells, as determined using the CCK-8 assay and Transwell assay. MicroRNA-22-3p targeted PHF8, as verified by a dual-luciferase reporter assay. MYC upregulated the protein expression of PHF8 but had no effect on PHF8 mRNA expression. MYC regulates PHF8 by affecting the stability of miR-22-3p. We identified a novel MYC/miR-22-3p/PHF8 regulatory axis in GC. Therefore, PHF8 may provide a new therapeutic target for patients with GC.
Keywords: gastric cancer, plant homeodomain finger protein 8, MYC, microRNA-22-3p, metastasis, proliferation
Introduction
Gastric cancer (GC) is one of the major causes of cancer-related deaths worldwide.1 Although the pathogenesis and treatment strategies of GC have been extensively studied, the mortality rate of gastric cancer patients remains high due to its recurrence and metastasis. According to statistics, more than 63% of gastric cancer patients were diagnosed with metastatic GC. Therefore, it is very important to elucidate the mechanisms involved in the pathogenesis of GC and develop new treatment strategies.
Histone methylation is a common pattern of epigenetic regulation, which is jointly controlled by methyltransferase and demethylase, and plays a fundamental role in many cellular processes.2 It has been reported that changes in histone modification patterns are involved in the occurrence and metastasis of tumors.3 For example, KDM5A/RBP2, belonging to the H3K4me3 demethylase family, promotes breast cancer metastasis by regulating tenascin-c (TNC).4 KDM1A promotes proliferation, migration, and invasion of non-small cell lung cancer,5 but inhibits metastasis of breast cancer cells.6
Plant homeodomain finger protein 8 (PHF8) is one of the members of the histone demethylase family,7 which has attracted increasing attention in recent years because of its expression characteristics and its role as a transcription activator. PHF8 can bind to the promoter sites of approximately one-third of human genes8,9; therefore, the abnormal expression of PHF8 may be related to human cancer and other genetic and environmental diseases. Studies have shown that upregulation of PHF8 is the key factor for malignant progression and metastasis of cancer, such as prostate cancer,10 breast cancer,11 lung cancer,12 and esophageal squamous cell cancer.13 However, little is known about the expression pattern and role of PHF8 in GC.
Studies have shown that the proto-oncogene, MYC, can drive PHF8 expression in prostate cancer10 and breast cancer,11 and plays an important role in cancer progression; however, the correlation between the 2 genes in GC remains unclear. Therefore, this study will explore the function of PHF8 in GC and the role of the MYC/miRNA/PHF8 axis as well as its influence on GC progression.
Materials and Methods
Clinical Samples and Cell Cultures
For GC samples, 10 sections of GC tissues and adjacent noncancerous tissues were obtained from the Tianjin Medical University Cancer Institute & Hospital. The samples were stored at −80°C prior to RNA extraction. All patients provided consent prior to enrollment in the study. Four GC cell lines (BGC-823, HGC-27, MKN45, and AGS) and human gastric mucosa cell line GES-1, obtained from the American Type Culture Collection (ATCC), were used in this study. The cells were cultured in RPMI1460 medium (Gibco, USA) containing 10% fetal bovine serum (FBS) (Gibco, USA) and incubated in 5% CO2 at 37°C.
Plasmid and siRNA Transfection
The MYC-overexpressing plasmids were transfected into GC cells using Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s protocol. PHF8 siRNAs, MYC siRNA, and miR-22-3p mimics or inhibitors were used to transfect GC cells using Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s protocol. The siRNA sequences were as follows: PHF8 siRNA1: AGGAGAGAAGACAGACAAGCATAAG; PHF8 siRNA2: CGAGCTCTATACCGCAGTAGAGGCC; MYC siRNA: AUGACGUGAAUGUGCAGACAGGAUC.
Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted using TRIzol Reagent (Invitrogen, CN) according to the manufacturer’s instructions. Next, 2 µg RNA was used to synthesize cDNA using the Advantage RT-for-PCR Kit (Clontech) according to the manufacturer’s protocol. cDNA was diluted and used for qRT-PCR with the HiScript® II One Step qRT-PCR SYBR® Green Kit (Takara, Japan). An All-in-One miRNA qRT-PCR Detection Kit (GeneCopoeia, USA) was used to detect the expression of miRNAs and pre-miRNAs. β-actin and U6 were used as internal controls for mRNA and miRNA, respectively. Gene expression was calculated using the 2-ΔΔCt method. The primer sequences used in qRT-PCR are shown in Table S1.
Western Blotting
The collected cells were lysed using RIPA buffer (Thermo Scientific, USA) to extract total protein. The protein concentration was determined using the Bradford method. The proteins were separated using 10% SDS-PAGE and electro-transferred to a PVDF membrane (Millipore, USA). The membrane was blocked in TBST mixed with 5% skim milk for 4 h at room temperature. Next, we incubated the membrane with primary antibody overnight at 4°C. After washing with TBST, the membrane was incubated with secondary antibody for 1 h at room temperature. The protein bands were visualized using the E-Gel Imager (Invitrogen, USA). The specific antibodies to PHF8 (ab36068), E-cadherin (ab15148), N-cadherin (ab18203), vimentin (ab137321), and β-actin (ab227387) were purchased from Abcam (UK).
Dual-Luciferase Reporter Gene Assay
Based on the miRNA target prediction analysis of bioinformatics databases (TargetScan, PITA, DIANA-microT, and miRanda), we found 5 miRNAs (miR-22-3p, miR-494-3p, miR-31-5p, miR-182-5p, and miR-495-3p) having highly conserved binding sites in the 3′-UTR of PHF8. Next, according to the manufacturer’s protocol, we performed a dual-luciferase reporter assay (GeneCopoeia) to confirm whether PHF8 was the target gene of miR-22-3p. The full-length sequence of PHF8 3′-UTR containing miR-22-3p binding sites at the position 663–670 of PHF8 3′-UTR (WT) or with a mutation site (MUT) were cloned into the KpnI and SacI sites of the pGL4.10 vector. Recombinant vectors (100 ng) were used to transfect the GC cells with 100 nM miR-22-3p mimic or NC mimic for 24 h. The luminescence intensity values of cells in each group were analyzed using a double luciferase reporting kit (Promega, USA).
The promoter sequence of PHF8 was used to replace the promoter of pGL3-basic, and the GC cells were transfected with a recombinant vector containing the PHF8 promoter and MYC-overexpressing vector. A dual-luciferase reporter assay (Promega, USA) was used to detect luciferase activity after transfection for 48 h. The assay was performed in triplicates.
The effects of MYC on PHF8 promoter activity were determined using a dual luciferase reporter gene assay.
MicroRNA Stability Assay
The cells were inoculated in 12-well plates and cultured at 37°C and 5% CO2 for 24 h. The cells were treated with actinomycin D (10 mg/L), and total RNA was isolated from the cells at 0, 4, 8, 12, and 24 h, respectively. Quantitative reverse transcription PCR was used to detect the relative abundance of miRNAs.
CCK-8 Assay
The function of PHF8 in GC cell proliferation was detected using the CCK-8 assay (GlpBio, USA). The cells (1 × 104 cells/well) were seeded in 96-well plates and the cells were transfected with PHF8 siRNA. CCK-8 solution was added to each well and the absorbance of each group was measured at 450 nm using a fluorescence microplate reader (Berthold Technologies, Germany).
Transwell Assay
To evaluate the ability of GC cell migration and invasion, a transwell assay was performed in accordance with the manufacturer’s instructions. First, the transwell chamber (BD Biosciences, USA) was placed in a 24-well plate (Corning, USA). The cells (2 × 105 cells/well) were seeded into the upper chamber containing serum-free DMEM culture mixed with 100 µL of diluted Matrigel. Medium (500 µL) containing 20% FBS was added to the lower chamber. After 12 h of incubation at 37°C and 5% CO2, the chamber was removed, fixed in pre-cooled methanol for 30 min, and stained with DAPI for 15 min. The cell images were captured and the number of fields was counted under an inverted microscope (Olympus, Japan).
Bioinformatics and Statistics
The expression data from the StarBase database was used to analyze the correlation. Statistical analysis was performed using GraphPad Prism. Data are expressed as the mean ± SD. The differences between 2 groups were analyzed using the Student’s t-tests. The differences for more than 3 groups were compared by one-way analysis of variance. P < 0.05 indicated a statistically significant difference.
Results
MYC Upregulated the Protein Expression of PHF8
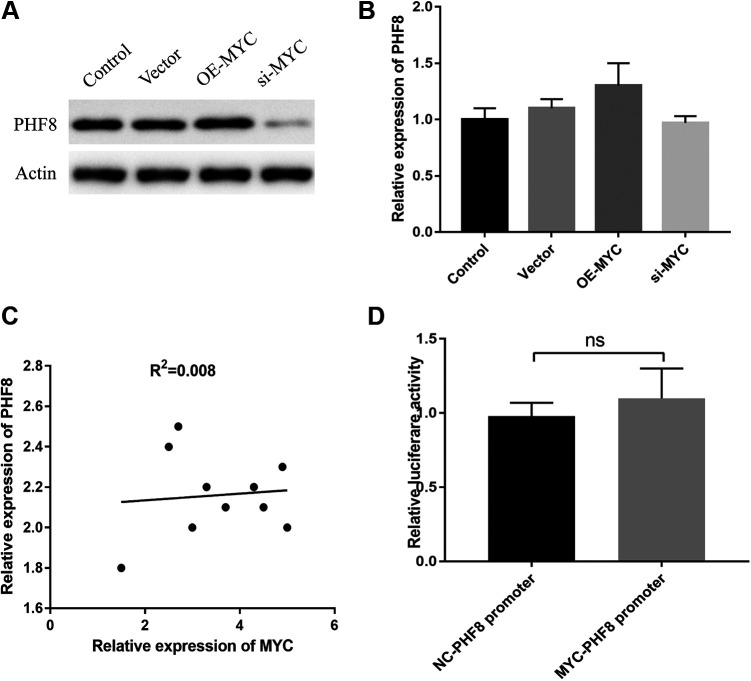
To investigate the relationship between MYC and PHF8, we performed MYC overexpression and knockdown. As shown in Figure 1A, PHF8 protein expression was increased or decreased based on MYC overexpression or knockdown, respectively. However, the mRNA expression of PHF8 was unaffected by MYC overexpression or MYC knockdown. Expression correlation analysis revealed no correlation between MYC and PHF8 expressions (Figure 1B and C). Thus, we speculated that PHF8 was not regulated by MYC at the transcriptional level. Further, we explored whether MYC acted on the promoter of PHF8 using the dual luciferase reporter gene assay. The results showed that there were no significant differences between the relative luciferase activities of the MYC-PHF8 promoter group and the NC-PHF8 group (Figure 1D), thereby indicating that MYC exerted no transcriptional regulation on PHF8. Therefore, regulation of PHF8 by MYC might be controlled by other mechanisms.
Figure 1.
MYC upregulated the protein expression of PHF8. A, The PHF8 protein expression was increased or decreased by MYC overexpression or knockdown, respectively. B, PHF8 mRNA expression was not affected by MYC. C, Correlation analysis demonstrated an extremely weak association between mRNA expression of MYC and PHF8, R2 = 0.008. D, Luciferase reporter gene assay revealed no interaction between MYC and the PHF8 promoter fragment.
MYC Regulated PHF8 by Decreasing the Stability of miR-22-3p
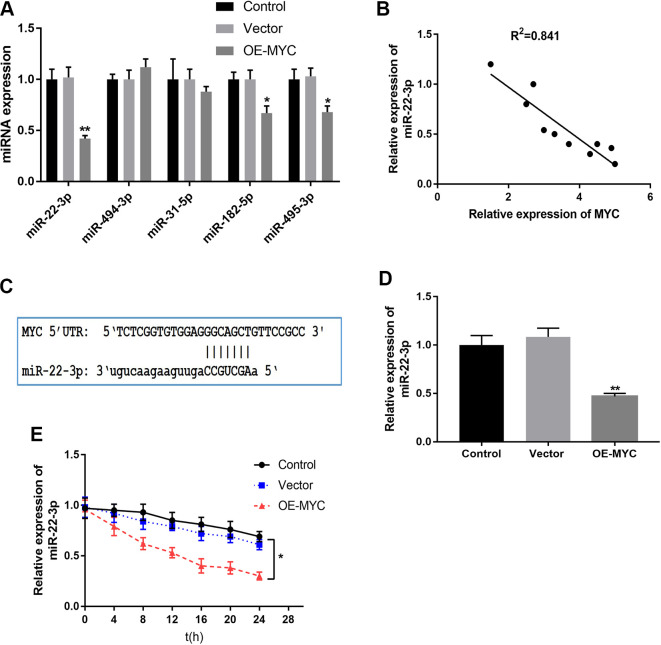
In order to further explore how MYC regulates PHF8, we speculated the probable role of miRNAs. It has been reported that MYC is involved in tumorigenesis through miRNA mediation.14 MicroRNA target predicted by TargetScan, PITA, DIANA-microT, and miRanda showed that five miRNAs, including miR-22-3p, miR-494-3p, miR-31-5p, miR-182-5p, and miR-495-3p had highly conserved binding sites in the 3′-UTR of PHF8 (Table S2). We overexpressed MYC to further detect whether the expression of miRNAs targeting PHF8 were affected by MYC. We found that the expression of miR-22-3p was significantly affected by MYC expression (Figure 2A) and that MYC decreased the expression miR-22-3p, which was consistent with a previous study.14 Expression correlation analysis revealed that the expression of MYC negatively correlated with the expression of miR-22-3p (Figure 2B). Although, there were no potential binding sites for miR-22-3p on the MYC 3′-UTR,15 miR-22-3p showed perfect complementarity with the 5′-UTR of MYC mRNA (Figure 2C). To further explore whether there was a direct interaction between MYC and miR-22-3p, we performed MYC overexpression to detect the stability of miR-22-3p. The results showed that the relative expression of miR-22-3p decreased significantly after MYC overexpression and thereby enhanced the degradation of miR-22-3p (Figure 2D and E). The stability of miR-22-3p decreased significantly in the MYC-overexpressing group compared to that in the vector control. Overall, MYC regulated the expression of PHF8 by decreasing the stability of miR-22-3p.
Figure 2.
MYC affected the stability of miR-22-3p. A, MYC overexpression downregulated miR-22-3p expression, one of the miRNAs targeting PHF8. B, Correlation analysis demonstrated a negative association between MYC and miR-22-3p expressions, R2 = 0.841, N = 10. C, Schematic representation of miR-22-3p binding to 5′-UTR of MYC mRNA. D, The relative level of miR-22-3p after overexpression of MYC was detected using qRT-PCR. E, The stability of miR-22-3p was examined at different time points with actinomycin D. *P < 0.05, **P < 0.01.
MiR-22-3p Mediated the Regulation of PHF8 by MYC
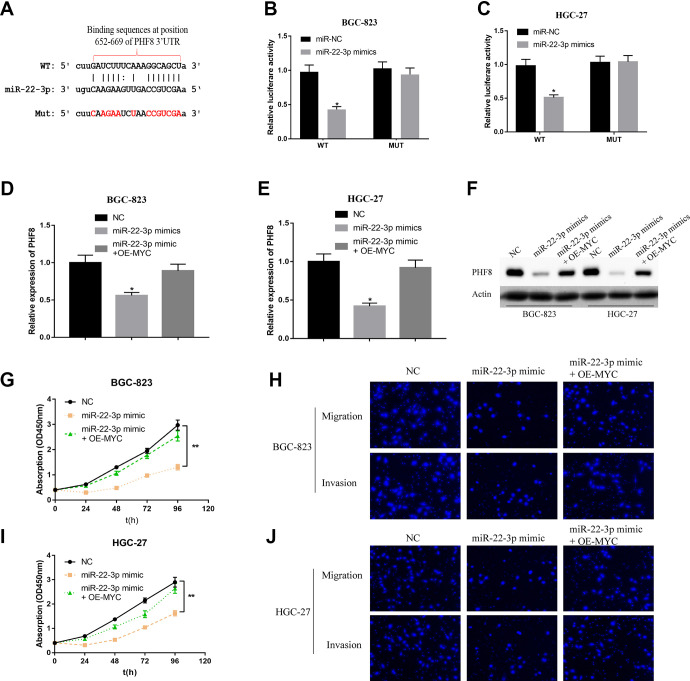
To further confirm the relationship between miR-22-3p and PHF8, miR-22-3p was predicted to bind the 3′-UTR of PHF8 through bioinformatics analysis (Targetscan; http://www.targetscan.org/vert_72/) (Figure 3A). A dual-luciferase reporter gene assay was performed to ascertain whether miR-22-3p regulated PHF8 by binding to the predicted target site in the 3′-UTR. The results revealed that miR-22-3p mimics significantly decreased the luciferase activity in the WT group compared to that in the MUT group. (Figure 3B and C). These results indicated that PHF8 was a target gene of miR-22-3p. At both the mRNA and protein levels, miR-22-3p inhibited the expression of PHF8 in the GC cells BGC-823 and HGC-27, but this effect was restored by the overexpression of MYC (Figure 3D-F). Moreover, exogenous miR-22-3p mimics significantly inhibited the proliferation, migration, and invasion abilities of GC cells, but MYC overexpression restored this effect by decreasing the stability of miR-22-3p (Figure 3G-J). Therefore, PHF8 is the target gene of miR-22-3p, and MYC regulates PHF8 by suppressing miR-22-3p.
Figure 3.
MicroRNA-22-3p mediated the regulation of PHF8 by MYC. A, The target site between miR-22-3p and PHF8 predicted by TargetScan (http://www.targetscan.org/vert_72/). B and C, The target relationship between miR-22-3p and PHF8 in BGC-823 and HGC-27 verified using dual-luciferase reporter gene assay. D and E, The mRNA expression of PHF8 in BGC-823 and HGC-27 was measured using qRT-PCR. F, Protein expression of PHF8 was determined using western blotting. G and I, The proliferation ability of GC cells BGC-823 and HGC-27 was analyzed using CCK-8 assay. H and J, The migration and invasion ability of GC cells BGC-823 and HGC-27 were analyzed using Transwell assay. *P < 0.05, **P < 0.01. Bar = 200 μm.
PHF8 Promoted Tumorigenesis and Metastasis of GC Cells In Vitro
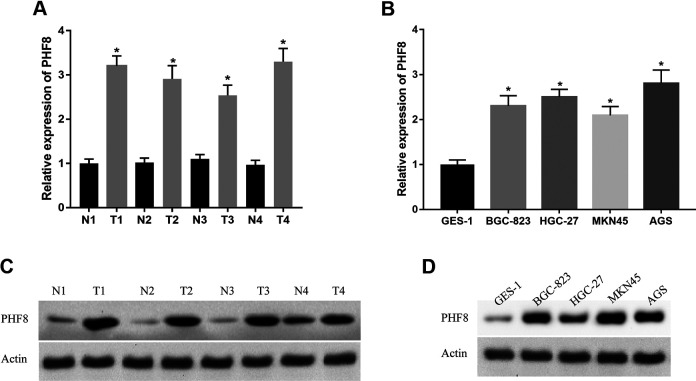
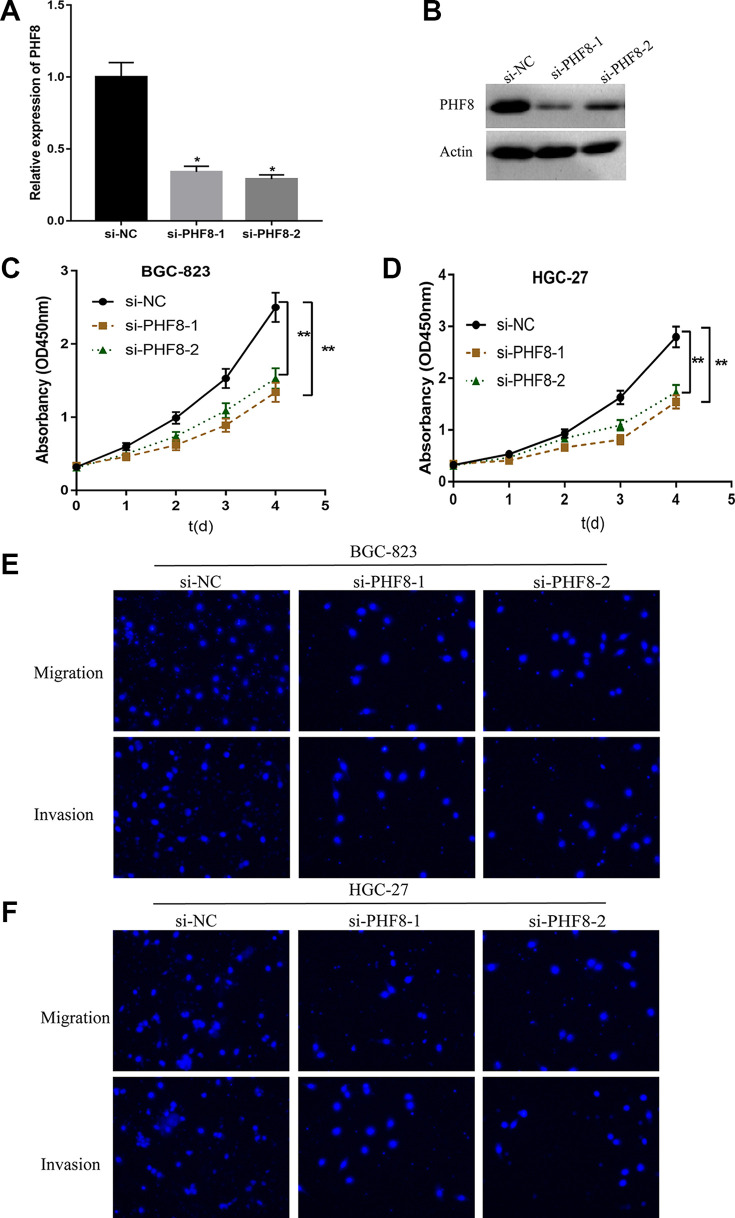
To explore the expression pattern and function of PHF8 in GC, we investigated the expression of PHF8 in four GC tissue sections and cell lines (BGC-823, HGC-27, MKN45, and AGS). The results demonstrated that PHF8 was highly expressed in GC tissues and cells, regardless of the mRNA or protein expression levels (Figure 4). Next, we transfected BGC-823 and HGC-27 GC cells with PHF8 siRNAs to determine the function of PHF8 in the tumorigenesis of GC. As shown in Figure 5A and B, PHF8 mRNA and protein expression was efficiently inhibited by PHF8 siRNA, as verified using qRT-PCR and western blotting. The proliferation ability of BGC-823 and HGC-27 GC cells decreased significantly after transfection with PHF8 siRNAs (Figure 5C and D). Furthermore, PHF8 knockdown suppressed the migration and invasion ability of GC cells (Figure 5E and F). These results suggested that PHF8-knockdown inhibited the proliferation, migration, and invasion of GC cells. Hence, PHF8 promoted tumorigenesis and metastasis of GC cells.
Figure 4.
High expression of PHF8 in GC tissues and cells. A and B, The mRNA expression of PHF8 in 4 sections of GC tissues and cells detected using qRT-PCR. C and D, The protein expression of PHF8 in 4 sections of GC tissues and cells measured using western blotting. *P < 0.05.
Figure 5.
PHF8 promoted the proliferation, migration, and invasion of GC cells. A and B, The mRNA and protein expression of PHF8 decreased after transfection with siRNA. C and D, CCK-8 assay was used to detect the effects of PHF8 on the proliferation of GC cells BGC-823 and HGC-27. E and F, Transwell assay measured the effects of PHF8 on the migration and invasion of GC cells BGC-823 and HGC-27. *P < 0.05, **P < 0.01. Bar = 200 μm.
Discussion
In recent years, an increasing number of studies on epigenetics and tumor formation have been conducted, providing a new theoretical basis for the occurrence and development of tumors, along with novel approaches for the diagnosis and treatment of tumors.16 Current epigenetic research mainly includes DNA methylation, covalent histone modification, and non-coding RNAs. Histone methylation is an important aspect of epigenetics, including methylation and demethylation.17 Several studies have confirmed the occurrence and development of various tumors related to histone methylation.4,5,18-21
PHF8 is a histone demethylase involved in the formation and metastasis of various tumors. Studies have shown that PHF8 is highly expressed in non-small cell lung cancer12 and prostate cancer,22 and promotes the proliferation, migration, and invasion of cancer cells. PHF8 is an oncogene in liver cancer,23 which can promote autophagy degradation, epithelial-mesenchymal transformation, and metastasis of hepatocellular carcinoma,24 and promote the occurrence and development of breast cancer and epithelial-mesenchymal transition.11 In our study, PHF8 was highly expressed in GC tissues and cells, and PHF8 knockout suppressed the proliferation, migration, and invasion of GC cells, which was consistent with existing studies.10,11 The data revealed that PHF8 was capable of promoting GC migration and invasion.
The results of this study showed that MYC regulates PHF8 expression by inhibiting miR-22-3p via a post-transcriptional regulatory mechanism. The oncogenic function of MYC in GC has been reported in many studies, and the high expression of MYC in GC is closely related to poor prognosis.25,26 Our study demonstrated that MYC knockdown or overexpression, decreased or increased the protein expression of PHF8 in GC, respectively, but showed no effect on the mRNA expression of PHF8. Next, we investigated how MYC regulates PHF8 expression based on the assumption that MYC mediates the miRNA regulation of PHF8. First, we determined the miRNAs that can bind to the 3′-UTR of PHF8 using four target prediction databases (TargetScan, PITA, DIANA-microT, and miRanda). We obtained miR-22-3p, miR-494-3p, miR-31-5p, miR-182-5p, and miR-495-3p, wherein miR-22-3p was significantly suppressed by MYC overexpression, and the expression of MYC was negatively correlated with miR-22-3p expression. The dual luciferase reporter assay and the use of miR-22-3p mimics were further used to verify that miR-22-3p targeted and regulated PHF8 in GC. Therefore, we propose that MYC upregulates PHF8 expression by repressing miR-22-3p.
It has been reported that MYC plays an important role in cancer progression by promoting PHF8 expression in prostate cancer (10) and breast cancer (11); however, the existence of a direct relation between these two genes in gastric cancer remains unknown. In our study, we found that the 5′-UTR of MYC mRNA contains the binding sites for miR-22-3p, and that MYC decreased the expression of miR-22-3p by affecting its stability. In addition, MYC overexpression restored the inhibitory effects of miR-22-3p mimics on PHF8. Thus, MYC can regulate the expression of PHF8 by suppressing the stability of miR-22-3p. In addition to the clinical applications of nanomaterials for cancer treatment,27 we anticipate the implementation of our molecular mechanism for the clinical treatment of gastric cancer using this technology.
Conclusion
We identified a novel MYC/miR-22-3p/PHF8 regulatory axis in GC. PHF8 is highly expressed and plays an important role in proliferation, migration, and invasion of GC cells. Moreover, MYC regulated PHF8 by suppressing the stability of miR-22-3p (Figure 6). Thus, the oncogenic role of PHF8 in GC provides a new therapeutic target for GC treatment.
Figure 6.
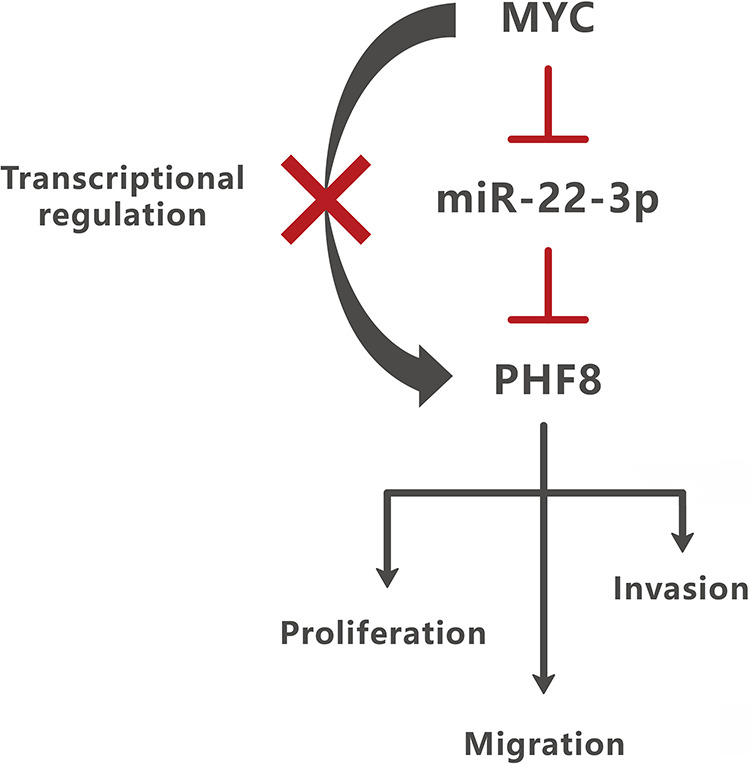
MYC regulated PHF8 by suppressing the stability of miR-22-3p. MYC regulated PHF8, not by transcriptional regulation, but by affecting the stability of miR-22-3p. PHF8 was a direct target of miR-22-3p and promotes the proliferation, migration, and invasion of GC cells.
Supplemental Material
Supplemental Material, sumplementary_materials for MYC Regulates PHF8, Which Promotes the Progression of Gastric Cancer by Suppressing miR-22-3p by Ming-Zhi Cai, Shao-Yan Wen, Xue-Jun Wang, Yong Liu and Han Liang in Technology in Cancer Research & Treatment
Footnotes
Authors’ Note: Han Liang designed this study. Ming-Zhi Cai and Shao-Yan Wen performed the experiments and wrote the manuscript. Xue-Jun Wang and Yong Liu collected information and analyzed the data. All authors read and approved this paper. The verbal consent from patients was obtained prior to enrollment in the study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Tianjin Medical University Clinical Medicine Research Project (11601501-2017KJ0402). This study was approved by the Tianjin Cancer Hospital Medical Ethical Committee (approval no. bc2018037).
ORCID iD: Han Liang  https://orcid.org/0000-0002-5674-0994
https://orcid.org/0000-0002-5674-0994
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2. Greer EL, Shi Y. Histone methylation: a dynamic mark in health, disease, and inheritance. Nat Rev Genet. 2012;13(5):343–357. doi:10.1038/nrg3173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Seligson DB, Horvath S, McBrian MA, et al. Global levels of histone modifications predict prognosis in different cancers. Am J Pathol. 2009;174(5):1619–1628. doi:10.2353/ajpath.2009.0808741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cao J, Liu Z, Cheung WK, et al. Histone demethylase RBP2 is critical for breast cancer progression and metastasis. Cell Rep. 2014;6(5):868–877. doi:10.1016/j.celrep.2014.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lv T, Yuan D, Miao X, et al. Over-expression of LSD1 promotes proliferation, migration, and invasion in non-small cell lung cancer. PLoS One. 2012;7(4):e35065 doi:10.1371/journal.pone.0035065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang Y, Zhang H, Chen Y, et al. LSD1 is a subunit of the NuRD complex and targets the metastasis programs in breast cancer. Cell. 2009;138(4):660–672. doi:10.1016/j.cell.2009.05.050 [DOI] [PubMed] [Google Scholar]
- 7. Klose RJ, Kallin EM, Zhang Y. JmjC-domain-containing proteins and histone demethylation. Nat Rev Genet. 2006;7(9):715–727. doi:10.1038/nrg1945 [DOI] [PubMed] [Google Scholar]
- 8. Fortschegger K, de Graaf P, Outchkourov NS, van Schaik FM, Timmers HT, Shiekhattar R. PHF8 targets histone methylation and RNA polymerase II to activate transcription. Mol Cell Biol. 2010;30(13):3286–3298. doi:10.1128/MCB.01520-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Qi HH, Sarkissian M, Hu GQ, et al. Histone H4K20/H3K9 demethylase PHF8 regulates zebrafish brain and craniofacial development. Nature. 2010;466(7305):503–507. doi:10.1038/nature09261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maina PK, Shao P, Liu Q, et al. C-MYC drives histone demethylase PHF8 during neuroendocrine differentiation and in castration-resistant prostate cancer. Oncotarget. 2016;7(46):75585–75602. doi:10.18632/oncotarget.12310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shao P, Liu Q, Maina PK, et al. Histone demethylase PHF8 promotes epithelial to mesenchymal transition and breast tumorigenesis. Nucleic Acids Res. 2017;45(4):1687–1702. doi:10.1093/nar/gkw1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shen Y, Pan X, Zhao H. The histone demethylase PHF8 is an oncogenic protein in human non-small cell lung cancer. Biochem Biophys Res Commun. 2014;451(1):119–125. doi:10.1016/j.bbrc.2014.07.076 [DOI] [PubMed] [Google Scholar]
- 13. Sun X, Qiu JJ, Zhu S, et al. Oncogenic features of PHF8 histone demethylase in esophageal squamous cell carcinoma. PLoS One. 2013;8(10):e77353 doi:10.1371/journal.pone.0077353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chang TC, Yu D, Lee YS, et al. Widespread microRNA repression by Myc contributes to tumorigenesis. Nat Genet. 2008;40(1):43–50. doi:10.1038/ng.2007.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xiong J, Du Q, Liang Z. Tumor-suppressive microRNA-22 inhibits the transcription of E-box-containing c-Myc target genes by silencing c-Myc binding protein. Oncogene. 2010;29(35):4980–4988. doi:10.1038/onc.2010.241 [DOI] [PubMed] [Google Scholar]
- 16. Taby R, Issa JP. Cancer epigenetics. CA Cancer J Clin. 2010;60(6):376–392. doi:10.3322/caac.20085 [DOI] [PubMed] [Google Scholar]
- 17. You JS, Jones PA. Cancer genetics and epigenetics: two sides of the same coin. Cancer Cell. 2012;22(1):9–20. doi:10.1016/j.ccr.2012.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meng Z, Liu Y, Wang J, et al. Histone demethylase KDM7A is required for stem cell maintenance and apoptosis inhibition in breast cancer. J Cell Physiol. 2019;235(2):932–943. doi:10.1002/jcp.29008 [DOI] [PubMed] [Google Scholar]
- 19. Zhang W, Sun W, Qin Y, et al. Knockdown of KDM1A suppresses tumor migration and invasion by epigenetically regulating the TIMP1/MMP9 pathway in papillary thyroid cancer. J Cell Mol Med. 2019;23(8):4933–4944. doi:10.1111/jcmm.14311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rissi VB, Glanzner WG, De Macedo MP, et al. The histone lysine demethylase KDM7A is required for normal development and first cell lineage specification in porcine embryos. Epigenetics. 2019;14(11):1088–1101. doi:10.1080/15592294.2019.1633864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhu L, Wang J, Kong W, et al. LSD1 inhibition suppresses the growth of clear cell renal cell carcinoma via upregulating P21 signaling. Acta Pharm Sin B. 2019;9(2):324–334. doi:10.1016/j.apsb.2018.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Björkman M, Östling P, Härmä V, et al. Systematic knockdown of epigenetic enzymes identifies a novel histone demethylase PHF8 overexpressed in prostate cancer with an impact on cell proliferation, migration, and invasion. Oncogene. 2012;31(29):3444–3456. doi:10.1038/onc.2011.512 [DOI] [PubMed] [Google Scholar]
- 23. Ye H, Yang Q, Qi S, Li H. PHF8 plays an oncogene function in hepatocellular carcinoma formation. Oncol Res. 2019;27(5):613–621. doi:10.3727/096504018X15410353669149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhou W, Gong L, Wu Q, et al. PHF8 upregulation contributes to autophagic degradation of E-cadherin, epithelial-mesenchymal transition, and metastasis in hepatocellular carcinoma. J Exp Clin Cancer Res. 2018;37(1):215 doi:10.1186/s13046-018-0890-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Taniguchi K, Iwatsuki A, Sugito N, et al. Oncogene RNA helicase DDX6 promotes the process of c-Myc expression in gastric cancer cells. Mol Carcinog. 2018;57(5):579–589. doi:10.1002/mc.22781 [DOI] [PubMed] [Google Scholar]
- 26. Wang X, Liu Y, Shao D, et al. Recurrent amplification of MYC and TNFRSF11B in 8q24 is associated with poor survival in patients with gastric cancer. Gastric Cancer. 2016;19(1):116–127. doi:10.1007/s10120-015-0467-2 [DOI] [PubMed] [Google Scholar]
- 27. Hou J, Aerts J, den Hamer B, et al. Gene expression-based classification of non-small cell lung carcinomas and survival prediction. PLoS One. 2010;5(4):e10312 doi:10.1371/journal.pone.0010312 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sumplementary_materials for MYC Regulates PHF8, Which Promotes the Progression of Gastric Cancer by Suppressing miR-22-3p by Ming-Zhi Cai, Shao-Yan Wen, Xue-Jun Wang, Yong Liu and Han Liang in Technology in Cancer Research & Treatment