Abstract
The clinical presentation of children and adolescents infected with severe acute respiratory syndrome coronavirus 2 can range from asymptomatic to mild or moderate manifestations. We present a case series of three adolescents who presented during the coronavirus disease 2019 (COVID-19) pandemic with symptoms concerning for COVID-19, including fever, abdominal symptoms, cough, respiratory distress, and hypoxemia. Their laboratory results showed elevated inflammatory markers that are also commonly seen in COVID-19. The chest imaging studies mimicked COVID-19 with non-specific ground glass opacities and interstitial prominence patterns. However, severe acute respiratory syndrome coronavirus 2 testing was negative and further questioning of these adolescents and their parents revealed a history of vaping marijuana-related products leading to the eventual diagnosis of e-cigarette, or vaping, product use–associated lung injury. Our patients were successfully treated with corticosteroids. The providers caring for pediatric patients, especially adolescents, should continue to have a high index of suspicion for e-cigarette, or vaping, product use–associated lung injury in patients presenting with unexplained respiratory failure, while ruling out COVID-19.
Keywords: Toxicology, respiratory medicine, critical care/emergency medicine, e-cigarette, or vaping, product use–associated lung injury, COVID-19, adolescents
Introduction
The coronavirus disease 2019 (COVID-19) pandemic caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is mostly known to cause mild to moderate manifestations in children.1 However, severe manifestations and fatalities are reported even in children, hence the need for increased awareness for possible COVID-19 when children and adolescents present with unexplained respiratory distress or respiratory failure.2 We introduce a case series of teenagers presenting with respiratory distress secondary to e-cigarette, or vaping, product use–associated lung injury (EVALI) closely mimicking the presentation of COVID-19.
Case 1
A 16-year-old Caucasian female with history of hypothyroidism and migraines presented to our pediatric intensive care unit (PICU) from an outside emergency department (ED) during late February 2020, with 10 days of dry cough, 3 days of fever, nausea, and vomiting. She was tachycardic (heart rate (HR): 116–128 beats/min), tachypneic (respiratory rate (RR): 26–48 breaths/min), and hypoxemic (saturation measured by pulse oximeter (SpO2): 89%) on admission with diminished breath sounds at the lung bases. The rest of her exam was within normal limits. The laboratory findings showed elevated white blood cell (WBC) count and inflammatory markers (Table 1). The common respiratory viral panel (RVP) and blood cultures were negative. Her initial chest radiograph (CXR) showed bilateral perihilar opacities and ill-defined left basilar opacity, suspicious for early pneumonia, which rapidly progressed in just more than 24 h to prominent bibasilar hazy opacities (Figure 1). The patient was started on supplemental oxygen and doxycycline for suspected atypical pneumonia that was soon changed to ceftriaxone and vancomycin due to worsening respiratory distress needing noninvasive positive airway pressure (NIPPV) support. At that time, given the heightened concern for COVID-19, communication was initiated with the Centers for Disease Control and Prevention (CDC) for COVID-19 testing as the testing resources were limited and testing decisions were made in conjunction with CDC at the beginning of the pandemic. It was decided to hold the testing since there was no history of travel or exposure to SARS-CoV-2. Simultaneously, the patient’s mother raised concerns about a possible vaping history since she had found marijuana-based vaping cartridges in the patient’s bedroom and revealed that the patient had positive tetrahydrocannabinol (THC) in her urine 2 weeks prior to presentation. Pulmonary consultation and further investigations such as urine toxicology, computerized tomography (CT) chest, and bronchoscopy were ordered at this time. The CT chest showed prominent bilateral ground glass and patchy consolidative opacities in the posterior lungs with areas of mild diffuse bronchial wall thickening and subpleural sparing (Figure 2). A bronchoscopy showed mild bronchial wall inflammation; the bronchoalveolar lavage (BAL) showed neutrophil predominance with negative cultures. Meanwhile, patient’s urine toxicology returned positive for THC suggesting recent marijuana use and supporting the diagnosis of EVALI. The patient admitted to vaping with her friends several months prior to the presentation but denied recent use. We assumed that the patient was lying that this was in fact a case of EVALI and started her on methylprednisolone 1 mg/kg/dose twice a day for 5 days. She showed significant improvement in clinical symptoms within 48 h. She was offered smoking cessation counseling and was discharged home with close primary care provider and pulmonologist monitoring.
Table 1.
Laboratory values for all three patients.
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| WBC (k/mm3) | 18.9 | 23.5 | 17.0 |
| Neutrophils (%), lymphocytes (%), monocytes (%), eosinophils (%), basophils (%) | 96, 2, 1, 0, 0 | 93, 6, 1, 0, 0 | 95.8, 3, 1, 0, 0.2 |
| Hemoglobin (g/dL)/hematocrit (%) | 12.0/35.2 | 12.8/37 | 12.6/37.7 |
| Platelets (k/mm3) | 344 | 412 | 334 |
| Alanine transferase, ALT (U/L) | 11 | 13 | 34 |
| Aspartate transferase, AST (U/L) | 22 | 23 | 35 |
| Total bilirubin (mg/dL) | 0.6 | 1 | < 0.3 |
| CRP (mg/dL) | 29.4 | 33.5 | 35.4 |
| Procalcitonin (ng/mL) | 3.17 | Not done | 0.81 |
| Blood gas (pH/pCO2/pO2/HCO3−) | 7.45/36/75/25 | 7.39/33/79/20 | 7.43/36/108/24 |
| Respiratory viral panela | Negative | Negative | Negative |
| Blood culture | Negative | Negative | Negative |
| SARS-CoV-2 RNAb | Not tested | Negative | Negative |
| BAL cell count (total count, % polys, % lymphocytes, % histiocytes, % macrophages) | 700, 50, 4, 12, 34 | Not done | 798, 86, 1, 10, 3 |
| BAL lipid laden macrophages (Oil red O stain) | <1% | Not done | 15%–20% |
| BAL cultures (bacterial, fungal, and mycobacterial) | Negative | Not done | Negative |
| Legionella cultures (BAL) | Negative | Not done | Negative |
| Streptococcal antigen (Urine) | Not done | Not done | Negative |
BAL: bronchoalveolar lavage; CRP: C-reactive protein; WBC: white blood cell.
Respiratory viral panel includes influenza A, influenza B, respiratory syncytial virus (RSV), parainfluenza, coronavirus (not including SARS-CoV-2), human metapneumovirus, rhinovirus/enterovirus, adenovirus, Chlamydia pneumoniae, Mycoplasma pneumoniae.
Internally developed Roche Diagnostics Cobas 6800 System that has 91% sensitivity and 100% specificity.
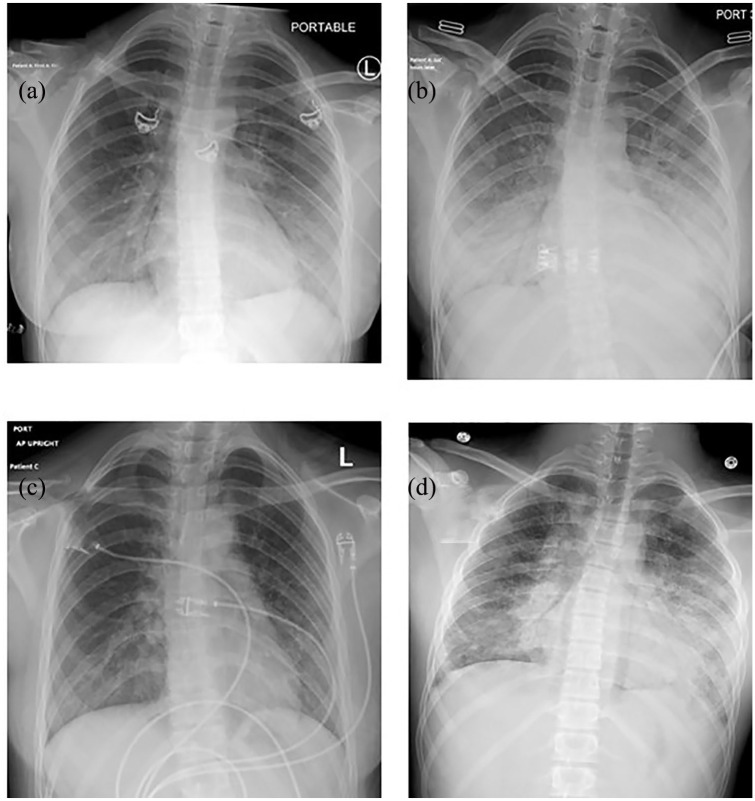
Figure 1.
Chest radiographs for case 1 (images a and b with just more than 24 h in between the two radiographs) demonstrating bilateral perihilar opacities and an ill-defined opacity at the left base (a), which rapidly progressed to prominent bibasilar hazy opacities (b). Chest radiograph for case 2 (image c) displays prominent perihilar and bibasilar interstitial opacities with subtle diffuse background reticular and linear interstitial prominence. Chest radiograph for case 3 (image d) shows bilateral perihilar and bibasilar consolidative and patchy opacities, left more so than right, with more diffuse prominence of the reticular pattern.
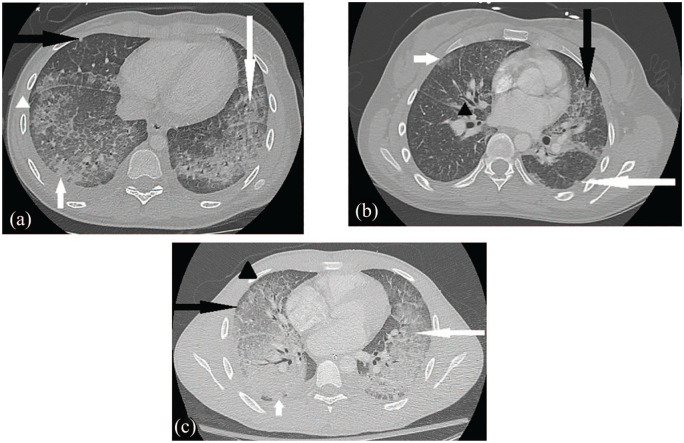
Figure 2.
Chest CT for case 1 (image a) demonstrates ground glass opacities (long white arrow), patchy consolidation (short white arrow), crazy paving appearance (white arrowhead) which describes ground glass opacities with septal thickening, nodular foci (black arrow), and subpleural sparing (also white arrowhead). Chest CT for case 2 (image b) displays scattered mostly peripheral ground glass lung opacities (short white arrow) with nodules (long white arrow), septal thickening (black arrow), and peribronchial wall thickening (black arrowhead). Chest CT for case 3 (image c) shows interlobular septal thickening (black arrowhead) and alveolar ground glass opacities (white arrow) throughout much of the lungs with notable subpleural sparing (black arrow), more consolidative opacities in dependent lung bases (small white arrow), and small bilateral pleural effusions.
Case 2
A 17-year-old Hispanic female with well-controlled asthma and seasonal allergies was transferred to our PICU for respiratory distress in early April 2020. She presented with 8 days of sore throat, cough, and pleuritic chest pain that was unresponsive to home albuterol. She additionally complained of 2 days of fever, post-tussive emesis, and diarrhea. Her physical examination was significant for tachycardia (HR: 122–134 beats/min), tachypnea (RR: 30–37 breaths/min) with bilateral rhonchi and hypoxemia (SpO2: 88% on room air). Her laboratory results showed elevated WBC and inflammatory markers (Table 1). Her CXR showed prominent perihilar and bibasilar interstitial opacities (Figure 1). A CT chest performed for tachycardia and chest pain, to exclude pulmonary embolism, showed scattered mostly peripheral ground glass and nodular lung opacities with septal thickening and peribronchial wall thickening (Figure 2). Her presentation raised concerns for COVID-19. The infectious workup included negative SARS-CoV-2 and RVP testing, as well as negative blood cultures (Table 1). She was appropriately isolated and treated with bronchodilators, supplemental oxygen and azithromycin, ceftriaxone, and vancomycin for community-acquired pneumonia. However, her symptoms progressed despite the treatment. With further questioning, she admitted to vaping nicotine-free marijuana-based pods, multiple times per week for 4–5 months prior to presentation. With this newly elicited vaping history, EVALI was diagnosed and she was started on 5 days of prednisone at 1 mg/kg twice daily. She received smoking cessation counseling and was advised to follow-up closely with her primary care physician and pulmonologist, then was discharged within 48 h.
Case 3
A 17-year-old previously healthy mixed-race male was transferred to our pediatric wards for respiratory distress in late April 2020. He presented with 5 days of fever, cough, post-tussive emesis, dyspnea, pleuritic chest pain, myalgia, and decreased appetite. When his symptoms first started, he had gone to the ED where he was diagnosed with community-acquired pneumonia and prescribed amoxicillin. He returned to the ED with worsening symptoms and was found to be febrile to 39.1°C, tachycardic (HR: 90–133 beats/min), and tachypneic (RR: 26–32 breaths/min) with hypoxemia (SpO2: 85%), raising suspicion for COVID-19. On initial exam, he was noted to be in significant respiratory distress with bilateral diminished breath sounds and coarse crackles. The laboratory findings were remarkable for elevated WBC with neutrophilia, elevated inflammatory markers, negative SARS-CoV-2, RVP, and blood cultures (Table 1). His CXR showed bilateral perihilar and bibasilar consolidative patchy opacities, more on the left side, with diffuse prominence of the reticular pattern (Figure 1). He endorsed intermittent use of THC-vaping products that he had acquired from his friends, 1 month prior to presentation raising concern for EVALI. His respiratory distress quickly worsened, and he required PICU level of care for NIPPV and eventual intubation. He was started on azithromycin, ceftriaxone, and vancomycin for community-acquired pneumonia. A bronchoscopy performed by the pulmonary service on day 2 of hospitalization showed moderate-to-severe airway inflammation, a neutrophil predominant BAL sample and negative cultures. His CT chest demonstrated diffuse alveolar ground glass opacities throughout the lungs with prominent subpleural sparing, septal thickening, bilateral small pleural effusions, and bibasilar dependent consolidation (Figure 2). The patient was started on 7 days of methylprednisolone 1 mg/kg twice daily due to high suspicion for EVALI. The patient was able to be extubated after 5 days of corticosteroids and was discharged home on fluticasone/salmeterol 115–21 μg twice daily with recommendation for close follow-up with his primary care physician and pulmonologist.
Discussion
These cases serve to remind us that the EVALI outbreaks persist and there is potential for increased incidence with social distancing and isolation precautions implemented during the current COVID-19 pandemic. There are similarities between EVALI and COVID-19 in clinical symptomatology, laboratory results, and radiologic findings. EVALI, although not suspected on initial presentation with our cases, was revealed upon further questioning and elicitation of a vaping history.
Since March 2019, there have been outbreaks of EVALI defined as using an e-cigarette (“vaping”) or dabbing within 90 days of symptom onset, and pulmonary infiltrate such as opacities on CXR or ground glass opacities on chest CT, and absence of pulmonary infection on initial work up and no evidence of alternative plausible diagnoses (e.g. cardiac, rheumatological, or neoplastic processes).3 Since the introduction of e-cigarettes in the United States in 2007, the incidence of vaping has increased significantly in adolescents, likely due to recent popularity of nondescript e-cigarettes shaped like a USB flash drive as well as flavors that appeal to youth. Approximately 4.9% of middle school students and 20.8% of high school students self-reported as current e-cigarette users, defined as use in the last 30 days.4 EVALI is most commonly associated with the use of cannabis-based vaping products, especially those obtained from informal sources like family, friends, and in-person or online dealers.5 Vitamin E acetate, an additive in some THC containing vaping products, is strongly linked to the EVALI outbreaks. The legalization of recreational marijuana use may have reduced the number of EVALI cases in some states, but most adolescents likely access these products from informal sources, thereby leading to continued outbreaks in this underage population.6,7 Since March 2019, over 2,600 EVALI cases and 60 deaths have been reported in the United States.8
During the EVALI outbreaks, COVID-19, a novel infectious disease caused by SARS-CoV-2, has emerged causing significant respiratory illness and death. The global outbreak of COVID-19 is a major public health issue that was declared a worldwide pandemic in March 2020. As of 14 September 2020, data from the CDC showed that there were 6,503,030 total cases and 193,705 total deaths in the United States.9 The clinical spectrum of COVID-19 varies from asymptomatic to respiratory failure requiring mechanical ventilation.10 Pediatric COVID-19 cases account for a small percentage of patients although the incidence is increasing; 2.4% of confirmed and suspected COVID-19 cases in China were reported in children as of February 2020.11 Children with COVID-19 generally present with milder symptoms when compared to adults.1,2
Both EVALI and COVID-19 share the clinical symptoms of fever, cough, and non-specific gastrointestinal symptoms such as nausea, abdominal pain, and diarrhea as well as the physical findings of respiratory distress and hypoxemia. Our cases presented with a varying range of pulmonary, gastrointestinal, and constitutional symptoms. The pulmonary symptoms were most striking with rapid progression within 48 h in two out of the three patients. There is elevation of inflammatory markers as well as mild transaminitis in both conditions, though leukocytosis and neutrophilia are more common in EVALI.3,12 Bilateral multifocal ground glass opacities, with or without consolidation, and lower lobe predominance are seen on chest CT imaging in both conditions.13 Subpleural sparing and possible “atoll sign” (central ground glass opacity surrounded by dense consolidation of crescentic shape) are commonly reported in EVALI, whereas more peripheral and subpleural distribution of opacities (“halo sign”) is common in COVID-19, though these findings are often intermixed.13–15 These similarities reinforce the importance of eliciting any vaping history in adolescents who present with unexplained respiratory distress to differentiate the underlying diagnosis. The treatment of EVALI differs from COVID-19, and early initiation of steroids in EVALI can be lifesaving and may minimize the duration of hospital stay as was seen with our cases.16,17
With implementation of social distancing and isolation precautions due to COVID-19, many adolescents are attending school remotely. Isolation from the school environment, loneliness, stress of the current pandemic, and lack of social support could increase potential for substance use including e-cigarette use in adolescents, particularly those with pre-existing mental health conditions.18 Therefore, during this COVID-19 pandemic, it is exceedingly important to obtain a thorough social history including use of e-cigarettes and vaping products, in adolescents presenting with signs and symptoms suspicious for EVALI. Clinicians should continue to advise against the use of e-cigarettes and vaping products as abstinence remains the best way to prevent lung injury. At the same time, there is no recommendation for withholding COVID-19 testing, as EVALI is a diagnosis of exclusion, and EVALI and COVID-19 may co-exist in some cases. Social distancing and isolation precautions should continue to be enforced to limit the spread of COVID-19 during the pandemic.
Conclusion
During the COVID-19 pandemic, providers caring for pediatric patients, especially adolescents, should continue to have a high index of suspicion for EVALI in patients presenting with unexplained respiratory failure, while excluding COVID-19. Providers should ask for pertinent social history and pursue the appropriate diagnostic routes as treatment of EVALI differs from COVID-19. The early initiation of steroids in EVALI can shorten the duration of illness and hospital stay. Appropriate vaping cessation counseling and close outpatient monitoring are needed to reduce the risk of recurrence of EVALI.
Footnotes
Author contributions: D.O.D. contributed to acquisition, drafted, and critically revised manuscript. K.G., B.D.G., and S.J. contributed to acquisition, critically revised manuscript. K.N. contributed to conception and design, and acquisition, drafted, and critically revised manuscript. All authors gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
IRB number: Our Institution does not require IRB approval for case reports or case series. We have obtained written informed consent from our patients as well as their parents for publishing their anonymized information in this article.
ORCID iD: Kiran Nandalike  https://orcid.org/0000-0002-0176-5617
https://orcid.org/0000-0002-0176-5617
References
- 1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. J Am Med Assoc, 2020, https://www.practiceupdate.com/content/characteristics-of-and-important-lessons-from-the-covid-19-outbreak-in-china/97018 [DOI] [PubMed]
- 2. Coronavirus Disease. 2019 in Children—United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep 2020; 69(14): 422–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Layden JE, Ghinai I, Pray I, et al. Pulmonary illness related to E-cigarette use in Illinois and Wisconsin—final report. N Engl J Med 2020; 382(10): 903–916. [DOI] [PubMed] [Google Scholar]
- 4. Cullen KA, Ambrose BK, Gentzke AS, et al. Notes from the field: use of electronic cigarettes and any tobacco product among middle and high school students—United States, 2011-2018. MMWR Morb Mortal Wkly Rep 2018; 67(45): 1276–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Navon L, Jones CM, Ghinai I, et al. Risk factors for E-cigarette, or vaping, product use-associated lung injury (EVALI) among adults who use E-cigarette, or vaping, products—Illinois, July-October 2019. MMWR Morb Mortal Wkly Rep 2019; 68(45): 1034–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wing C, Bradford AC, Carroll AE, et al. Association of state marijuana legalization policies for medical and recreational use with vaping-associated lung disease. JAMA Netw Open 2020; 3(4): e202187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ellington S, Salvatore PP, Ko J, et al. Update: product, substance-use, and demographic characteristics of hospitalized patients in a nationwide outbreak of E-cigarette, or vaping, product use-associated lung injury—United States, August 2019-January 2020. MMWR Morb Mortal Wkly Rep 2020; 69(2): 44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Werner AK, Koumans EH, Chatham-Stephens K, et al. Hospitalizations and deaths associated with EVALI. N Engl J Med 2020; 382(17): 1589–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. 2020,https://www.cdc.gov/coronavirus/2019-ncov/
- 10. Pascarella G, Strumia A, Piliego C, et al. COVID-19 diagnosis and management: a comprehensive review. J Intern Med 2020; 288(2): 192–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hasan A, Mehmood N, Fergie J. Coronavirus disease (COVID-19) and pediatric patients: a review of epidemiology, symptomatology, laboratory and imaging results to guide the development of a management algorithm. Cureus 2020; 12(3): e7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang B, Liu S, Zhang J, et al. Children hospitalized for coronavirus disease 2019 (COVID-19): a multicenter retrospective descriptive study. J Infect 2020; 81(2): e74–e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Foust AM, Winant AJ, Chu WC, et al. Pediatric SARS, H1N1, MERS, EVALI, and now coronavirus disease (COVID-19) pneumonia: what radiologists need to know. AJR Am J Roentgenol 2020; 215(3): 736–744. [DOI] [PubMed] [Google Scholar]
- 14. Artunduaga M, Rao D, Friedman J, et al. Pediatric chest radiographic and CT findings of electronic cigarette or vaping product use-associated lung injury (EVALI). Radiology 2020; 295: 192778. [DOI] [PubMed] [Google Scholar]
- 15. Panse PM, Feller FF, Butt YM, et al. Radiologic and pathologic correlation in EVALI. AJR Am J Roentgenol 2020; 2: 1–8. [DOI] [PubMed] [Google Scholar]
- 16. Jatlaoui TC, Wiltz JL, Kabbani S, et al. Update: interim guidance for health care providers for managing patients with suspected E-cigarette, or vaping, product use-associated lung injury—United States, November 2019. MMWR Morb Mortal Wkly Rep 2019; 68(46): 1081–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Galo J, Celli D, Gross D, et al. A presentation of E-cigarette vaping associated lung injury (EVALI) caused by THC-containing electronic smoking device. Respir Med Case Rep 2020; 31: 101154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zuckermann AME, Williams GC, Battista K, et al. Prevalence and correlates of youth poly-substance use in the COMPASS study. Addict Behav 2020; 107: 106400. [DOI] [PubMed] [Google Scholar]