Abstract
Background:
Because high failure rates have frequently been reported after arthroscopic rotator cuff repair (ARCR) of massive rotator cuff tears (mRCTs), we introduced the technique of ARCR with supraspinatus and infraspinatus muscle advancement (MA). However, for cases where the original footprint cannot be completely covered, additional surgery using an approved artificial biomaterial is performed.
Purpose:
To investigate the postoperative clinical outcomes and failure rate after MA-ARCR, with and without our reinforcement technique.
Study Design:
Cohort study; Level of evidence, 3.
Methods:
A total of 74 patients (mean ± SD age, 68.7 ± 7.7 years) diagnosed with mRCT with a minimum postoperative follow-up of 2 years were included in the current study. Of these patients, 47 underwent MA-ARCR with polyglycolic acid (PGA) sheet reinforcement (study group), and 27 patients underwent MA-ARCR alone (control group). PGA reinforcement was performed when full coverage of the footprint could not be achieved by MA alone, but where the latter was possible, reinforcement was not required. Thus, the study group had significantly worse muscle quality than the control group (P < .05). The pre- and postoperative range of motion (ROM), isometric muscle strength, acromiohumeral interval, and clinical outcomes were evaluated and compared between these 2 groups. Cuff integrity during the last follow-up period was assessed with magnetic resonance imaging, and the failure rate was calculated. In addition, the postoperative foreign body reaction was investigated in the study group.
Results:
In both groups, significant postoperative improvements were seen in acromiohumeral interval, clinical scores, ROM in anterior flexion, and isometric muscle strength in abduction, external rotation, and internal rotation (P < .001 for all). The failure rate of the study group was 12.8% (6 patients) and that of the control group was 25.9% (7 patients). No significant differences were noted between the 2 groups on any of the data findings, even regarding the failure rate. Foreign body reactions in the early period were found in 3 patients, although these spontaneously disappeared within 3 months.
Conclusion:
Patients who underwent PGA patch reinforcement for MA-ARCR when the footprint could not be completely covered had clinical results similar to isolated MA-ARCR when the footprint could be covered. Both procedures resulted in significant improvement in symptoms and function compared with preoperatively.
Keywords: massive rotator cuff tear, muscle advancement, artificial degradable biomaterial, reinforcement
Rotator cuff tears (RCTs) are among the main causes of shoulder pain, and they occur frequently in elderly people and athletes. Repair of RCTs has been shown to improve pain and restore shoulder function. Although good to excellent clinical outcomes are reported by arthroscopic rotator cuff repair (ARCR) for small- to medium-sized RCTs, in the case of massive-sized RCTs (mRCTs), high rates of failure (retear on magnetic resonance imaging [MRI] scans) have been reported.7,14,18,43 Because previous reports have shown a direct correlation between postoperative clinical outcomes and anatomic healing of RCTs, reduction of the failure rate after ARCR might be crucial to achieving an excellent outcome.19 We therefore devised a method of treatment using ARCR with supraspinatus (SSP) and infraspinatus (ISP) muscle advancement (MA-ARCR) to perform complete repair for mRCTs.47 The purpose of the procedure is to reduce the failure rate by reducing tension on the tendons during repair.8 However, the procedure is not effective for all cases, and there are still some cases where the original footprint cannot be completely covered even after MA-ARCR.
In previous reports, various factors have been cited as being the cause of failure after ARCR for mRCTs, including the low healing capacity of repaired cuff tendons38 and the concentration of tension on the tendons undergoing repair.15 Previous reports have described the use of reinforcing materials to enhance the repair capability4,5,11,33–35,38,39 and to reduce excessive tension at the repair site.1,6,24 Previously, it was reported that a tendon-like insertion was regenerated by the use of an artificial biodegradable material, a polyglycolic acid (PGA) sheet at the site of the rotator cuff defect in a rabbit model.49 The PGA sheets can potentially serve not only as a reinforcing material for the repair site but also as a source of rotator cuff repair enhancement and regeneration. This gave us the idea of using this PGA sheet for reinforcement and enhancement of rotator cuff tendon healing. The purpose of our study was to investigate the clinical and radiologic outcomes and failure rates of PGA sheet reinforcement for rotator cuff healing after MA-ARCR.
Methods
This study was approved by our institutional review board. We retrospectively evaluated patients who had undergone MA-ARCR with or without PGA sheet reinforcement for mRCTs (≥2 tendon tears involved, as defined by Gerber et al16) between October 2011 and December 2017. The exclusion criteria were as follows: isolated subscapularis (SSC) or SSP tendon tear, post–acute trauma cases, revision cases after repair failure or infection, RCT with a neurologic lesion (eg, cervical problems), and osteoarthritis or rheumatoid arthritis. We also excluded patients who showed indications (according to Japanese guidelines) of reverse shoulder arthroplasty, such as anterosuperior escape of the humeral head, Hamada classification grade ≥4,17 or high fatty degeneration (grade 4 Goutallier classification on MRI12) of both SSP and ISP muscles >70 years of age for Japanese RSA indication. Isolated MA-ARCR was performed only for cases where full coverage of the footprint was possible by adding muscle advancement alone, and MA-ARCR with PGA sheet reinforcement was performed for cases where full coverage was impossible by muscle advancement. The final composition of the study group was 47 patients who underwent MA-ARCR with PGA sheet reinforcement; the control group consisted of 27 patients who underwent MA-ARCR alone. The descriptive data are summarized in Table 1. Tendon retraction was categorized according to the Boileau classification system,42 SSC tendon findings were categorized according to the Lafosse method of classification,26 the long head of biceps brachii (LHB) findings were categorized using a modified version of the Lafosse classification method,25 and the degree of fatty degeneration was categorized according to the Goutallier classification system.12 Furthermore, the global fatty degeneration index (GFDI) was calculated according to the report by Fuchs et al.12
Table 1.
Descriptive Data of the Study Group and the Control Groupa
| Variable | Study Group (n = 47) | Control Group (n = 27) | P Value |
|---|---|---|---|
| Sex | .28 | ||
| Male | 27 | 12 | |
| Female | 20 | 15 | |
| Age, y | 68.3 ± 8.1 | 69.4 ± 7.1 | .73 |
| Affected arm | .91 | ||
| Right | 36 | 21 | |
| Left | 11 | 6 | |
| Supraspinatus retraction | .03b | ||
| Stage 3 | 21 | 19 | |
| Stage 4 | 26 | 8 | |
| Subscapularis lesion | .32 | ||
| Type 0 | 8 | 1 | |
| Type 1 | 15 | 11 | |
| Type 2 | 13 | 11 | |
| Type 3 | 10 | 4 | |
| Type 4 | 1 | 0 | |
| Type 5 | 0 | 0 | |
| Long head of biceps brachii lesion | .42 | ||
| Grade 1 | 9 | 5 | |
| Grade 2 | 7 | 8 | |
| Grade 3 | 10 | 2 | |
| Grade 4 | 12 | 7 | |
| Grade 5 | 9 | 5 | |
| Fatty degeneration, grade | |||
| Subscapularis | 1.3 ± 1.5 | 0.9 ± 1.0 | .5 |
| Supraspinatus | 2.1 ± 1.2 | 1.5 ± 0.6 | .02b |
| Infraspinatus | 2.0 ± 1.3 | 1.1 ± 0.9 | .01b |
| Global fatty degeneration index | 1.8 ± 1.0 | 1.2 ± 0.5 | .01b |
| Follow-up period, mo | 24.2 ± 1.0 | 24.3 ± 0.7 | .12 |
aValues are expressed as number of participants or mean ± SD.
bSignificant difference between the study group and the control group (P < .05).
Surgery
Arthroscopic Assessment of the Cuff Excursion
For mRCTs, we performed a release of the superficial and deep layers of the torn cuff where possible using a radiofrequency device. We then used a suture hook to penetrate the stump of the rotator cuff with No. 0 nylon string, pulling each thread outward with a force of 30 N measured by a tension meter. We then checked whether the stump could cover the entire footprint at the position of 30° of abduction (Figure 1). The number of threads was usually 3, depending on the tear size. The value of 30 N was determined from the study by Davidson and Rivenburgh,8 which showed that failure often occurs in repairs with a force of ≥30 N.
Figure 1.

Arthroscopic findings after cuff release. Each No. 0 nylon string was pulled outward through the cuff stump with a force of 30 N measured by a tension meter. We then checked whether the stump could cover the entire footprint at 30° of abduction.
Suprascapular Nerve Release
After confirming that full coverage of the footprint was not possible, we proceeded with arthroscopic suprascapular nerve (SSN) release under the same procedure as previously reported47 before performing muscle advancement. When an electrothermal device or shaver was used to progress inward along the anterior edge of the SSP muscle, the superior transverse scapular ligament, which runs across the scapular notch, was detected. Then, arthroscopic SSN release was performed by cutting the ligament with a blunt switching rod.
Muscle Advancement
Next, we moved to mini-open SSP and ISP muscle advancement, as previously reported.47 A 4-cm transverse skin incision was applied along the medial border of the scapular spine. The trapezius was released from the spine, and the SSP and ISP muscle belly was elevated from the scapular body without any continuity between the rhomboid muscles (Figure 2). Care was taken not to injure the SSN on the lateral aspect. This procedure enabled us to mobilize the retracted cuff tendon by about 2 cm.
Figure 2.
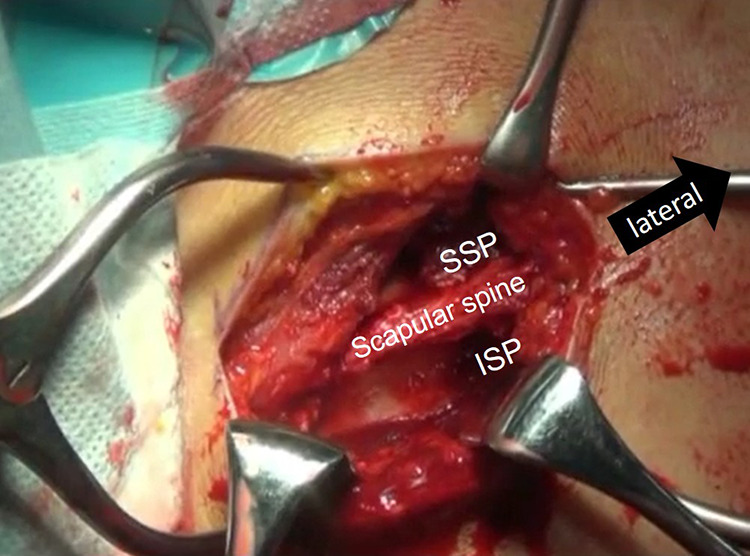
Muscle advancement surgery being performed. After removal of the trapezius muscle from the scapular spine, the supraspinatus (SSP) and infraspinatus (ISP) muscles were elevated bluntly, with care taken not to injure the suprascapular nerve. Black arrow indicates lateral direction.
Cuff Repair
After footprint decortication was performed, 2 or 3 double-loaded medial anchors (Healicoil PK or RG suture anchor; Smith & Nephew Endoscopy) were inserted at regular intervals, depending on the tear size, along the medial edge of the footprint. No medialization of the footprint was performed in these patients. All 4 suture limbs from each anchor penetrated the cuff tendons through use of a retrograde retrieving device such as the Banana Lasso (Arthrex). Then, we performed our modified medial double-pulley technique (Figure 3A) (described in a previous study47), so as to avoid too-frequent penetration of the cuff tendons and to increase the contact area and pressure between the sutures and cuffs. The suture limbs of the double pulley were locked by pulling each of the limbs in opposite directions from each of the corresponding anchor knots (Figure 3B).
Figure 3.
(A) Schematic drawing of a modified double pulley. (B) Picture of tied suture limbs of each medial anchor, forming a modified double pulley. Black arrows indicate the directions for locking the knots from each anchor.
PGA Reinforcement
For patients in whom the footprint could not be completely covered even after we performed muscle advancement, a double-folded 0.5–mm thick PGA sheet (Neoveil; Gunze Medical) was inserted along the sutures of the medial anchors and spread over the cuff tendons, with the aim of dispersing the stress on the cuff with thread or tape and repair reinforcement. The size of the PGA sheet was 20-30 mm × 20 mm, depending on the area of the footprint uncovered with the cuff stump. We fixed the torn tendon and PGA sheet according to the suture-bridge technique so that the repair site could be reinforced by the sheet (Figures 4 and 5).
Figure 4.
Arthroscopic findings during polyglycolic acid (PGA) reinforcement. (A) The torn cuff stump was well-mobilized after the muscle advancement. (B) Suture limbs from the medial anchors were retrieved from the lateral portal. (C) The sheet was inserted along the suture limbs of the medial anchors. (D) The torn tendon and PGA sheet were fixed together by the bridging sutures.
Figure 5.

Schematic drawing of the suture bridge with polyglycolic acid (PGA) reinforcement.
Rehabilitation
After surgery, all patients wore abduction braces. Postoperative rehabilitation was performed using the same protocol for both groups as follows: Passive range of motion (ROM) was commenced from 1 week, active ROM began at 4 weeks, and abduction braces were removed at 6 weeks. When biceps tenotomy or tenodesis was performed, elbow ROM was prohibited for 3 weeks. Rotator cuff and deltoid muscle strengthening exercises were permitted from 12 weeks postoperatively.
Evaluation
Authors (Y.H., H.N, R.M, N.M.) who were not involved in the surgery and who were blinded as to the procedure evaluated the pre- and postoperative ROM in anterior flexion (AF), external rotation (ER), and internal rotation (IR). The IR was expressed as the highest vertebral level able to be reached, and this was converted to a numeric value. Furthermore, the quantitative isometric muscle strengths of abduction, ER, and IR were measured by a handheld dynamometer (MicroFet 2; Nihon Medix Co Ltd). Using previously reported methods,47 we measured the muscle strength in seated patients; abduction strength was measured at 45° of abduction in the scapular plane, and the ER and IR strengths were measured with the patient’s upper arm at the side of body and the elbow at 90° of flexion in ER/IR neutral position. We also compared the pre- and postoperative clinical outcomes using the Constant score and the University of California Los Angeles (UCLA) Shoulder Rating Scale in both groups.
For improvement of superior migration, the pre- and postoperative acromiohumeral interval (AHI) was measured from the true anteroposterior view on plain radiographs taken in a standing and ER/IR neutral position. Using a previously reported mothod,47 we defined the AHI as the shortest distance between the undersurface of the acromion and the top of the humeral head.
MRI scans performed at the final follow-up, together with the Sugaya classification method, were used to calculate and evaluate the failure rate and cuff integrity in both groups. Sugaya types 4 and 5 were regarded as failures. Postoperative evaluation of all findings was carried out at 2 years after surgery. In the study group, we also investigated whether there were cases of foreign body reactions that occurred around 2 months after surgery, including fever, shoulder swelling, or elevated C-reactive protein with normal white blood cell count. Such reactions usually decrease after a couple of weeks with the usual anti-inflammatory and analgesic treatment without any antibiotics. We therefore regarded this as a foreign body reaction, not as an infection.
Statistical Analysis
For the statistical analyses, the descriptive data were assessed via the chi-square test and Fisher exact test for categorical variables and the Mann-Whitney U test for numeric variables. Preoperative and postoperative values were compared statistically using the paired t test. All findings for the study group and the control group were compared using the Mann-Whitney U test, except those relating to the failure rate. This comparison was performed using the chi-square test. P < .05 was set as a significant difference.
Results
According to the patients’ descriptive data (Table 1), the study group had a significantly more severe SSP retraction according to the Boileau classification method (P = .03) and more severe fatty degeneration of the SSP, ISP, and GFDI (P = .02, .01, and .01, respectively) according to the Goutallier classification system, when compared with the control group. No significant differences were seen between the 2 groups regarding preoperative ROM, muscle strength, clinical outcomes, or AHI (Table 2). In total, 29 patients from the study group underwent LHB surgery (21 tenotomy and 8 tenodesis), and 12 patients from the control group underwent the same surgery (10 tenotomy and 2 tenodesis).
Table 2.
Pre- and Postoperative Values of Range of Motion, Isometric Muscle Strength, Clinical Scores, and AHIa
| Variable | Study Group | Control Group | P Value (Study vs Control) | |||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| Range of motion | ||||||
| AF angle, deg | 111 ± 43 | 140 ± 26b | 122 ± 37 | 148 ± 21b | .23 | .21 |
| ER angle, deg | 39.8 ± 29.3 | 42.7 ± 22.3 | 51.4 ± 22.3 | 50.2 ± 13.6 | .08 | .16 |
| IR level, Th spine | 11.3 ± 3.1 | 11.4 ± 2.5 | 12.2 ± 2.7 | 11.8 ± 2.4 | .15 | .65 |
| Muscle strength, N | ||||||
| Abduction | 26.0 ± 17.5 | 44.8 ± 22.6b | 23.3 ± 15.6 | 41.7 ± 21.4b | .61 | .61 |
| ER | 31.4 ± 22.4 | 49.7 ± 23.7b | 26.3 ± 14.9 | 48.9 ± 21.7b | .69 | .79 |
| IR | 80.0 ± 43.2 | 103 ± 44.0b | 61.2 ± 28.0 | 91.0 ± 38.3b | .14 | .2 |
| Clinical outcomes | ||||||
| Constant score | 41.2 ± 16.1 | 70.4 ± 18.0b | 42.7 ± 15.0 | 69.3 ± 14.3b | .33 | .59 |
| UCLA score | 14.6 ± 5.2 | 29.6 ± 6.4b | 12.6 ± 3.1 | 29.0 ± 6.4b | .23 | .72 |
| AHI, mm | 6.9 ± 2.8 | 9.1 ± 2.7b | 7.4 ± 2.0 | 9.3 ± 2.3b | .4 | .99 |
aValues are expressed as mean ± SD. AF, anterior flexion; AHI, acromiohumeral interval; ER, external rotation; IR, internal rotation; Pre, preoperative; Post, postoperative; Th spine, thoracic spine; UCLA, University of California Los Angeles.
bSignificant difference between the pre- and postoperative values (P < .001).
The follow-up was at a mean of 24.2 ± 1.0 months (range, 24-30 months) in the study group and 24.3 ± 0.7 months (range, 24-26 months) in the control group. Although the postoperative AF ROM in both the study and control groups showed a significant improvement compared with preoperative values (P < .001 for both groups), neither ER nor IR ROM showed any significant improvement after surgery (P = .32 in the study group; P = .63 in the control group). Muscle strength in abduction, ER, and IR improved significantly after surgery in both groups (P < .001 for all). Similarly, the Constant and UCLA scores as well as the AHI improved significantly after surgery in both groups (P < .001 for all) (Table 2). Comparisons between the study group and the control group after surgery resulted in no significant differences regarding any of the variables (Table 2).
The results of cuff integrity are shown in Table 3. MRI appearance for healed rotator cuffs in the study group was similar to that in the control group. Sugaya types 4 and 5, regarded as failures, comprised 12.8% (6 failures) of the study group and 25.9% (7 failures) of the control group. Figure 6 shows the arthroscopic findings in a patient at second-look surgery 3 months after MA-ARCR with PGA sheet reinforcement. This patient underwent osteosynthesis because of an acromion fracture 2 months after the MA-ARCR surgery (performed with patient consent).
Table 3.
Results of Cuff Integrity According to Sugaya Classification
| Variable | Study Group (n = 47) | Control Group (n = 27) |
|---|---|---|
| Sugaya classification, n | ||
| Type 1 | 3 | 4 |
| Type 2 | 31 | 13 |
| Type 3 | 7 | 3 |
| Type 4 | 0 | 2 |
| Type 5 | 6 | 5 |
| Failure rate, %a | 12.80 | 25.90 |
aThe failure rate between groups was not significant (P = .21).
Figure 6.

Arthroscopic image taken at the second-look surgery 3 months after polyglycolic acid sheet reinforcement.
Overall, 3 of the 47 patients (6.4%) in the study group had fever and swelling of the affected shoulder and elevated C-reactive protein (average, 3.52 mg/dL; range, 2.14-15.44 mg/dL), which seemed to signify a foreign body reaction due to PGA and which started around 2 to 3 months after surgery.. However, the symptoms disappeared spontaneously in 1 to 2 weeks and did not leave any sequelae such as osteolysis.
Discussion
This is the first study to examine the postoperative results of ARCR with muscle advancement and artificial biodegradable materials as a reinforcement for mRCTs. This technique significantly improved not only the ROM of anterior flexion, isometric muscle strength, and clinical results but also the AHI, which was the sole indication of the humeral head’s depressor effect. Although the PGA reinforcement group had a significantly more severe preoperative rotator cuff pathology, the same failure rate could be achieved as in the group in which complete coverage was possible with MA-ARCR alone, despite relatively modest Constant scores in both groups. Although 3 of the 47 patients who underwent MA-ARCR with PGA experienced local inflammatory responses, which appeared to be a foreign body reaction, these adverse responses were resolved spontaneously after 1 or 2 weeks without causing any major complications.
Regarding ARCR for mRCTs, high failure rates have been reported by many authors.8,14,16,18,43,47 Cho and Rhee7 reported that even with elderly patients, in whom larger tears and severe fatty degeneration pose considerable risk factors for failure after ARCR, pain relief was achieved successfully and disruption of daily activities was minimal even in cases of failure. In contrast, Heuberer et al19 reported that clinical results were significantly better in the complete repair group compared with the results for arthroscopic debridement and partial repair of the mRCTs. Various treatment methods have been developed and performed, aiming for complete healing of such tears, but several problems have accompanied each procedure. Partial repair has been reported to produce good short-term results,22 but there is concern about long-term results. Medialized repair for retracted RCTs has been able to achieve good clinical outcomes and lower failure rates.23 However, it is possible that abduction muscle strength may be inferior owing to a decrease in the moment arm by the footprint medialization.31 Patch grafts or superior capsular reconstructions (which often use fascia lata27,28 to span the rotator cuff defect) sacrifice normal tissues, with a strong possibility that the free transplanted tissue will be necrotic. In a systematic review, Jordan et al21 reported that despite significant clinical outcomes achieved for mRCTs treated by superior capsular reconstruction, graft tears after superior capsular reconstruction were concerning enough to warrant longer term follow-up. Latissimus dorsi muscle transfer for mRCTs also sacrifices normal tissue, has poor recovery of muscle strength after surgery, and may cause osteoarthritis in the future.20,30 However, good outcomes have been reported from reverse shoulder arthroplasty2,10,45 and from SSC tendon transfer and small-head hemiarthroplasty.44 Because these replacement surgeries use an artificial prosthesis, there are concerns about future loosening of the components and deterioration of the outcomes.
We believe that complete anatomic repair can provide good, long-lasting clinical results. Hence, we reported on a technique of ARCR combined with “living” SSP and ISP muscle advancement for mRCTs for which it has been difficult to achieve primary repair, and we showed that the failure rate can be significantly reduced.47 Debeyre et al9 first reported open muscle advancement with acromio-osteotomy, and Morihara et al29 modified this procedure arthroscopically without acromio-osteotomy. Morihara et al performed the procedures while maintaining medial fascial continuity and added arthroscopic SSN to prevent the postoperative SSN palsy described by Warner et al.46 We also performed muscle advancement with arthroscopic SSN release to avoid postoperative SSN palsy and without medial fascial continuity to extract the torn cuff tendon more laterally. We have never experienced obvious postoperative SSN palsy, except for in 1 patient who had some paresthesia around her shoulder girdle. Although good surgical results were obtained, the failure rate in our study was still about 23%,47 and further improvement of the procedure is needed.
Many reports of reinforcement are drawn from basic research on rotator cuff repair using commercially available scaffolds, whether biomaterial or graft.41 Regarding artificial synthetic materials, Proctor35 reported successful long-term results (postoperative American Shoulder and Elbow Surgeons shoulder score of 82 at 42 months without any failure cases) and a comparable failure rate (4 of 18; 22.2%) for mRCTs with a poly-l-lactic acid synthetic patch. Petrie and Ismaiel34 also reported good clinical and structural outcomes (2 revisions in 31 cases) for rotator cuff repair with a polyester artificial ligament (ligament augmentation and reconstruction system). Petriccioli et al33 reported good short-term clinical outcomes (80% of patients had a good or excellent result) and structural outcomes (90% of patients revealed structurally intact repair on ultrasound imaging) by means of SportMech, a readily available synthetic degradable poly(urethane urea) scaffold. However, none of these synthetic materials have been approved for use in rotator cuff repair in Japan. Barber et al1 described achieving good clinical and structural outcomes using allografts or xenografts and acellular human dermal matrix (GraftJacket; Wright Medical Technology) for the augmentation of large cuff tear repairs. They achieved good structural outcomes (intact cuffs in 85% of the augmented group) and clinical outcomes (28.2 on the UCLA score). In a randomized clinical trial, porcine small intestinal submucosa-augmented rotator cuff repair (Restore Orthobiologic Implant; DePuySynthes) did not provide superior outcomes in patients with moderate RCTs.4 Chalmers et al5 reported on the surgical technique of using porcine dermis to augment the biological parameters of cuff repair. However, these materials have not been approved for clinical use in Japan either, on ethical grounds. Recently, an interesting technique using a biodegradable balloon-type material (InSpace) for rotator cuff defects has been reported, although the results do not seem to be stable.37,40 Although polytetrafluoroethylene felt39 was previously used as a patch for mRCTs in Japan, its use has been withheld in recent years because of reports of bone resorption.13 Because a PGA sheet can be used in thoracic surgery, even in Japan,32 we decided to use this material for augmentation at the repair site after conducting some animal experiments to confirm its safety and effectiveness for rotator cuff repair.48,49 Kokubu et al24 reported on this same method, using autologous fascia lata as reinforcement for the repair site. However, the use of fascia lata requires autograft tissue and donor site morbidity, as described above.
Our method achieved the same ROM, muscle strength, improved clinical outcomes, and failure rate for significantly more severe cases where complete coverage could not be achieved with MA alone, compared with patients in whom complete coverage was achieved with MA. Compared with previous reports,16,18,19,43 our failure rate of 12.8% was remarkably lower, considering that all patients had mRCTs. The reason may be that the tension produced by the anchor sutures or tapes was dispersed, with the PGA sheet covering the surface of the repaired torn rotator cuff. Another reason is that the PGA sheet has good biocompatibility and fiber-inducing ability,48,49 which enables a lot of cufflike fibrous tissue to be produced on the surface of the rotator cuff.
Previous studies have reported that a foreign body reaction becomes a problem when using PGA. Böstman and Pihlajamäki3 reported a 5.3% probability of a foreign body reaction due to PGA-based absorbable pins or screws, with an average appearance at 11 weeks postoperatively. Rokkanen et al36 reported that about 2% of noninfectious inflammation occurred 2 to 3 months after surgery when using PGA absorbable pins. We had a 6.4% rate of noninfectious reaction 2 to 3 months after surgery, but it disappeared spontaneously with no significant sequelae.
There are some limitations of this study. First, it was retrospective and not randomized. The number of the participants was relatively small and the follow-up period was short; more patients and a longer follow-up period are required. The slightly higher failure rate in the control group than in the study group (25.9% vs 12.8%) meant that power analysis was necessary because of the increased risk of type II error. Second, different indications for surgery in the 2 groups meant that there were differences in patient background. However, patients in the study group had a significantly larger tear size (P = .03), and the degree of fatty degeneration of the SSP and ISP was significantly more severe (P = .01-.02). Finally, it was also a limitation to exclude severe mRCTs, for which reverse shoulder arthroplasty is indicated in Japan.
Conclusion
We performed ARCR with SSP and ISP muscle advancement and PGA sheet reinforcement for mRCTs. Significant postoperative improvements were seen in AHI, clinical scores, ROM in anterior flexion, and isometric muscle strength in abduction, ER, and IR. The MA-ARCR with PGA method achieved the same ROM muscle strength, improvement in clinical outcomes, and failure rate as MA-ARCR alone, for significantly more severe cases where complete footprint coverage could not be achieved with MA. Foreign body reactions in the early period were found in 3 patients, although these spontaneously disappeared within 3 months.
Acknowledgment
We thank the researchers of Kyoto Furitsu Medical University, whose work is the basis of the technique of muscle advancement. We also thank our colleague, Dr Yoshihiro Nakamura, for his tremendous efforts in developing and improving surgical techniques. S.Y. expresses his deep gratitude to President Mitsuo Ochi for guidance and encouragement.
Footnotes
Final revision submitted April 30, 2020; accepted May 18, 2020.
The authors declared that there are no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from Hiroshima University (ref No. E-1842).
References
- 1. Barber FA, Burns JP, Deutsch A, Labbé MR, Litchfield RB. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy. 2012;28:8–15. [DOI] [PubMed] [Google Scholar]
- 2. Boileau P, Baba M, McClelland WB, Jr, Thélu CÉ, Trojani C, Bronsard N. Isolated loss of active external rotation: a distinct entity and results of L’Episcopo tendon transfer. J Shoulder Elbow Surg. 2018;27:499–509. [DOI] [PubMed] [Google Scholar]
- 3. Böstman OM, Pihlajamäki HK. Adverse tissue reactions to bioabsorbable fixation devices. Clin Orthop Relat Res. 2000;371:216–227. [PubMed] [Google Scholar]
- 4. Bryant D, Holtby R, Willis K, et al. A randomized clinical trial to compare the effectiveness of rotator cuff repair with or without augmentation using porcine small intestine submucosa for patients with moderate to large rotator cuff tears: a pilot study. J Shoulder Elbow Surg. 2016;25:1623–1633. [DOI] [PubMed] [Google Scholar]
- 5. Chalmers PN, Frank RM, Gupta AK, et al. All-arthroscopic patch augmentation of a massive rotator cuff tear: surgical technique. Arthrosc Tech. 2013;2:e447–e451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chaudhury S, Holland C, Thompson MS, Vollrath F, Carr AJ. Tensile and shear mechanical properties of rotator cuff repair patches. J Shoulder Elbow Surg. 2012;21:1168–1176. [DOI] [PubMed] [Google Scholar]
- 7. Cho NS, Rhee YG. The factors affecting the clinical outcome and integrity of arthroscopically repaired rotator cuff tears of the shoulder. Clin Orthop Surg. 2009;1:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davidson PA, Rivenburgh DW. Rotator cuff repair tension as a determinant of functional outcome. J Shoulder Elbow Surg. 2000;9:502–506. [DOI] [PubMed] [Google Scholar]
- 9. Debeyre J, Patie D, Elmelik E. Repair of ruptures of the rotator cuff of the shoulder. J Bone Joint Surg Br. 1965;47:36–42. [PubMed] [Google Scholar]
- 10. Ernstbrunner L, Andronic O, Grubhofer F, Camenzind RS, Wieser K, Gerber C. Long-term results of reverse total shoulder arthroplasty for rotator cuff dysfunction: a systematic review of longitudinal outcomes. J Shoulder Elbow Surg. 2019;28:774–781. [DOI] [PubMed] [Google Scholar]
- 11. France EP, Paulos LE, Harner CD, Straight CB. Biomechanical evaluation of rotator cuff fixation methods. Am J Sports Med. 1989;17:176–181. [DOI] [PubMed] [Google Scholar]
- 12. Fuchs B, Weishaupt D, Zanetti M, Hodler J, Gerber C. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg. 1999;8:599–605. [DOI] [PubMed] [Google Scholar]
- 13. Fukubayashi T, Ikeda K. Follow-up study of Gore-Tex artificial ligament—special emphasis on tunnel osteolysis. J Long Term Eff Med Implants. 2010;10:267–277. [PubMed] [Google Scholar]
- 14. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2003;85:1084–1089. [DOI] [PubMed] [Google Scholar]
- 15. Gerber C, Schneeberger AG, Perren SM, Nyffeler RW. Experimental rotator cuff repair: a preliminary study. J Bone Joint Surg Am. 1999;81:1281–1290. [DOI] [PubMed] [Google Scholar]
- 16. Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82:505–515. [DOI] [PubMed] [Google Scholar]
- 17. Hamada K, Yamanaka K, Uchiyama Y, Mikasa T, Mikasa M. A radiographic classification of massive rotator cuff tear arthritis. Clin Orthop Relat Res. 2011;469:2452–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Henry P, Wasserstein D, Park S, et al. Arthroscopic repair for chronic massive rotator cuff tears: a systematic review. Arthroscopy. 2015;31:2472–2480. [DOI] [PubMed] [Google Scholar]
- 19. Heuberer PR, Kölblinger R, Buchleitner S, et al. Arthroscopic management of massive rotator cuff tears: an evaluation of debridement, complete, and partial repair with and without force couple restoration. Knee Surg Sports Traumatol Arthrosc. 2016;24:3828–3837. [DOI] [PubMed] [Google Scholar]
- 20. Irlenbusch U, Bracht M, Gansen HK, Lorenz U, Thiel J. Latissimus dorsi transfer for irreparable rotator cuff tears: a longitudinal study. J Shoulder Elbow Surg. 2008;17:527–534. [DOI] [PubMed] [Google Scholar]
- 21. Jordan RW, Sharma N, Daggett M, Saithna A. The role of superior capsule reconstruction in the irreparable rotator cuff tear—a systematic review. Orthop Traumatol Surg Res. 2019;105:1535–1542. [DOI] [PubMed] [Google Scholar]
- 22. Kim SJ, Lee IS, Kim SH, Lee WY, Chun YM. Arthroscopic partial repair of irreparable large to massive tears. Arthroscopy. 2012;28:761–768. [DOI] [PubMed] [Google Scholar]
- 23. Kim YK, Jung KH, Won JS, Cho SH. Medialized repair for retracted rotator cuff tears. J Shoulder Elbow Surg. 2017;26:1432–1440. [DOI] [PubMed] [Google Scholar]
- 24. Kokubu T, Mifune Y, Inui A, Kuroda R. Arthroscopic rotator cuff repair with graft augmentation of fascia lata for large and massive tears. Arthrosc Tech. 2016;5(6):e1235–e1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lafosse L, Reiland Y, Baier GP, Toussaint B, Jost B. Anterior and posterior instability of the long head of the biceps tendon in rotator cuff tears: a new classification based on arthroscopic observations. Arthroscopy. 2007;23:73–80. [DOI] [PubMed] [Google Scholar]
- 26. Lafosse L, Lanz U, Saintmard B, Campens C. Arthroscopic repair of subscapularis tear: surgical technique and results. Orthop Traumatol Surg Res. 2010;96(8 suppl):S99–S108. [DOI] [PubMed] [Google Scholar]
- 27. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tear. Arthroscopy. 2013;29:459–470. [DOI] [PubMed] [Google Scholar]
- 28. Mori D, Funakoshi N, Yamashita F. Arthroscopic surgery of irreparable large or massive rotator cuff tears with low-grade fatty degeneration of the infraspinatus: patch autograft procedure versus partial repair procedure. Arthroscopy. 2013;29:1911–1921. [DOI] [PubMed] [Google Scholar]
- 29. Morihara T, Kida Y, Furukawa R, et al. Therapeutic outcomes of muscular advancement by an arthroscopic-assisted modified Debeyre-Patte procedure for irreparable large and massive rotator cuff tears. J Orthop Sci. 2018;23:495–503. [DOI] [PubMed] [Google Scholar]
- 30. Namdari S, Voleti P, Baldwin K, Glaser D, Huffman GR. Latissimus dorsi tendon transfer for irreparable rotator cuff tears: a systematic review. J Bone Joint Surg Am. 2012;94:891–898. [DOI] [PubMed] [Google Scholar]
- 31. Neviaser JS, Neviaser RF, Nejiaser TJ. The repair of chronic massive ruptures of the rotator cuff of the shoulder by use of a freeze-dried rotator cuff. J Bone Joint Surg Am. 1978;60:681–684. [PubMed] [Google Scholar]
- 32. Ozawa Y, Sakai M, Ichimura H. Covering the staple line with polyglycolic acid sheet versus oxidized regenerated cellulose mesh after thoracoscopic bullectomy for primary spontaneous pneumothorax. Gen Thorac Cardiovasc Surg. 2018;66:419–424. [DOI] [PubMed] [Google Scholar]
- 33. Petriccioli D, Bertone C, Marchi G, Mujahed I. Open repair of isolated traumatic subscapularis tendon tears with a synthetic soft tissue reinforcement. Musculoskelet Surg. 2013;97(suppl 1):63–68. [DOI] [PubMed] [Google Scholar]
- 34. Petrie MJ, Ismaiel AH. Treatment of massive rotator-cuff tears with a polyester ligament (LARS) patch. Acta Orthop Belg. 2013;79:620–625. [PubMed] [Google Scholar]
- 35. Proctor CS. Long-term successful arthroscopic repair of large and massive rotator cuff tears with a functional and degradable reinforcement device. J Shoulder Elbow Surg. 2014;23:1508–1513. [DOI] [PubMed] [Google Scholar]
- 36. Rokkanen PU, Böstman O, Hirvensalo E, et al. Bioabsorbable fixation in orthopaedic surgery and traumatology. Biomaterials. 2000;21:2607–2613. [DOI] [PubMed] [Google Scholar]
- 37. Ruiz Ibán MA, Lorente Moreno R, Ruiz Díaz R, et al. The absorbable subacromial spacer for irreparable posterosuperior cuff tears has inconsistent results. Knee Surg Sports Traumatol Arthrosc. 2018;26:3848–3854. [DOI] [PubMed] [Google Scholar]
- 38. Schlegel TF, Hawkins RJ, Lewis CW, Motta T, Turner AS. The effects of augmentation with swine small intestine submucosa on tendon healing under tension: histologic and mechanical evaluations in sheep. Am J Sports Med. 2006;34:275–280. [DOI] [PubMed] [Google Scholar]
- 39. Seker V, Hackett L, Lam PH, Murrell GAC. Evaluating the outcomes of rotator cuff repairs with polytetrafluoroethylene patches for massive and irreparable rotator cuff tears with a minimum 2-year follow-up. Am J Sports Med. 2018;46:3155–3164. [DOI] [PubMed] [Google Scholar]
- 40. Senekovic V, Poberaj B, Kovacic L, et al. The biodegradable spacer as a novel treatment modality for massive rotator cuff tears: a prospective study with 5-year follow-up. Arch Orthop Trauma Surg. 2017;137:95–103. [DOI] [PubMed] [Google Scholar]
- 41. Smith RD, Carr A, Dakin SG, Snelling SJ, Yapp C, Hakimi O. The response of tenocytes to commercial scaffolds used for rotator cuff repair. Eur Cell Mater. 2016;31:107–118. [DOI] [PubMed] [Google Scholar]
- 42. Spencer EE, Dunn WR, Wright RW, et al. Interobserver agreement in the classification of rotator cuff tears using magnetic resonance imaging. Am J Sports Med. 2008;36:99–103. [DOI] [PubMed] [Google Scholar]
- 43. Sugaya H, Maeda K, Matsuki K, Moriishi J. Repair integrity and functional outcome after arthroscopic double-row rotator cuff repair: a prospective outcome study. J Bone Joint Surg Am. 2007;89:953–960. [DOI] [PubMed] [Google Scholar]
- 44. Urita A, Funakoshi T, Suenaga N, Oizumi N, Iwasaki N. A combination of subscapularis tendon transfer and small-head hemiarthroplasty for muff tear arthropathy: a pilot study. Bone Joint J. 2015;97:1090–1095. [DOI] [PubMed] [Google Scholar]
- 45. Wall B, Nové-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89:1476–1485. [DOI] [PubMed] [Google Scholar]
- 46. Warner JP, Krushell RJ, Masquelet A, Gerber C. Anatomy and relationships of the suprascapular nerve: anatomical constraints to mobilization of the supraspinatus and infraspinatus muscles in the management of massive rotator-cuff tears. J Bone Joint Surg Am. 1992;74:36–45. [PubMed] [Google Scholar]
- 47. Yokoya S, Nakamura Y, Harada Y, Ochi M, Adachi N. Outcomes of arthroscopic rotator cuff repair with muscle advancement for massive rotator cuff tears. J Shoulder Elbow Surg. 2019;28:445–452. [DOI] [PubMed] [Google Scholar]
- 48. Yokoya S, Mochizuki Y, Natsu K, Omae H, Nagata Y, Ochi M. Rotator cuff regeneration using a bioabsorbable material with bone marrow-derived mesenchymal stem cells in a rabbit model. Am J Sports Med. 2012;40:1259–1268. [DOI] [PubMed] [Google Scholar]
- 49. Yokoya S, Mochizuki Y, Nagata Y, Deie M, Ochi M. Tendon-bone insertion repair and regeneration using polyglycolic acid sheet in the rabbit rotator cuff injury model. Am J Sports Med. 2008;36:1298–1309. [DOI] [PubMed] [Google Scholar]




