Abstract
Background:
Posaconazole prophylaxis during remission induction chemotherapy not only decreases the incidence of invasive aspergillosis (IA) but also improves the overall survival rate among patients with acute myeloid leukemia (AML). However, it remains debatable whether this result applies to patients in a real-world setting.
Methods:
We retrospectively assessed 208 adult patients with newly diagnosed AML who underwent remission induction therapy. These 208 patients were stratified into the posaconazole prophylaxis group (n = 58) and no antifungal prophylaxis group (n = 150).
Results:
Multivariate analyses showed that induction failure significantly increased the risk of proven or probable IA during the first induction chemotherapy [hazard ratio (HR), 10.47; 95% confidence interval (CI), 1.73–63.45; p = 0.011] and the entire course of AML treatment (HR, 4.48; 95% CI, 1.71–11.75; p = 0.002). However, posaconazole prophylaxis did not reduce the risk of IA during the first induction chemotherapy (HR, 1.47; 95% CI, 0.14–15.04; p = 0.746) and during the entire course of AML treatment (HR, 1.09; 95% CI, 0.29–4.09; p = 0.896). Furthermore, there was no significant difference in overall survival between these two groups of patients (514 versus 689 days; p = 0.454).
Conclusion:
Successful induction remains fundamental to reducing the risk of IA among AML patients undergoing remission induction chemotherapy.
Keywords: acute myeloid leukemia, antifungal agent, aspergillosis, azole, chemotherapy, treatment failure
Introduction
With an incidence of approximately 1.3 per 100,000 population, acute myeloid leukemia (AML) is a hematological malignancy with complex disease behavior.1 The diagnosis of AML is confirmed using the World Health Organization criteria when more than 20% of all the nucleated cells in either the bone marrow or peripheral blood are immature myeloblasts.2 Due to population ageing and a higher prevalence of comorbidities, about 25% of patients with newly diagnosed AML receive only best supportive care without intent-to-cure therapies.3 For patients receiving treatment with curative intent, the first step is the achievement of complete remission (CR) via remission induction therapy. Patients’ clinical characteristics and risk stratification may lead to further therapeutic strategies. High-risk cytogenetic abnormalities and genetic mutations can be indications for allogeneic hematopoietic stem cell transplantation (allo-HSCT).4 The standard of care for patients with low-risk features is consolidation chemotherapy after remission induction chemotherapy.
During the entire course of AML treatment, various complications may occur, with infection being the leading one. Among all the infectious complications of AML, invasive aspergillosis (IA) is crucial because it impacts negatively on the outcome of the overall treatment of AML.5 However, the epidemiology of IA in AML has changed significantly over the past two decades. There has been a progressive reduction in the rate of IA-associated mortality in AML.6 This is probably due to increased awareness and utility of the galactomannan antigen test in not only sera but also bronchoalveolar lavage fluid samples, which enhances the diagnostic ability and results in more appropriate antifungal treatment.7 In addition, more extensive use of prophylactic antifungal therapies may lead to a further decline in the incidence of IA during AML treatment.
Recent practice guidelines proposed by the Infectious Diseases Society of America recommended posaconazole and voriconazole for IA prevention in AML.8 The evidence for this recommendation mainly comes from the studies conducted by Cornely et al.9 and Chabro et al.10 Compared with fluconazole or itraconazole, posaconazole has demonstrated superiority not only in IA prevention but also in survival among AML patients undergoing intensive chemotherapy.9 Voriconazole prophylaxis significantly decreased the incidence of IA among AML patients receiving remission induction chemotherapy. However, the survival benefit was not assessed.10
Antifungal prophylaxis with posaconazole in AML patients undergoing induction chemotherapy has become a standard of care at our institution since January 2012. However, it is unclear whether this preventive strategy reduces the incidence of IA and further improves the overall survival outside clinical trial settings. Therefore, we conducted this retrospective study to clarify this issue.
Methods
Patients
We retrospectively reviewed the medical records of 323 consecutive adult AML patients diagnosed at our hospital between January 2005 and May 2019. We excluded patients who did not receive intent-to-cure induction therapy (n = 99) and those who did not undergo follow-up regularly (n = 16). Finally, a total of 208 cases were analyzed. The median age of this study cohort was 51 years. One hundred and thirty-two (70.6%) of the 208 patients achieved CR after the first induction chemotherapy. Sixty-eight of the 208 (32.7%) patients received allo-HSCT. The incidence of IA during the entire course of treatment was 26.4% (55/208). To investigate the impact of posaconazole prophylaxis, these 208 patients were further stratified into the posaconazole prophylaxis group (n = 58) and the no antifungal prophylaxis group (n = 150) according to the antifungal prophylaxis intervention received during the first remission induction chemotherapy. The criteria for reimbursement were the primary reason for posaconazole prophylaxis or otherwise. For most patients not receiving preventive posaconazole it was because posaconazole was not reimbursed for the prophylactic setting when these patients underwent their induction chemotherapy. There were no significant differences in age (p = 0.808), sex (p = 0.503), and proportion of patients who received allo-HSCT (p = 0.501) between these two groups of patients. However, patients in the no antifungal prophylaxis group had a longer median follow-up time than did patients in the posaconazole prophylaxis group (20.3 versus 10.6 months; p = 0.001) (Table 1). The study protocol was approved by the institutional review board of Taichung Veterans General Hospital. This study was conducted in accordance with the tenets of the current version of the Declaration of Helsinki.
Table 1.
Patients’ characteristics and comparison of outcomes.
| Total n = 208 |
Posaconazole prophylaxis n = 58 |
No antifungal prophylaxis n = 150 |
p-value | ||||
|---|---|---|---|---|---|---|---|
| Age, years, median (range) | 51 | 21–79 | 52 | 23–73 | 51 | 21–79 | 0.808† |
| Sex, n (%) | 0.503‡ | ||||||
| Male | 116 | (55.8) | 35 | (60.3) | 81 | (54.0) | |
| Female | 92 | (44.2) | 23 | (39.7) | 69 | (46.0) | |
| Disease status after first induction chemotherapy, n (%) | 1.000‡ | ||||||
| CR | 132 | (70.6) | 36 | (70.6) | 96 | (70.6) | |
| Non-CR | 55 | (29.4) | 15 | (29.4) | 40 | (29.4) | |
| Follow-up time, months, median (range) | 15.9 | (0.1–177.4) | 10.6 | (0.5–53.4) | 20.3 | (0.1–177.4) | 0.001† |
| Allogeneic HSCT, n (%) | 68 | (32.7) | 21 | (36.2) | 47 | (31.3) | 0.501‡ |
| Invasive aspergillosis infection, n (%) | 55 | (26.4) | 11 | (19.0) | 44 | (29.3) | 0.129‡ |
| Types of aspergillosis infection, n (%) | 0.574‡ | ||||||
| Proven | 4 | (1.9) | 0 | (0.0) | 4 | (2.7) | |
| Probable | 15 | (7.2) | 3 | (5.2) | 12 | (8.0) | |
| Possible | 36 | (17.3) | 8 | (13.8) | 28 | (18.7) | |
| Timing of aspergillosis infection, n (%) | 0.863‡ | ||||||
| At diagnosis | 1 | (0.5) | 0 | (0.0) | 1 | (0.7) | |
| During first induction therapy | 23 | (11.1) | 4 | (6.9) | 19 | (12.7) | |
| During consolidation therapy | 6 | (2.9) | 1 | (1.7) | 5 | (3.3) | |
| At relapse | 13 | (6.3) | 2 | (3.4) | 11 | (7.3) | |
| After allogeneic HSCT | 3 | (1.4) | 1 | (1.7) | 2 | (1.3) | |
| Others | 9 | (4.3) | 3 | (5.2) | 6 | (4.0) | |
| Survival, n (%) | 0.150‡ | ||||||
| Yes | 84 | (40.3) | 28 | (48.3) | 56 | (37.3) | |
| No | 124 | (59.6) | 30 | (51.7) | 94 | (62.7) | |
|
Causes of death, n (%)
n = 124 |
0.644‡ | ||||||
| Acute myeloid leukemia | 87 | (70.2) | 20 | (66.7) | 67 | (71.3) | |
| Induction death | 21 | (16.9) | 7 | (23.3) | 14 | (14.9) | |
| Sepsis | 2 | (1.6) | 1 | (3.3) | 1 | (1.1) | |
| Aspergillosis | 1 | (0.8) | 0 | (0.0) | 1 | (1.1) | |
| Allogeneic HSCT related | 9 | (7.3) | 2 | (6.7) | 7 | (7.4) | |
| Others | 4 | (3.2) | 0 | (0.0) | 4 | (4.3) | |
Mann–Whitney U test.
Chi-squared test.
CR, complete remission; HSCT, hematopoietic stem cell transplantation.
Definitions and outcome measurements
The present study assessed only the first episode of IA. Cases of invasive pulmonary aspergillosis were categorized as proven, probable, or possible, according to the criteria of the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group.11 Cases of IA other than invasive pulmonary aspergillosis needed to be proven by either surgical pathology or fungal culture. Finally, 94.5% (52/55) of the patients in our study cohort were diagnosed with invasive pulmonary aspergillosis. The other three patients had IA sinusitis.
For the outcome measures, the overall survival time was defined as the period from the date of AML diagnosis to the date of the end of the analysis (20 August 2019) or death due to any reason. In terms of causes of death, we defined AML as the cause of death if it remained active at the time of death. Death during induction chemotherapy, without evidence of residual AML or active IA, was defined as induction death. Deaths among patients due to graft versus host disease or cytomegalovirus infection after allo-HSCT were considered to be allo-HSCT related.
Antifungal prophylaxis protocol
Patients in the no antifungal prophylaxis group did not receive any antifungal prophylaxis therapy during the entire course of AML treatment. For patients in the posaconazole prophylaxis group, posaconazole prophylaxis therapy was initiated on the first day of remission induction chemotherapy and was terminated only when the white blood cell count was restored to 1000/mm3 or intolerable adverse events occurred. All instances of unexpected discontinuation of posaconazole were defined as clinical failure. We treated the patients with different formulations of prophylactic posaconazole at various time points according to the prevailing conditions for reimbursement. Briefly, 200 mg of posaconazole as a liquid suspension administered thrice daily was used between January 2012 and June 2015. Posaconazole tablets (300 mg per day) had been administered since July 2015. Therapeutic drug monitoring of posaconazole was not practiced routinely.
Statistical analysis
Continuous and categorical variables were compared between the posaconazole prophylaxis and no antifungal prophylaxis groups using the Mann–Whitney U test and the chi-squared test, respectively. Risk factors for IA during the first induction treatment and the entire course of AML treatment were quantified as hazard ratios (HRs) and their accompanying 95% confidence intervals (CIs) and investigated using Cox proportional hazards regression. The comparison of overall survival was performed using the log-rank test. Analysis items with p < 0.05 were considered statistically significant.
Results
Comparison of IA incidence between patients who received posaconazole prophylaxis and no antifungal prophylaxis
The incidences of IA in the posaconazole prophylaxis and no antifungal prophylaxis groups were 19.0% and 29.3%, respectively (p = 0.129). Among the patients with IA, there was no significant difference in the diagnostic level of IA between these two groups (p = 0.574). Notably, the majority of patients were diagnosed as possible cases of IA. The patients may have been infected with IA at different time points of treatment. Most cases of IA occurred during the first induction chemotherapy or disease relapse (65.5%, 36/55). These results were observed in both groups of patients (p = 0.863) (Table 1).
Risk factors for proven or probable IA in patients with AML
Next, we investigated the possible risk factors for proven or probable IA during AML treatment. Regarding the risk factors for proven or probable IA during the first induction chemotherapy, the univariate analysis revealed that first induction failure [hazard ratio (HR), 11.02; 95% confidence interval (CI), 1.93–62.99; p = 0.007)] was associated with a higher incidence of IA. However, posaconazole prophylaxis did not significantly reduce the risk of proven or probable IA during remission induction chemotherapy (HR, 1.29; 95% CI, 0.13–13.25; p = 0.833). The multivariate analysis further validated the initial result that first induction chemotherapy failure was the only parameter associated with a higher incidence of IA during the first induction therapy (HR, 10.47; 95% CI, 1.73–63.45; p = 0.011) (Table 2).
Table 2.
Results of Cox regression analysis of risk factors for proven or probable invasive aspergillosis after first induction chemotherapy.
| Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age | 1.04 | 0.98–1.11 | 0.225 | 1.06 | 0.98–1.14 | 0.156 |
| Sex (male versus female) | 0.18 | 0.02–1.55 | 0.118 | 0.18 | 0.02–1.66 | 0.130 |
| Posaconazole prophylaxis | 1.29 | 0.13–13.25 | 0.833 | 1.47 | 0.14–15.04 | 0.746 |
| First induction chemotherapy failure | 11.02 | 1.93–62.99 | 0.007 | 10.47 | 1.73–63.45 | 0.011 |
CI, confidence interval; HR, hazard ratio.
When we investigated the risk of proven or probable IA during the entire course of AML treatment, the univariate analysis revealed that first induction chemotherapy failure (HR, 5.06; 95% CI, 1.96–13.03; p = 0.001) was associated with a higher incidence of IA. Multivariate analysis revealed that first induction chemotherapy failure was the only factor that significantly increased the risk of proven or probable IA during the entire course of AML treatment (HR, 4.48; 95% CI, 1.71–11.75; p = 0.002). Posaconazole prophylaxis during the first induction chemotherapy was not found to significantly reduce the risk of IA in either the univariate (HR, 1.07; 95% CI, 0.30–3.91; p = 0.913) or the multivariate (HR, 1.09; 95% CI, 0.29–4.09; p = 0.896) analyses (Table 3).
Table 3.
Results of Cox regression analysis of risk factors for proven or probable invasive aspergillosis during the entire course of acute myeloid leukemia treatment.
| Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| HR | 95 % CI | p-value | HR | 95 % CI | p-value | |
| Age | 1.03 | 0.99–1.06 | 0.140 | 1.04 | 1.00–1.08 | 0.072 |
| Sex (male versus female) | 0.67 | 0.27–1.67 | 0.388 | 0.86 | 0.34–2.21 | 0.758 |
| Posaconazole prophylaxis | 1.07 | 0.30–3.91 | 0.913 | 1.09 | 0.29–4.09 | 0.896 |
| First induction chemotherapy failure | 5.06 | 1.96–13.03 | 0.001 | 4.48 | 1.71–11.75 | 0.002 |
| Allogeneic HSCT | 2.31 | 0.90–5.90 | 0.080 | 2.45 | 0.90–6.69 | 0.080 |
CI, confidence interval; HR, hazard ratio; HSCT, hematopoietic stem cell transplantation.
Survival analysis
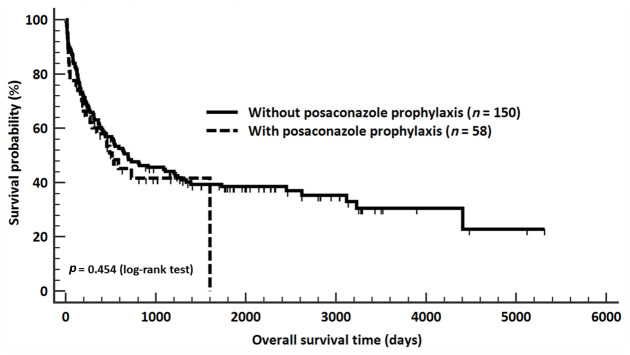
We compared the overall survival between patients who received posaconazole prophylaxis and those who did not receive any antifungal prophylaxis during their first induction chemotherapy. The overall survival rates of these two groups of patients were 48.3% and 37.3%, respectively (p = 0.150) (Table 1). The median overall survival time among patients who received posaconazole prophylaxis and those who received no antifungal prophylaxis was 514 (95% CI, 270–1602) and 689 (95% CI, 423–1243) days, respectively (p = 0.454) (Figure 1).
Figure 1.
A comparison of overall survival between the study groups. The median overall survival times among patients who received posaconazole prophylaxis and those who did not receive any antifungal prophylaxis were 514 (95% confidence interval (CI), 270–1602) and 689 (95% CI, 423–1243) days, respectively (p = 0.454). There was no significant difference in overall survival time between these two groups of acute myeloid leukemia patients.
Further analysis of the causes of death revealed that patients who received posaconazole prophylaxis and those who received no antifungal prophylaxis had similar causes of death (p = 0.644). Acute myeloid leukemia remained the leading cause of mortality in both groups of patients (66.7% and 71.3%, respectively). The second leading cause of death was induction death, which accounted for 23.3% and 14.9% of deaths, respectively (Table 1).
Clinical failure of posaconazole prophylaxis during AML induction therapy
As posaconazole prophylaxis was not found to significantly reduce the incidence of IA among AML patients, we studied the prevalence and causes of unexpected posaconazole discontinuation. The analysis yielded a clinical failure rate of 34.5% (20 of 58 patients). Among these 20 patients who did not complete posaconazole prophylaxis therapy during their first induction chemotherapy, impaired liver function was found to be the leading cause of clinical failure, which accounted for 50.0% (10/20) of cases. Breakthrough fungal infection occurred in six of the 20 patients (30.0%). Other causes of clinical failure were allergy (3/20, 15.0%) and unknown (1/20, 5.0%).
Discussion
In this study, we found that induction failure significantly increased the risk of proven or probable IA among AML patients receiving remission induction therapy. Humans regularly inhale hundreds of aspergillosis spores every day. However, IA remains uncommon in the healthy population. Both the innate and cellular immune mechanisms are responsible for the host defense against IA.12 Deficiencies in host factors are considered the most critical risk factors for IA. Individuals who received chemotherapy, allo-HSCT, and solid organ transplantation have a high risk for IA because these treatments damage not only the innate immune mechanism but also the T cell function.13 The data reported by Tang et al.14 showed that failure to achieve CR increased the risk of invasive fungal infection in AML patients receiving remission induction chemotherapy without primary antifungal prophylaxis. Our study further revealed that induction failure was an independent risk factor for IA in AML, even with posaconazole prophylaxis. Prolonged neutropenia and failure of recovery of immunity could be possible explanations. Lien et al.15 demonstrated that a neutropenic period of >30 days was associated with a higher risk of invasive fungal infection in AML patients. Because more than 80% of neutrophil recovery occurs within 35 days in AML patients who achieve CR after intensive induction therapy,16 delayed neutrophil recovery usually suggests induction failure.
The incidence of IA in our study cohort without any antifungal prophylaxis was 29.3%, which was similar to the data reported by Lien et al. (33%).15 This high incidence raises the clinical need for antifungal prophylaxis in AML patients receiving remission induction chemotherapy. A randomized-controlled study conducted by Cornely et al.9 showed that compared with primary prophylaxis with either fluconazole or itraconazole, posaconazole significantly reduces the incidence of proven or probable invasive fungal infections. Our study, however, did not show similar results. On the contrary, our data revealed that, compared with patients who received no systemic antifungal prophylaxis, posaconazole prophylaxis during induction therapy did not reduce the incidence of proven or probable IA either during induction or during the entire course of AML treatment. Differences in the spectrum for itraconazole compared with posaconazole would partially explain the conflicting results. Another possible reason for the data discrepancy could be the difference in diagnostic power. The majority of IA cases in our cohort were possible cases (65.5%, 36/55), suggesting that the confirmation of IA diagnosis remains a challenge in real-world clinical practice. A more aggressive approach to diagnosis is still encouraged.
Adequate therapeutic posaconazole concentration is another critical issue in prophylaxis. An insufficient therapeutic level is associated with a higher risk of breakthrough IA. On the other hand, an overdose may result in a higher incidence of adverse events. In our analysis, 34.5% (20/58) of patients experienced clinical failure. The clinical failure rate in our study was quite similar to that reported in the cohort considered in the study conducted by Cornely et al. (35.6%). In contrast, the 65.0% clinical failure rate recorded in our study was due to intolerable adverse events, of which impaired liver function was the commonest. However, the clinical failure rate due to unacceptable adverse events was only 22.9% in the study conducted by Cornely et al. Posaconazole is metabolized through the CYP pathway. A study conducted in Japan showed that the Asian population has a higher incidence of CYP2C19 genetic polymorphisms, which impairs the metabolism of azoles and further impairs the liver function.17 This could at least partially explain why impaired liver function was more common in our cohort than in the Western group.
Besides genetic polymorphisms, differences in the absorption of drugs with different formulations could be another problem. In the present study, posaconazole in the form of oral suspension or delayed-release tablets was administered to patients during different periods according to prevailing conditions for reimbursement. Posaconazole delayed-release tablets are currently more recommended than the oral suspension form because the delayed-release tablet makes it easier to achieve the target average steady-state concentration.18 Unfortunately, the serum concentration of posaconazole was not assessed routinely in our practice, and so we could not confirm the pharmacokinetics of the drug in the present study. Nevertheless, regular posaconazole therapeutic drug monitoring is highly recommended among AML patients receiving posaconazole prophylaxis, particularly among those who experience extreme adverse events or breakthrough IA.8
In terms of the overall survival, posaconazole prophylaxis did not significantly improve the overall survival of AML patients in the present study. The primary reason is that more than 80% of the patients eventually died of relapsed/refractory AML or experienced induction death. Moreover, a better approach to the diagnosis and treatment of IA would yield progress towards reducing the associated mortality rate.6 Taking these together, besides effective IA prophylaxis and treatment, a better therapeutic strategy against AML remains a key factor for improving survival.19
In summary, our study showed that induction failure was the most critical risk factor for IA in AML. Compared with AML patients who received no systemic antifungal prophylaxis, those who received prophylactic posaconazole had neither improved risk of IA nor overall survival outside the clinical trial setting. Impaired liver function was the leading cause of unexpected discontinuation of posaconazole prophylaxis. These results could be due to the lack of routine therapeutic drug monitoring. Different follow-up time between these two groups of patients, retrospective study design, and lack of therapeutic drug monitoring were the major limitations in the current study. Prospective and randomized studies with large numbers of patients are needed to validate our data. In addition to appropriate aspergillosis prophylaxis, the identification of an effective therapeutic strategy for induction success without further relapse remains fundamental to reducing the incidence of IA among AML patients.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Ethical statement: The study protocol was approved by the institutional review board of Taichung Veterans General Hospital. This study was conducted in accordance with the tenets of the current version of the Declaration of Helsinki.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was partially supported by grants from TCVGH-1083701C and TCVGH-1083702D.
ORCID iD: Chieh-Lin Jerry Teng  https://orcid.org/0000-0001-9744-0368
https://orcid.org/0000-0001-9744-0368
Contributor Information
Tsung-Chih Chen, Department of Medicine, Division of Hematology/Medical Oncology, Taichung Veterans General Hospital, Taiwan; Graduate Institute of Clinical Medicine, College of Medicine, National Taiwan University, Taipei, Taiwan.
Ren Ching Wang, Department of Pathology, Taichung Veterans General Hospital, Taiwan; Department of Nursing, College of Nursing, Hungkuang University, Taichung, Taiwan.
Yu-Hui Lin, Department of Medicine, Division of Infectious Disease, Taichung Veterans General Hospital, Taiwan.
Kuang-Hsi Chang, Department of Medical Research, Tungs’ Taichung Metroharbor Hospital, Taiwan; Graduate Institute of Biomedical Sciences, China Medical University, Taichung, Taiwan; General Education Center, Jen-Teh Junior College of Medicine, Nursing and Management, Miaoli, Taiwan.
Li-Ya Hung, Section of Medical Records, Taichung Veterans General Hospital, Taiwan.
Chieh-Lin Jerry Teng, Department of Medicine, Division of Hematology/Medical Oncology, Taichung Veterans General Hospital, No. 1650, Sec. 4, Taiwan Blvd., Taichung, 40705, Taiwan; Department of Life Science, Tunghai University, Taichung, Taiwan; School of Medicine, Chung Shan Medical University, Taichung, Taiwan.
References
- 1. De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J 2016; 6: e441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 2009; 114: 937–951. [DOI] [PubMed] [Google Scholar]
- 3. Chang KH, Hwang WL, Muo CH, et al. Outcome and late effects among acute myeloid leukemia survivors: a nationwide population-based study. Support Care Cancer 2016; 24: 4993–5000. [DOI] [PubMed] [Google Scholar]
- 4. Burnett A, Wetzler M, Lowenberg B. Therapeutic advances in acute myeloid leukemia. J Clin Oncol 2011; 29: 487–494. [DOI] [PubMed] [Google Scholar]
- 5. Cattaneo C, Gramegna D, Malagola M, et al. Invasive pulmonary aspergillosis in acute leukemia: a still frequent condition with a negative impact on the overall treatment outcome. Leuk Lymphoma 2019; 60: 3044–3050. [DOI] [PubMed] [Google Scholar]
- 6. Dragonetti G, Criscuolo M, Fianchi L, et al. Invasive aspergillosis in acute myeloid leukemia: are we making progress in reducing mortality? Med Mycol 2017; 55: 82–86. [DOI] [PubMed] [Google Scholar]
- 7. Hardak E, Fuchs E, Leskes H, et al. Diagnostic role of polymerase chain reaction in bronchoalveolar lavage fluid for invasive pulmonary aspergillosis in immunocompromised patients - a retrospective cohort study. Int J Infect Dis 2019; 83: 20–25. [DOI] [PubMed] [Google Scholar]
- 8. Patterson TF, Thompson GR, 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 63: e1–e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med 2007; 356: 348–359. [DOI] [PubMed] [Google Scholar]
- 10. Chabrol A, Cuzin L, Huguet F, et al. Prophylaxis of invasive aspergillosis with voriconazole or caspofungin during building work in patients with acute leukemia. Haematologica 2010; 95: 996–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European organization for research and treatment of cancer/invasive fungal infections cooperative group and the national institute of allergy and infectious diseases mycoses study group (EORTC/MSG) consensus group. Clin Infect Dis 2008; 46: 1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Segal BH. Aspergillosis. N Engl J Med 2009; 360: 1870–1884. [DOI] [PubMed] [Google Scholar]
- 13. Camargo JF, Husain S. Immune correlates of protection in human invasive aspergillosis. Clin Infect Dis 2014; 59: 569–577. [DOI] [PubMed] [Google Scholar]
- 14. Tang JL, Kung HC, Lei WC, et al. High incidences of invasive fungal infections in acute myeloid leukemia patients receiving induction chemotherapy without systemic antifungal prophylaxis: a prospective observational study in Taiwan. PLoS One 2015; 10: e0128410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lien MY, Chou CH, Lin CC, et al. Epidemiology and risk factors for invasive fungal infections during induction chemotherapy for newly diagnosed acute myeloid leukemia: a retrospective cohort study. PLoS One 2018; 13: e0197851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murphy T, Zou J, Daher-Reyes GS, et al. Impact of preleukemic mutations and their persistence on hematologic recovery after induction chemotherapy for AML. Blood Adv 2019; 3: 2307–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Suzuki Y, Tokimatsu I, Sato Y, et al. Association of sustained high plasma trough concentration of voriconazole with the incidence of hepatotoxicity. Clin Chim Acta 2013; 424: 119–122. [DOI] [PubMed] [Google Scholar]
- 18. Mellinghoff SC, Panse J, Alakel N, et al. Primary prophylaxis of invasive fungal infections in patients with haematological malignancies: 2017 update of the recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society for Haematology and Medical Oncology (DGHO). Ann Hematol 2018; 97: 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chiu YC, Hsiao TH, Tsai JR, et al. Integrating resistance functions to predict response to induction chemotherapy in de novo acute myeloid leukemia. Eur J Haematol 2019; 103: 417–425. [DOI] [PubMed] [Google Scholar]