Abstract
Objective
To compare the prognostic values of metabolic parameters from pretreatment PET/CT between limited disease (LD) and extensive disease (ED) small cell lung cancer (SCLC) patients.
Methods
Data on 118 newly diagnosed SCLC patients (50 LD and 68 ED) who underwent pretreatment positron emission tomography–computed tomography (PET/CT) were reviewed. For PET, metabolic parameters were measured for: (1) primary tumor, maximum standardized uptake value (SUVmax), metabolic tumor volume, and total lesion glycolysis; and (2) all tumor lesions, SUVmax of the hottest tumor, whole body metabolic tumor volume (WBMTV), and whole body total lesion glycolysis (WBTLG). Prognostic values of metabolic parameters and other clinical variables were analyzed to predict overall survival (OS).
Results
In LD, SUVmax of the primary tumor was an independent prognostic factor for OS. Patients with high SUVmax showed significantly worse OS than those with low SUVmax. In ED, WBMTV and WBTLG were independent prognostic factors for OS. Patients with high WBMTV or WBTLG showed significantly worse OS than those with low WBMTV or WBTLG.
Conclusions
SUVmax of primary tumor was the only independent prognostic factor for OS in LD SCLC patients. WBMTV and WBTLG were independent prognostic factors in ED SCLC patients.
Keywords: Small cell lung carcinoma, fluorodeoxyglucose F18, positron emission tomography–computed tomography, prognosis, limited disease, extensive disease
Introduction
Small cell lung cancer (SCLC) accounts for 13% to 20% of all lung cancers, and is characterized by rapid growth rate and widespread metastases.1 The Veterans Administration Lung Study Group classifies SCLC as a limited disease (LD) or an extensive disease (ED) according to the extent of disease and its spread.2
LD, diagnosed in approximately 30% of patients, is confined to one hemithorax and can be encompassed within a single radiation port, while ED, affecting the remaining 70% of patients, extends beyond the boundaries of a single radiation field.3 In patients with LD, the addition of thoracic radiotherapy to chemotherapy results in a good survival outcome or even a cure for some patients, while chemotherapy alone is the standard of care for SCLC patients with ED. The prognosis of SCLC is poor, yielding 2-year survival rates of 20% to 40% and 5% to 13% for LD and ED patients, respectively.1 However, little progress has been made during the past 20 years regarding the treatment of SCLC in contrast to targeted therapy in non-small cell lung cancer (NSCLC).
Although lactate dehydrogenase (LDH) has been considered a useful prognostic indicator since the late 1980s,4 some investigations have reported non-significant results regarding the prognostic value of LDH in SCLC patients.5,6 Therefore, there have been attempts to identify alternative effective prognostic factors for SCLC. Identifying imaging biomarkers that can predict prognosis in SCLC would be helpful in clinical decision and management, and several studies have been conducted to evaluate the prognostic role of metabolic parameters from 2-deoxy-2-[18F]fluoro-D-glucose (FDG) positron emission tomography–computed tomography (PET/CT).7,8 Although the prognostic role of PET/CT parameters has been studied in SCLC, inconsistent results have been reported.9,10 Moreover, because patients with LD and ED have different survival outcomes, it may be helpful to understand the prognostic factors of PET/CT parameters at each stage of cancer to predict outcomes and select appropriate treatment options. In this study, therefore, we evaluated the prognostic value of metabolic parameters from pretreatment PET/CT scans in patients with LD and ED SCLC.
Materials and methods
Study population
This study was approved by the institutional review board at our medical center. Informed consent was waived because of the retrospective nature of the study. We retrospectively reviewed the medical records of 141 consecutive patients with SCLC who underwent pretreatment staging with PET/CT between February 2010 and December 2016. All patients were clinically assessed by laboratory findings, chest CT, abdomen CT, brain magnetic resonance imaging, and PET/CT.
Eligible patients had pathologically confirmed SCLC and no previous history of other malignancy, and underwent PET/CT before the start of initial treatment. We excluded 23 of the 141 patients for the following reasons: refusal of chemotherapy (n = 19), and indiscernible FDG uptake of primary lung cancer (n = 2) and double primary malignancies (n = 2). Finally, 118 patients were enrolled in the study and were divided into LD or ED groups. LD was defined as disease confined to one hemithorax and regional or ipsilateral supraclavicular lymph nodes, whereas ED was defined as disease beyond these boundaries.
18 F-FDG PET/CT
All PET/CT scans were performed on a dedicated PET/CT scanner (Discovery STe, General Electric Healthcare, Milwaukee, WI, USA). All patients fasted for at least 6 hours and blood glucose levels were lower than 140 mg/dL before intravenous administration of FDG. A dose of approximately 5.5 MBq/kg of FDG was intravenously administered. PET images were acquired from the cerebellum to the proximal thighs in 3-D mode 60 minutes after the injection of FDG immediately after acquiring a pre-contrast CT scan. PET images were reconstructed by an iterative reconstruction algorithm using CT images for attenuation correction.
Image analysis
All PET/CT images were transferred to the IntelliSpace Portal 9.0 (Philips Healthcare, Cleveland, OH, USA) and were reviewed by two board-certified nuclear medicine physicians (JKO and EKC) who were blinded to the patients’ survival information. If there was any discrepancy between the readers, it was resolved by consensus. Quantitative analysis was performed of two spherical volumes of interest (VOI) in primary tumors and metastatic tumors in the entire body. For primary tumors, a spherical VOI was drawn by the nuclear medicine physicians. In vivo assessment of glucose metabolism was estimated by the experienced medical physicist (MJP) using three metabolic parameters: the maximal standardized uptake value (SUVmax), metabolic tumor volume (MTV), and total lesion glycolysis (TLG). Raw SUV values and physical locations of each voxel were extracted in ASCII format using A Medical Imaging Data Examiner. The MTV was calculated using a fixed SUV threshold of 3.0. TLG was calculated as the volume of MTV multiplied by the average SUV of the MTV. Primary tumor SUVmax, primary tumor MTV (PMTV), and primary tumor TLG (PTLG) were also calculated. We also measured the SUVmax of the hottest tumor lesion for both primary tumors and metastatic tumors. To measure the metabolic tumor burden in the entire body, whole body MTV (WBMTV) and whole body TLG (WBTLG) were determined by summing the corresponding values for all tumor lesions (primary tumor, regional metastatic node, and all distant metastatic tumors).
Statistical analysis
The primary endpoint of this study was the duration of overall survival (OS), which was measured from the date of the PET/CT scan to the date of death from any cause, with surviving patients censored at the time of last follow-up until December 2018. For the purposes of statistical analysis, all continuous variables except age and metabolic parameters were dichotomized according to clinical settings. For univariate analysis, the Cox proportional hazards regression test was performed to assess the prognostic value for OS using the following factors: age, sex, smoking history, staging, LDH, carcinoembryonic antigen, and metabolic parameters (SUVmax, MTV, and TLG) from PET/CT. The multivariate Cox proportional hazards model was performed with variables that were significant in univariate analyses after adjusting for the effects of sex and age. Because TLG is calculated by multiplying the mean SUV and MTV, there was a significant correlation between MTV and TLG so they were assessed separately. Survival curves were estimated using the Kaplan–Meier method, and differences between subgroups according to staging or the optimal cutoff value were compared with the log-rank test. Optimal cutoff values were determined using receiver-operating characteristic curve analysis. All statistical analysis was performed using the statistical software SPSS (version 20; IBM Corp., Armonk, NY, USA), in which a P value of less than 0.05 was considered statistically significant.
Results
Patient characteristics
The characteristics of 118 patients and primary tumors are shown in Table 1. Fifty (42.4%) patients were classified as LD and 68 (57.6%) as ED. In patients with LD, the median values of primary tumor SUVmax, PMTV, PTLG, WBMTV, and WBTLG were 10.9 (range, 3.3–29.9), 27.8 (range, 2.4–165.1), 244.5 (range, 10.4–1229.7), 76.1 (range, 1.4–340), and 410.5 (range, 4.3–1824), respectively. In patients with ED, the median values of primary tumor SUVmax, PMTV, PTLG, WBMTV, and WBTLG were 10.8 (range, 5.1–57.3), 41.2 (range, 1.3–181.3), and 312.4 (range, 8.3–5073.3), 157.1 cm3 (range, 8.61–1243 cm3), and 744.1 (range, 38.1–11040), respectively. In LD patients, the hottest lesion was located in the primary tumor. In ED patients, the median SUVmax of the hottest tumor was 11.0 (range, 5.1–57.3). In five ED patients, the hottest tumors were located in metastatic tumors (four in the mediastinum and one in bone). There was no significant difference in LDH, SUVmax of the primary tumor and hottest tumor, PMTV, or PTLG between patients with LD and ED. Eleven LD patients were scheduled for concurrent chemoradiotherapy but received only induction chemotherapy because of rapid disease progression or deterioration of their general condition. Three ED patients received only prophylactic radiation therapy.
Table 1.
Patient characteristics (N = 118).
| Characteristics | Overall patients | LD (n = 50) | ED (n = 68) | P |
|---|---|---|---|---|
| Age (years) | ||||
| Median (range) | 65 (47–87) | 66 (52–87) | 65 (47–84) | 0.8658 |
| Sex, n (%) | ||||
| Male | 93 (78.8%) | 38 (76%) | 55 (81%) | 0.521 |
| Female | 25 (21.2%) | 12 (24%) | 13 (19%) | |
| Smoking history | ||||
| Presence | 105 (89%) | 46 (92%) | 59 (86.8%) | 0.369 |
| Absence | 13 (11%) | 4 (8%) | 9 (13.2%) | |
| LDH, n (%) | ||||
| <450 U/l | 70 (59%) | 34 (68%) | 36 (53%) | 0.1 |
| ≥450 U/l | 48 (41%) | 16 (32%) | 32 (47%) | |
| *CEA, n (%) | ||||
| <5 (ng/mL) | 58 (50%) | 30 (60%) | 28 (42.4%) | 0.061 |
| ≥5 (ng/mL) | 58 (50%) | 20 (40%) | 38 (57.6%) | |
| SUVmax, primary tumor | ||||
| Median (range) | 10.9 (3.3–57.3) | 10.9 (3.3–29.9) | 10.8 (5.1–57.3) | 0.777 |
| PMTV (cm3) | ||||
| Median (range) | 34.9 (1.3–181.3) | 27.8 (2.4–165.1) | 41.2 (1.3–181.3) | 0.0618 |
| PTLG | ||||
| Median (range) | 277 (8.3–5073.3) | 244.5 (10.4–1229.7) | 312.4 (8.3–5073.3) | 0.0723 |
| SUVmax, hottest tumor | ||||
| Median (range) | 11.0 (5.1–57.3) | |||
| WBMTV (cm3) | ||||
| Median (range) | 157.1 (8.61–1243) | |||
| WBTLG | ||||
| Median (range) | 744.1 (38.1–11040) | |||
| Treatment, n (%) | ||||
| Chemotherapy only | 76 (64.4%) | 11 (22%) | 65 (95.6%) | |
| Radiotherapy only | 4 (3.4%) | 1 (2%) | 3 (4.4%) | |
| CCRT | 38 (32.2%) | 38 (76%) | 0 |
*Two cases had no information on CEA.
LD, limited disease; ED, extensive disease; CCRT, concurrent chemoradiotherapy; LDH, lactate dehydrogenase; CEA, carcinoembryonic antigen; SUVmax, maximum standardized uptake value; PMTV, primary tumor metabolic tumor volume; PTLG, primary tumor total lesion glycolysis; WBMTV, whole body metabolic tumor volume; WBTLG, whole body total lesion glycolysis.
Prognostic factor analyses for overall survival
The median duration of follow-up was 13.4 months (range, 0.6–59.8 months). During follow-up, 81 (68.6%) of the 118 patients died, and the median OS duration was 9.3 months. The estimated median OS times for LD and ED patients were 11.8 months and 8.2 months, respectively.
In LD, SUVmax of primary tumor was an independent predictor of OS. High SUVmax was a significant prognostic factor, with a 1.24-fold increase in the risk of death (hazard ratio [HR], 1.24; 95% confidence interval [CI], 1.06–1.45; P = 0.007, Table 2). WBMTV and WBTLG were significant prognostic factors in univariate analysis (HR, 1.005 and 1.0009; 95% CI 1.0009–1.01 and 1.0001–1.001; P = 0.019 and 0.016, respectively), but were not significant in multivariate analysis. No other metabolic or clinical variable were identified as a significant prognostic factor for OS.
Table 2.
Univariate and multivariate analysis of prognostic factors for overall survival in LD patients (n = 50).
| Variables |
Univariate |
Multivariate |
||
|---|---|---|---|---|
| HR (95% CI) | P | Adjusted HR (95% CI) | P | |
| Age | 1.006 (0.964–1.05) | 0.757 | 1.016 (0.971–1.062) | 0.482 |
| Sex (male vs female) | 0.592 (0.205–1.704) | 0.332 | 0.924 (0.289–2.95) | 0.894 |
| Smoking history | 3.314 (0.939–11.691) | 0.062 | ||
| LDH (<450 vs ≥450) | 1.0 (0.998–1.003) | 0.5 | ||
| CEA (<5 vs ≥5) | 0.716 (0.34–1.505) | 0.378 | ||
| SUVmax, primary tumor | 1.177 (1.061–1.306) | 0.007 | 1.24 (1.06–1.45) | 0.007 |
| PMTV | 1.006 (0.997–1.015) | 0.186 | ||
| PTLG | 1.0 (0.999–1.002) | 0.107 | ||
| WBMTV | 1.005 (1.0009–1.01) | 0.019 | 1.004 (0.999–1.01) | 0.076 |
| WBTLG | 1.0009 (1.0001–1.001) | 0.016 | 1.006 (0.999–1.001) | 0.132 |
HR, hazard ratio; CI, confidence interval; LD, limited disease; LDH, lactate dehydrogenase; CEA, carcinoembryonic antigen; SUVmax, maximum standardized uptake value; PMTV, primary tumor metabolic tumor volume; PTLG, primary tumor total lesion glycolysis; WBMTV, whole body metabolic tumor volume; WBTLG, whole body total lesion glycolysis.
In ED, WBMTV and WBTLG were significant predictors of OS in both univariate and multivariate analyses (HR, 1.001 and 1.0003; 95% CI, 1.001–1.003 and 1.0001–1.0004; P = 0.025 and 0.006, respectively, Table 3). Regarding the hottest tumor, none of the metabolic parameters were significant predictors of OS. While LDH was significant in univariate analysis (HR, 1.001; 95% CI 1.0001–1.002; P = 0.022), it was not significant in multivariate analysis.
Table 3.
Univariate and multivariate analysis of prognostic factors for overall survival in ED patients (n = 68).
| Variables |
Univariate |
Multivariate |
||
|---|---|---|---|---|
| HR (95% CI) | P | Adjusted HR (95% CI) | P | |
| Age | 1.01 (0.971–1.051) | 0.608 | 1.019 (0.976–1.063) | 0.386 |
| Sex (male vs female) | 0.778 (0.349–1.737) | 0.541 | 0.782 (0.35–1.748) | 0.55 |
| Smoking history | 1.14 (0.511–2.566) | 0.741 | ||
| LDH (<450 vs ≥450) | 1.001 (1.0001–1.002) | 0.022 | 1.0 (0.99–1.002) | 0.285 |
| CEA (<5 vs ≥5) | 1.012 (0.574–1.784) | 0.965 | ||
| SUVmax, hottest tumor | 1.028 (0.995–1.062) | 0.09 | ||
| MTV, hottest tumor | 1.002 (0.997–1.008) | 0.303 | ||
| TLG, hottest tumor | 1.0 (0.999–1.006) | 0.118 | ||
| WBMTV | 1.002 (1.001–1.003) | 0.001 | 1.001 (1.0001–1.003) | 0.025 |
| WBTLG | 1.0003 (1.0001–0.0004) | <0.001 | 1.0003 (1.0001–.0006) | 0.006 |
HR, hazard ratio; CI, confidence interval; ED, extensive disease; LDH, lactate dehydrogenase; CEA, carcinoembryonic antigen; SUVmax, maximum standardized uptake value; MTV, metabolic tumor volume; TLG, total lesion glycolysis; WBMTV, whole body metabolic tumor volume; WBTLG, whole body total lesion glycolysis.
Kaplan–Meier survival analyses according to tumor FDG uptake
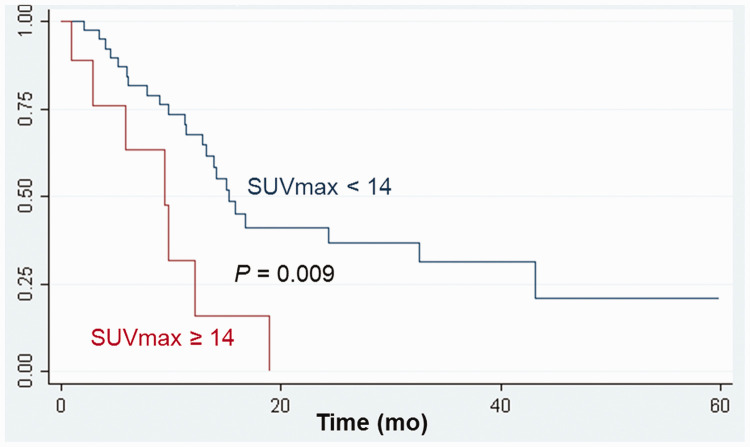
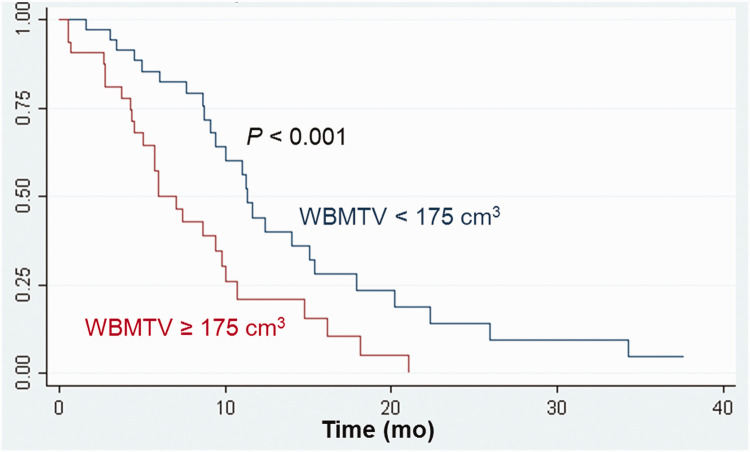
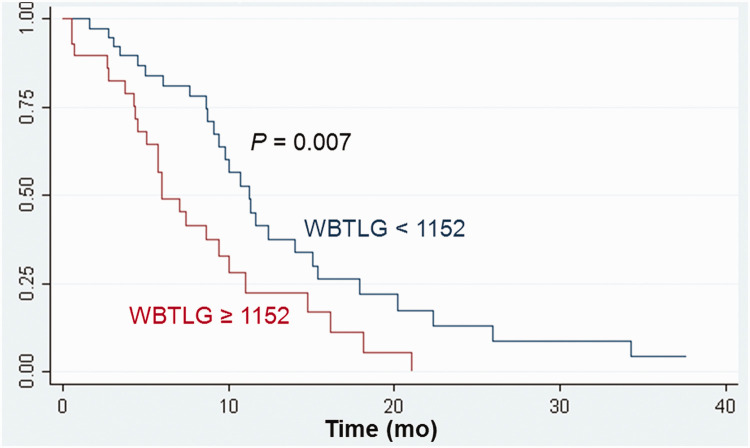
For comparisons of survival, the optimal SUVmax cutoff value in LD patients was 14; the median OS differed according to the SUVmax cutoff value: 13.2 months versus 8.1 months (P = 0.009, Figure 1). In ED patients, the optimal WBMTV cutoff value was 175 cm3; the median OS differed according to the WBMTV cutoff value: 9.3 months versus 5.9 months (P < 0.001, Figure 2). The optimal WBTLG cutoff value was 1152, and the median OS differed according to WBTLG cutoff value: 9.1 months versus 6 months (P = 0.007, Figure 3).
Figure 1.
Cumulative overall survival curves according to SUVmax in patients with limited disease.
Figure 2.
Cumulative overall survival curves according to WBMTV in patients with extensive disease.
Figure 3.
Cumulative overall survival curves according to WBTLG in patients with extensive disease.
Discussion
Although pretreatment PET/CT has prognostic value in NSCLC, prognostic stratification of SCLC patients with PET/CT remains controversial.11,12 Additionally, because the prognosis varies widely according to the stage of SCLC, a more relevant question might be which stage of SCLC would benefit from PET/CT as a tool of prognosis stratification. In this regard, we evaluated the prognostic value of metabolic parameters from PET/CT in LD and ED SCLC.
In the present study, SUVmax of the primary tumor was a significant prognostic factor in patients with LD, but not in patients with ED. In ED patients, WBMTV and WBTLG were prognostic factors as indices of whole body tumor burden, rather than metabolic parameters of primary tumors. This study also revealed that the prognosis factors for OS in LD patients differed from those in ED patients. This suggests that biological aggressiveness of the primary tumor is important for prognosis in LD, whereas the extent of whole body tumor burden is associated with prognosis in ED.
Several studies that have explored metabolic parameters of PET/CT as prognostic factors in SCLC have combined LD and ED for analysis instead of analyzing them separately.6,13 However, because LD and ED are considered to have different characteristics and prognosis, there might be a problem of either one of the groups affecting overall results if they are combined for analysis. Indeed, a previous study showed that the results were different when comparing the prognostic value of SUVmax for primary tumors in the combined analysis versus the separate analysis of LD and ED.14
In a study that investigated metabolic parameters in 150 patients with LD, the highest SUVmax was identified as the only significant prognostic factor for OS.15 Moreover, volumetric parameters such as WBMTV and WBTLG were shown to be significant prognostic factors in univariate analysis, but not in multivariate analysis. Our results for LD were in accordance with this. In this previous study, highest SUVmax was measured in primary or metastatic tumors. Although SUVmax was also measured for all primary and metastatic tumors in our study, the hottest lesion was always found in the primary tumor.
In patients with LD, concurrent chemoradiotherapy is an attractive treatment modality that enhances the effects of local radiotherapy, leading to better tumor control and improved survival.16 It is likely that employing PET/CT scans in radiotherapy planning will help selective nodal irradiation and more accurate gross tumor volume delineation to reduce treatment-related toxicity.17 Moreover, because SCLC with aggressive features and rapid tumor growth has a high FDG uptake, the efficacy of higher dose radiation might be more prominent in SCLC with higher FDG uptake. Therefore, information on geographic distribution of FDG uptake could be useful in radiation planning. Further studies are needed to explore the clinical role of primary tumor SUVmax in radiotherapy planning in LD.
Serum LDH level is an important prognostic factor in predicting the response to chemotherapy and survival in SCLC patients,18 and to reflect tumor burden in solid tumors including SCLC.19 In this study, a high level of LDH was significant for OS in the univariate analysis of ED, but not in multivariate analysis. Only WBMTV and WBTLG were significant prognostic factors in ED regardless of age, sex, and serum LDH levels. Therefore, WBMTV or WBTLG are assumed to represent the whole body tumor burden better than serum LDH levels.
Several researchers have attempted to evaluate whether volumetric metabolic parameters such as MTV and TLG have predictive values in newly diagnosed SCLC patients.7,8 For example, Oh et al. investigated the prognostic value of whole body tumor burden on PET/CT in LD and ED groups of SCLC patients, and reported that WBMTV was a better predictor of survival than SUVmax of the hottest tumor.7 However, in our study, WBMTV only had a prognostic value for OS in the ED group, and the MTV and TLG of the primary tumor or whole body tumor were not associated with OS in LD patients. We propose that biological aggressiveness of the primary tumor is associated with prognosis in LD, and that the extent of whole body tumor burden might affect prognosis in ED.
There are several limitations in this study. First, because of the retrospective nature of the study design, selection bias may have been introduced. Second, there was no standardization in measuring MTV and TLG. Although some studies have used an absolute value of threshold SUV or 50% of SUVmax for segmentation of the target lesion,20,21 no definite threshold for MTV and TLG measurement has been established. However, an SUV value of 3.0 is generally accepted as a cutoff threshold value to measure MTV and TLG for differentiating between malignant and benign lesions in various malignancies.7
Conclusion
In patients with LD, SUVmax of the primary tumor was the only significant prognostic factor for OS. In patients with ED, WBMTV and WBTLG were significant independent prognostic factors for OS. LD and ED groups of SCLC had different prognostic factors from PET/CT.
Acknowledgments
None.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research was supported by the National Research Foundation of Korea (NRF), and was funded by the Ministry of Science and ICT (2017R1C1B2011802), the Catholic Medical Center Research Foundation made in the program year of 2016 and a Grant from the Translational R&D Project through the Institute for Bio-Medical convergence, Incheon St. Mary’s Hospital, The Catholic University of Korea.
ORCID iDs
Yong-An Chung https://orcid.org/0000-0003-4004-4019
Jin Kyoung Oh https://orcid.org/0000-0001-9820-4770
References
- 1.van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet 2011; 378: 1741–1755. [DOI] [PubMed] [Google Scholar]
- 2.Kalemkerian GP, Akerley W, Bogner P, et al. Small cell lung cancer. J Natl Compr Canc Netw 2013; 11: 78–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morabito A, Carillio G, Daniele G, et al. Treatment of small cell lung cancer. Crit Rev Oncol Hematol 2014; 91: 257–270. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Guo M, Fan J, et al. Prognostic significance of serum LDH in small cell lung cancer: a systematic review with meta-analysis. Cancer Biomark 2016; 16: 415–423. [DOI] [PubMed] [Google Scholar]
- 5.Ando S, Suzuki M, Yamamoto N, et al. The prognostic value of both neuron-specific enolase (NSE) and Cyfra21-1 in small cell lung cancer. Anticancer Res 2004; 24: 1941–1946. [PubMed] [Google Scholar]
- 6.Kim H, Yoo IR, Boo SH, et al. Prognostic value of pre- and post-treatment FDG PET/CT parameters in small cell lung cancer patients. Nucl Med Mol Imaging 2018; 52: 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh JR, Seo JH, Chong A, et al. Whole-body metabolic tumour volume of 18F-FDG PET/CT improves the prediction of prognosis in small cell lung cancer. Eur J Nucl Med Mol Imaging 2012; 39: 925–935. [DOI] [PubMed] [Google Scholar]
- 8.Yu X, Zhu Y, Wang J, et al. Pretreatment metabolic parameters measured by 18F-FDG-PET to predict the outcome of first-line chemotherapy in extensive-stage small-cell lung cancer. Nucl Med Commun 2017; 38: 193–200. [DOI] [PubMed] [Google Scholar]
- 9.Kim SJ, Chang S. Limited prognostic value of SUV max measured by F-18 FDG PET/CT in newly diagnosed small cell lung cancer patients. Oncol Res Treat 2015; 38: 577–585. [DOI] [PubMed] [Google Scholar]
- 10.Zhu D, Ma T, Niu Z, et al. Prognostic significance of metabolic parameters measured by (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with small cell lung cancer. Lung Cancer 2011; 73: 332–337. [DOI] [PubMed] [Google Scholar]
- 11.Liao S, Penney BC, Wroblewski K, et al. Prognostic value of metabolic tumor burden on 18F-FDG PET in nonsurgical patients with non-small cell lung cancer. Eur J Nucl Med Mol Imaging 2012; 39: 27–38. [DOI] [PubMed] [Google Scholar]
- 12.Ong LT, Dunphy M, Foster A, et al. Prognostic value of preradiotherapy (18)F-FDG PET/CT volumetrics in limited-stage small-cell lung cancer. Clin Lung Cancer 2016; 17: 184–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yilmaz Demirci N, Yilmaz U, Biner Uslu I, et al. Prognostic significance of standardised uptake value (SUVmax) measured on 18F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with small cell lung cancer. Eur J Cancer Care (Engl) 2017; 26. [DOI] [PubMed] [Google Scholar]
- 14.van der Leest C, Smit EF, Baas J, et al. SUVmax during 18FDG-PET scanning in small cell lung cancer: similar information as in non-small cell lung cancer? Lung Cancer 2012; 76: 67–71. [DOI] [PubMed] [Google Scholar]
- 15.Kwon SH, Hyun SH, Yoon JK, et al. The highest metabolic activity on FDG PET is associated with overall survival in limited-stage small-cell lung cancer. Medicine (Baltimore) 2016; 95: e2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gazdar AF, Bunn PA, Minna JD. Small-cell lung cancer: what we know, what we need to know and the path forward. Nat Rev Cancer 2017; 17: 725–737. [DOI] [PubMed] [Google Scholar]
- 17.van Loon J, De Ruysscher D, Wanders R, et al. Selective nodal irradiation on basis of (18)FDG-PET scans in limited-disease small-cell lung cancer: a prospective study. Int J Radiat Oncol Biol Phys 2010; 77: 329–336. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Zhang W, Yin W, et al. The prognostic value of the serum neuron specific enolase and lactate dehydrogenase in small cell lung cancer patients receiving first-line platinum-based chemotherapy. Medicine (Baltimore) 2017; 96: e8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walenta S, Mueller-Klieser WF. Lactate: mirror and motor of tumor malignancy. Semin Radiat Oncol 2004; 14: 267–274. [DOI] [PubMed] [Google Scholar]
- 20.Frings V, de Langen AJ, Smit EF, et al. Repeatability of metabolically active volume measurements with 18F-FDG and 18F-FLT PET in non-small cell lung cancer. J Nucl Med 2010; 51: 1870–1877. [DOI] [PubMed] [Google Scholar]
- 21.Kaida H, Toh U, Hayakawa M, et al. The relationship between 18F-FDG metabolic volumetric parameters and clinicopathological factors of breast cancer. Nucl Med Commun 2013; 34: 562–570. [DOI] [PubMed] [Google Scholar]