Abstract
We present a case of vertebral osteomyelitis in a previously healthy, adolescent Caucasian female athlete. After months of lower back pain, spinal imaging demonstrated phlegmon and suspected osteomyelitis of the L4 vertebral body. A bone biopsy was obtained, and microbiologic cultures yielded pure growth of Salmonella enterica subsp. enterica serovar Poona (S. Poona), a member of the nontyphoid Salmonella group associated with food-borne gastroenteritis in the United States. This case represents the first reported association of S. Poona with osteomyelitis and is interesting in that the infection developed in a patient without traditional risk factors for invasive Salmonella disease (i.e. sickle cell disease). This case highlights the importance of keeping a broad differential diagnosis for lower back pain and emphasizes the value of obtaining specimens for microbiologic culture to aid in diagnosing non-traditional and potentially emerging bacterial pathogens.
Keywords: Pediatrics, adolescent, infectious disease, vertebral osteomyelitis, Salmonella Poona
Introduction
Osteomyelitis is an inflammatory process of the bone and bone marrow that can result in local bone destruction, necrosis, and apposition of new bone.1,2 In children and adolescents, the bones most commonly involved include the long-bones of upper and lower extremities, with osteomyelitis of the vertebral bodies of the spine being much less common, accounting for only 1%–2% of osteomyelitis episodes in children.3,4 Osteomyelitis is commonly due to pyogenic bacteria with Staphylococcus aureus accounting for 32%–67% of cases among all ages.1,3–6 Among pediatric populations, S. aureus and Streptococcus species, including Group B Streptococcus, are the predominate pathogens.3,6 Gram-negative bacilli, such as Escherichia coli, are associated with vertebral osteomyelitis arising from a urinary tract or intra-abdominal source.6,7 Immunosuppressed patients, including those infected with HIV, diabetes, and the elderly, carry an increased risk of infection with more exotic bacterial pathogens, including Mycobacterium tuberculosis or Brucella species, or fungus with Histoplasma, Aspergillus, or Candida.8 Salmonella are rare causative organisms of vertebral osteomyelitis but are more frequently implicated in patients with sickle cell disease.9 This report describes an unusual case of vertebral osteomyelitis in a previously healthy adolescent Caucasian female due to Salmonella Poona.
Case report
A 14-year-old Caucasian female presented with a 3-month history of progressively worsening lower back pain. Her back pain quality was sharp, with 9/10 pain at its worst. The pain would sometimes radiate to her hips and down her legs and worsen with prolonged walking or activity. She was an otherwise healthy, competitive volleyball player. She had no fevers but did recall some occasional night sweats. There was no appreciable weight loss, no abdominal pain, no urinary or fecal incontinence. She had no recent diarrhea; in contrast, she reported experiencing more constipation in the weeks preceding her diagnosis. She had no significant past medical history and no known medication allergies. She was a freshman in high school and lived on a farm in southeast Michigan, raising chickens, turkeys, and cattle. Her only travel was to Key West, Florida, 3 months earlier. She had no specific risk factors and no known contacts with possible exposure to tuberculosis.
Her symptoms were initially attributed to a musculoskeletal injury secondary to playing volleyball. Anti-inflammatory treatment with non-steroidal anti-inflammatory drugs (NSAIDs) and physical therapy (PT) only provided moderate relief. Her back pain continued to worsen to the point that she was unable to walk without assistance. X-ray imaging of the spine was obtained by her primary care physician and was interpreted as normal, with no abnormalities of the vertebral bodies or disk spaces identified. A chest X-ray showed no pulmonary infiltrates and no masses concerning tuberculosis or other invasive infection. An orthopedic surgeon obtained a magnetic resonance imaging (MRI; Figure 1) of her spine to screen for possible musculoskeletal injury with nerve involvement. Surprisingly, the imaging demonstrated lesions consistent with pre- and para-vertebral phlegmon with associated osteomyelitis of the L4 vertebra and L4-L5 discitis. Blood work was obtained and showed a normal complete blood cell count but elevated inflammatory markers with erythrocyte sedimentation rate (ESR) of 43 mm (normal < 20) and C-reactive protein (CRP) of 1.7 mg/dL (normal < 0.6). She was subsequently referred to a quaternary care children’s hospital for further evaluation.
Figure 1.
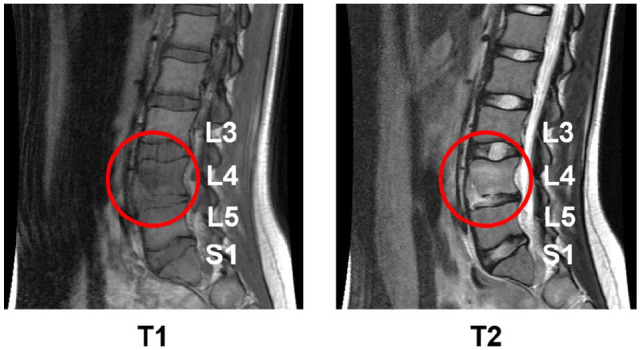
Magnetic resonance imaging of the spine with T1 and T2 images shown demonstrating lesion consistent with pre- and para-vertebral phlegmon, with associated osteomyelitis of the L4 vertebra, and L4-L5 discitis.
She was admitted to the pediatric general medicine service for presumed vertebral osteomyelitis, and pediatric orthopedic surgery and pediatric infectious diseases were consulted. Upon admission, she was afebrile, and her physical exam was notable for tenderness to palpation over her lower back about the area of L4. She hyperextended her spine with ambulation and was unable to flex her spine without significant pain. Laboratory studies showed a white blood cell (WBC) count of 11.9 K/mcL with 63% neutrophils, hemoglobin of 10.6 g/dL, and platelet count of 370 K/mcL. A procalcitonin level was obtained at 0.1 ng/mL (normal < 0.25 ng/mL). Her inflammatory markers were repeated with ESR 41 mm/Hr and CRP 1.4 mg/dL. Lactate dehydrogenase (LDH) and uric acid (UA) were within normal limits, reducing the likelihood of malignancy. Blood culture was obtained; note that she had not received antibiotics before her arrival. Given the prolonged duration of symptoms, her osteomyelitis was categorized as subacute to chronic, necessitating a longer course of antibiotic therapy of likely 6–12 weeks. A biopsy was requested to obtain specimens for microbiologic cultures to assist with organism identification and susceptibility testing. A computed tomography (CT)-guided tissue biopsy of the vertebral lesion was performed to obtain culture specimens for aerobic, fungal, and acid-fast bacilli (AFB). She was subsequently started on IV cefazolin for empiric treatment of typical organisms causing vertebral osteomyelitis in healthy children, including methicillin-susceptible Staphylococcal aureus (MSSA) and Streptococcus species. Cefazolin was chosen for empiric MSSA coverage, as our institution, as have many medical centers across the United States, has noted an increased incidence of MSSA as opposed to methicillin-resistant S. aureus (MRSA) in pediatric bone and joint infections.4 Surprisingly, the tissue biopsy culture resulted in the pure growth of a gram-negative rod, initially identified as Salmonella species, with subsequent identification by the Michigan State Health Department Microbiology Laboratory as Salmonella enterica subsp. enterica serovar Poona (S. Poona).
Given the recovery of Salmonella species and unknown susceptibilities initially, cefazolin was transitioned to ceftriaxone for more reliable empiric coverage. A stool specimen was sent for nucleic acid testing. It was negative for the detection of Salmonella, suggesting she was not an active carrier. However, we cannot exclude the possibility that antibiotic treatment for 2 days before the stool specimen submission may have eradicated the infection. The isolate was susceptible to ampicillin, ceftriaxone, trimethoprim-sulfamethoxazole (TMP-SMX), and ciprofloxacin. All other biopsy and blood cultures remained negative for growth. After 5 days of IV antibiotics as an inpatient, she was clinically stable, afebrile, and had adequate oral intake. Given her clinical course and her known isolate susceptibilities, she was an appropriate candidate for an early transition to oral therapy with a highly bioavailable antibiotic. She was discharged home to continue treatment with oral TMP-SMX.
One week after hospital discharge, she developed a pruritic rash worrisome for possible sulfa allergy, and she was transitioned to ciprofloxacin. Her rash resolved, and she completed a 10-week course of antibiotics in total. She wore a supportive back brace for approximately 2 months. With PT, she underwent a gradual build-up of core strength-building and flexibility exercises. By 3 months after the initiation of antibiotics and PT, she resumed limited physical activity and some volleyball practice participation. At the 3-month clinic visit, her lower back pain was entirely resolved, she had a full range of motion on physical exam, and her ESR and CRP had normalized. By 6 months post-treatment, she had completed all PT sessions, and she had resumed full volleyball activity. As of 12 months post-treatment, she remains asymptomatic with normal inflammatory markers.
Discussion
Salmonella species are rare causative organisms of osteomyelitis, responsible for only 0.45% of all cases, and are both clinically and radiologically indistinguishable from osteomyelitis caused by other organisms.10,11 Patients with hemoglobinopathies are believed to be predisposed to infection with Salmonella species due to repetitive vaso-occlusive crises causing devitalization of gut and bone, red cell breakdown products of chronic hemolysis saturating the macrophage system, and underlying splenic and hepatic dysfunction.9,12
Risk factors for Salmonella infections include food-borne exposures and handling of reptiles or live poultry. This patient was known to have interacted with chickens and turkeys on her parents’ farm, and we speculate that was her source of exposure; in fact, she admitted to eating raw cookie dough from eggs raised on her farm. She had no exposure to reptiles. Our patient had no initial gastrointestinal symptoms other than constipation. She must have had bacteremia at some point to transit the Salmonella from her gastrointestinal (GI) tract into her vertebral body, and it is possible her night sweats were symptomatic of this. The S. Poona serovar has been associated with the consumption of contaminated cucumbers.13 A sizable multi-state outbreak of S. Poona occurred in the United States in 2015, when there were 907 cases reported in at least 40 states. According to the Centers for Disease Control and Prevention (CDC), about 75% of cases had reported consuming cucumbers.13 Since that epidemic, there have been no significant outbreaks related to S. Poona in the United States.
Most reports of disease due to S. Poona are associated with gastrointestinal illness and food-borne exposures.13 Invasive disease with S. Poona bacteremia has been documented in children and adults, often associated with reptile exposures.14 There have been case reports of invasive disease with osteomyelitis due to other nontyphoid Salmonella species.6,11 However, to our knowledge, this is the first report of serovar S. Poona as an etiology of osteomyelitis. This case report of osteomyelitis in a healthy young woman, along with reports of S. Poona bacteremia in otherwise healthy individuals, may suggest enhanced virulence of S. Poona, even in patients without traditional risk factors like sickle cell disease.14
The differential diagnosis of low back pain in pediatric patients is broad, including direct trauma, muscle strain, myalgias related to viral syndromes, and urinary tract infections, in addition to less common, but severe, conditions such as discitis, osteomyelitis, spondylolisthesis, Scheuermann disease, and even malignancies.15 In this case, CT-guided biopsy and culture were critical in confirming the diagnosis of bacterial osteomyelitis and initiation of appropriate antibiotic treatment, especially given her need for prolonged antibiotic therapy. In the absence of bacterial identification, her treatment may have been changed to vancomycin or clindamycin for presumed S. aureus osteomyelitis, which may not have been successful. A multidisciplinary approach by a team including a Pediatric Hospitalist, with subspecialty input from Pediatric Orthopedic Surgery, Pediatric Interventional Radiology, and Pediatric Infectious Disease, led to a successful outcome for this patient with a full recovery and return to competitive sports activity.
In conclusion, a multidisciplinary approach and vertebral biopsy with culture were imperative in determining appropriate long-term antibiotic therapy and the successful management of this patient. Thus, for clinicians, we recommend considering obtaining a biopsy with culture for appropriate treatment of vertebral osteomyelitis of prolonged presentation, especially in the setting of possible exotic exposures. S. Poona may be an emerging pathogen and may need to be considered in bacterial infections, even in previously healthy individuals with potential risk factors for exposure.
Footnotes
Author contributions: Y.T., S.T., and M.E.W. drafted the manuscript and conducted a literature search. All authors participated in the clinical care of this patient and critically reviewed the manuscript. All authors gave final approval and agreed to be held accountable for all aspects of the work’s integrity.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require Institutional Review Board approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from the patient’s legal guardian for patient information and images to be published prior to manuscript preparation. Written informed consent was obtained from a legally authorized representative(s) for anonymized patient information to be published in this article.
ORCID iD: Michael E Watson  https://orcid.org/0000-0001-5930-3290
https://orcid.org/0000-0001-5930-3290
References
- 1. Birt MC, Anderson DW, Bruce Toby E, et al. Osteomyelitis: recent advances in pathophysiology and therapeutic strategies. J Orthop 2017; 14(1): 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Issa K, Diebo BG, Faloon M, et al. The epidemiology of vertebral osteomyelitis in the United States from 1998 to 2013. Clin Spine Surg 2018; 31(2): E102–E108. [DOI] [PubMed] [Google Scholar]
- 3. Tyagi R. Spinal infections in children: a review. J Orthop 2016; 13: 254–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weiss L, Lansell A, Figueroa J, et al. Declining prevalence of methicillin-resistant Staphylococcus aureus septic arthritis and osteomyelitis in children: implications for treatment. Antibiotics 2020; 9: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lew DP, Waldvogel FA. Osteomyelitis. Lancet 2004; 364: 369–379. [DOI] [PubMed] [Google Scholar]
- 6. Fernandez M, Carrol CL, Baker CJ. Discitis and vertebral osteomyelitis in children: an 18-year review. Pediatrics 2000; 105(6): 1299–1304. [DOI] [PubMed] [Google Scholar]
- 7. Zimmerli W. Clinical practice: vertebral osteomyelitis. N Engl J Med 2010; 362: 1022–1029. [DOI] [PubMed] [Google Scholar]
- 8. Nickerson EK, Sinha R. Vertebral osteomyelitis in adults: an update. Br Med Bull 2016; 117(1): 121–138. [DOI] [PubMed] [Google Scholar]
- 9. Onwubalili JK. Sickle cell disease and infection. J Infect 1983; 7: 2–20. [DOI] [PubMed] [Google Scholar]
- 10. Berbari EF, Kanj SS, Kowalski TJ, et al. 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis 2015; 61: e26–e46. [DOI] [PubMed] [Google Scholar]
- 11. Vynichakis G, Chandrinos M, Angelis S, et al. Salmonella osteomyelitis of the proximal tibia in a previously healthy adolescent: a case report. Cureus 2019; 11: e5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murphy JB. Bone and joint disease in relation to typhoid fever. Surg Gynecol Obstet 1916; 23: 119–143. [Google Scholar]
- 13. Laughlin M, Bottichio L, Weiss J, et al. Multi-state outbreak of Salmonella Poona infections associated with imported cucumbers, 2015-2016. Epidemiol Infect 2019; 147: e270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fukushima K, Yanagisawa N, Sekiya N, et al. Bacteremia caused by Salmonella Poona in a healthy adult in Tokyo, Japan. Intern Med 2020; 59: 289–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Taxter AJ, Chauvin NA, Weiss PF. Diagnosis and treatment of low back pain in the pediatric population. Phys Sportsmed 2014; 42(1): 94–104. [DOI] [PMC free article] [PubMed] [Google Scholar]


