Abstract
Over the past 20 years, management of patients with metastatic colorectal cancer (mCRC) has improved considerably, leading to increased overall survival and more patients eligible for third- or later-line therapy. Currently, two oral therapies are recommended in the third-line treatment of mCRC, regorafenib and trifluridine/tipiracil. Selecting the most appropriate treatment in the third-line setting poses different challenges compared with treatment selection at earlier stages. Therefore, it is important for physicians to understand and differentiate between available treatment options and to communicate the benefits and challenges of these to patients. In this narrative review, practical information on regorafenib is provided to aid physicians in their decision-making and patient communications in daily practice. We discuss the importance of appropriate patient selection and adverse events management through close patient monitoring and dose adjustments to ensure patients stay on treatment for longer and receive as much benefit as possible. We also highlight key physician–patient communication points to facilitate shared decision-making.
Keywords: adverse events, metastatic colorectal cancer, practical management, regorafenib, third line
Introduction
The management of patients with metastatic colorectal cancer (mCRC) has improved considerably over the past 20 years, and the median overall survival (OS) from the start of systemic therapy is now approximately 30 months.1,2 Choosing the optimal first-line treatment, tailored to the clinical characteristics of the patient and molecular features of the tumors, is an essential step in achieving disease control and facilitating subsequent treatment.3 First-line treatment typically consists of chemotherapy with oxaliplatin-containing or irinotecan-containing regimens in combination with an anti-epidermal growth factor receptor (anti-EGFR) antibody (only in patients with RAS wild-type tumors) or an angiogenesis inhibitor.1 Other key factors contributing to improvements in survival are availability and access to subsequent lines of therapy. To increase the likelihood of survival, it is important that patients are given the opportunity to receive all available treatment options as part of the ‘continuum of care’.1
In addition to appropriate and effective systemic treatment, local treatment of metastases is increasingly important, especially in the oligometastatic setting, emphasizing the need for a multidisciplinary approach. Local treatment can include metastasectomy, thermal ablation, radioembolization, or stereotactic radiotherapy for some metastases (e.g. liver, lung, and spine metastases, and peritoneal carcinomatosis).4,5
As OS increases, more patients become eligible for third- or later-line therapy [classically after progression or intolerance to standard therapies: fluoropyrimidine, oxaliplatin, irinotecan, bevacizumab, and an anti-EGFR antibody (in case of RAS wild-type tumors)].1,6 Therefore, it is important for physicians to understand and differentiate between available treatment options and to communicate the benefits and challenges of these to patients. The issues faced by physicians and patients when selecting treatment in third line differ from those at earlier stages: the expectations of treatment shift from objective response to disease control (tumor response or stabilization), while patient preference, tolerability, and quality of life (QoL) become more important.6 Two options recommended in current guidelines for third-line treatment are regorafenib and trifluridine/tipiracil (TAS-102), which are both oral therapies1; therefore, patients can continue active treatment without the disadvantages associated with intravenous chemotherapy. Nevertheless, the transition from intravenous to oral therapies creates other challenges, including patient responsibility for medication adherence and monitoring, and the need for frequent communication with healthcare professionals to pre-empt and manage toxicity.
This narrative review provides expert opinion on treatment with regorafenib and patient management to aid physicians in their decision-making and communications with patients in daily clinical practice.
Regorafenib activity
Regorafenib is an oral tyrosine kinase inhibitor targeting angiogenesis, the tumor microenvironment, and tumor immunity,7,8 and is approved for the treatment of mCRC after progression on standard therapies.9,10 Regorafenib is also approved for third-line use in locally advanced, unresectable, or metastatic gastrointestinal stromal tumors and for second-line use in hepatocellular carcinoma.9,10 Regorafenib has also shown activity in phase II trials in chemotherapy-refractory biliary tract adenocarcinoma,11 advanced non-adipocytic soft-tissue sarcomas,12 progressive metastatic osteosarcoma,13,14 and relapsed glioblastoma.15
Approval in mCRC was based on results of the pivotal phase III CORRECT trial, which demonstrated significantly longer OS with regorafenib compared with placebo in patients who had progressed after all approved standard therapies16; these results were confirmed in the phase III CONCUR trial in Asian patients.17 Meta-analysis of the data from these two studies showed a significantly longer OS and progression-free survival (PFS) with regorafenib compared with placebo and best supportive care [hazard ratios (HRs) 0.67 and 0.40, respectively].18 Regorafenib is recommended as third-line therapy in the National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology guidelines1,19; NCCN guidelines also recommend that either regorafenib or trifluridine/tipiracil can be used as second-line therapy after FOLFOXIRI chemotherapy (5-fluorouracil, leucovorin, oxaliplatin, irinotecan).19 Data from single-arm phase IIIb and real-world observational studies of regorafenib in over 3500 patients support the results observed in the randomized phase III trials (Table 1; REBECCA,20 CONSIGN,21 CORRELATE,22 RECORA,23 Japanese post-marketing surveillance study,24 and the CORECT Czech Registry25).
Table 1.
Efficacy of regorafenib across phase III clinical trials and real-world studies.
| Study | Patients | Treatment | OS | PFS | Tumor response |
|---|---|---|---|---|---|
| CORRECT16
[ClinicalTrials.gov identifier: NCT01103323] RCT phase III n = 760 |
mCRC progression within 3 mo of standard therapy; ECOG PS 0–1 All had received previous anti-VEGF therapy |
Regorafenib [160 mg/day, n = 505 (ITT population)/n = 500 (safety population)], Days 1–21 of 28-day cycle, versus placebo [n = 255 (ITT population)/n = 253 (safety population)] | OS (primary endpoint): 6.4 versus 5.0 mo (HR 0.77; p = 0.0052) | PFS*: 1.9 versus 1.7 mo (HR 0.49; p < 0.0001) | CR*: 0% versus 0% PR*: 1% versus 0.4% ORR*: 1.0% versus 0.4% (p = 0.19) DCR ⩾6 wks*: 41% versus 15% (p < 0.0001) |
| CONCUR17
[ClinicalTrials.gov identifier: NCT01584830] RCT, phase III n = 204 |
Previously treated mCRC (Asian population); ⩾2 previous treatment lines or intolerance to standard therapy Biologic naïve (regorafenib: 41%/placebo: 38%) Previous anti-VEGF therapy only (regorafenib: 24%/placebo: 19%) Previous anti-EGFR therapy only (regorafenib: 18%/placebo: 25%) |
Regorafenib (160 mg/day, n = 136) Days 1–21 of 28-day cycle, versus placebo (n = 68) | OS (primary endpoint): 8.8 versus 6.3 mo (HR 0.55; p = 0.00016) | PFS*: 3.2 versus 1.7 mo (HR 0.31; p < 0.0001) | CR*: 0% versus 0% PR*: 4% versus 0% ORR*: 4% versus 0% (p = 0.045) DCR ⩾6 wks*: 51% versus 7% (p < 0.0001) |
| CONSIGN21
[ClinicalTrials.gov identifier: NCT01538680] Open-label, single-arm, phase IIIb n = 2872 |
mCRC progression within 3 mo of standard therapy; ECOG PS 0–1 | Regorafenib (160 mg/day, n = 2872) Days 1–21 of 28-day cycle | NR | PFS†: 2.7 mo (95% CI 2.6–2.7) | NR |
| REBECCA20
[ClinicalTrials.gov identifier: NCT02310477] Observational cohort n = 690 |
Refractory mCRC | Regorafenib in real-life clinical practice (n = 654) | OS: 5.6 mo (IQR 2.4–11.4) | PFS‡: 2.7 mo (IQR 1.6–4.6) | NR |
| CORRELATE22
[ClinicalTrials.gov identifier: NCT02042144] Observational cohort n = 1037 |
Previously treated mCRC | Regorafenib in real-life clinical practice (n = 1037) | OS: 7.6 mo (95% CI 7.1–8.2) | PFS‡: 2.8 mo (95% CI 2.6–2.8) | DCR‡: 21% PR*: 3.1% |
| RECORA23
[ClinicalTrials.gov identifier: NCT01959269] Observational cohort n = 481 |
Previously treated mCRC | Regorafenib in real-life clinical practice in Germany [n = 464 (safety); n = 463 (efficacy)] | OS: 5.8 mo (95% CI 5.3–6.6) | PFS‡: 3.1 mo (95% CI 2.8–3.3) | DCR‡: 26.7% |
| Japanese post-marketing surveillance study24
[ClinicalTrials.gov identifier: NCT01843400] Observational cohort n = 1301 |
Previously treated mCRC (Japanese pts) | Regorafenib in real-life clinical practice in Japan (n = 1227) | OS: 6.9 mo (95% CI 6.4–7.4) | TTF‡: 2.2 mo (95% CI 2.1–2.3) | NR |
| CORECT Czech registry25 | Previously treated mCRC (Czech pts) | Regorafenib in real-life clinical practice in the Czech Republic (n = 148) | OS: 9.3 mo | PFS‡: 3.5 mo | PR‡: 2.7% SD‡: 34.5% |
Radiologically evaluated using RECIST version 1.1.
Investigator assessed using radiologic and/or clinical tumor assessment according to local standards.
Assessment criteria/method of assessment are not reported.
CI, confidence interval; CR, complete response; DCR, disease control rate; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, estimated glomerular filtration rate; HR, hazard ratio; IQR, interquartile range; ITT, intention-to-treat; mCRC, metastatic colorectal cancer; mo, months; NR, not reported; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; PR, partial response; pts, patients; RCT, randomized controlled trial; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease; TTF, time to treatment failure; VEGF, vascular endothelial growth factor; wks, weeks.
In most cases, patients treated with regorafenib achieved a survival benefit with disease stabilization rather than tumor shrinkage (CORRECT: no complete responses, 1% partial responses; CONCUR: no complete responses, 4% partial responses).16,17 In routine clinical practice, response to treatment is usually assessed with regular computed tomography (CT) scans using Response Evaluation Criteria in Solid Tumors (RECIST).26 However, because patients may benefit from anti-angiogenic therapy without experiencing a response based on a reduction in tumor size, a number of studies are evaluating alternative techniques that assess changes in tumor metabolism or morphology rather than shrinkage of target lesions.26 Cavitation of lung metastases in response to regorafenib treatment has been observed using CT scans, potentially caused by anti-angiogenic therapy-induced necrosis or arterial thrombosis, and may be a novel radiologic marker of favorable outcome.27 The observation of non-size-based morphologic changes in liver metastases using contrast-enhanced CT have also been identified as potential markers for early response to regorafenib.28,29 Collectively, these studies suggest that Week 8 CT assessment can provide useful information that may help predict outcome.27–29 In addition, positron emission tomography (PET), dynamic contrast enhanced-magnetic resonance imaging, and diffusion-weighted imaging are being evaluated in translational studies; these studies include the phase II TEXCAN study [ClinicalTrials.gov identifier: NCT02699073],30 which evaluated CT texture analysis as a marker for response to regorafenib, and RegARd-C, a multicenter prospective study that used serial fluorodeoxyglucose PET-CT imaging to identify patients who are unlikely to respond to regorafenib [ClinicalTrials.gov identifier: NCT01929616].31
Clinical outcomes with regorafenib
Treatment with regorafenib has resulted in long-term response or disease stabilization in patients with mCRC in several phase III trials. In CORRECT, 19% of patients with mCRC receiving regorafenib experienced PFS >4 months,32 and the proportion of patients with PFS in CONSIGN was similar (23%).21 A higher proportion of patients (34%) achieved PFS >4 months in CONCUR, and an extended tail is visible on the Kaplan–Meier survival curve (PFS HR = 0.31), possibly because fewer patients in this trial had received prior biological therapy.33 While this information regarding long-term responders is of value, communicating these data to patients can be challenging because there is currently no standardized method of identifying patients with mCRC most likely to benefit from regorafenib for an extended period. Although an extended tail on the survival curve has generally not been seen with regorafenib (unlike immunotherapy in microsatellite unstable mCRC in a minority of patients),34 it is an important option for heavily pretreated patients, particularly for the majority of patients with mCRC who have microsatellite stable tumors, and who are therefore not eligible for immunotherapy. For most patients, it could be helpful to explain, in advance of treatment initiation, that achieving disease stabilization is a reasonable and positive treatment outcome. In other words, treatment could result in a halting of tumor progression and that the 2-month re-evaluation landmark is an appropriate time for taking further decisions.
Factors such as appropriate patient selection and management [including optimal management of adverse events (AEs)] can maximize the treatment benefit. Firstly, in CORRECT, patients who achieved PFS >4 months tended to have a better baseline Eastern Cooperative Oncology Group performance status (ECOG PS) than patients with shorter PFS (⩽4 months),32 highlighting the importance of using regorafenib early during third line before deterioration of ECOG PS. While the CORRECT trial only included patients with an ECOG PS of 0 and 1, real-world studies have confirmed that patients with ECOG PS 0–1 achieved better outcomes than those with ECOG PS 2.20,24 Secondly, it is important to consider the optimal treatment sequence. As mentioned earlier, the longer OS observed in CONCUR compared with CORRECT may be related to the fact that patients in CONCUR had received fewer prior treatments, including targeted agents.16,17 In CORRECT, all patients had received prior bevacizumab, and 52% had received prior anti-EGFR therapy versus 41% and 35%, respectively, in CONCUR.16,17,35 The results of a small phase II Japanese study, REVERCE, in patients with KRAS wild-type tumors also support regorafenib use earlier in the treatment sequence, although these results need to be confirmed in additional studies. OS after relapse on chemotherapy was longer with the sequence of regorafenib followed by cetuximab +/– irinotecan compared with cetuximab +/– irinotecan followed by regorafenib.36 In line with these results, and due to lack of available high-quality evidence, we believe that any rechallenge or recycling of a treatment given previously should be reserved for later lines of treatment. This is consistent with the conclusions from a recent systematic review stating that regorafenib or trifluridine/tipiracil are appropriate choices for third-line treatment and that rechallenge should be reserved for later use in patients with good ECOG PS who are willing to receive further lines of treatment.6 Finally, appropriate dose modifications and proactive management of AEs (discussed later) are essential to ensure that patients are able to maintain a good QoL and remain on therapy long enough to receive benefit.
AE profile of regorafenib
Regorafenib has a consistent and predictable AE profile, typical of tyrosine kinase inhibitors, characterized by hand–foot skin reaction (HFSR), hypertension, diarrhea, and fatigue (Table 2).18,37 The AEs reported during regorafenib treatment differ from those of conventional chemotherapy (e.g., unlike chemotherapy agents, including trifluridine/tipiracil, regorafenib does not cause bone marrow suppression, and skin toxicity is different from that seen with capecitabine and other chemotherapy agents). This distinctive pattern of AEs, which also differs from other targeted agents, may present challenges to clinicians who are unfamiliar with their management.
Table 2.
Most frequent regorafenib-related grade ⩾3 AEs.
| AEs (%) | HFSR | Hypertension | Diarrhea | Fatigue |
|---|---|---|---|---|
| CORRECT16 | 17 | 7 | 7 | 10 |
| CONCUR17 | 16 | 11 | 1 | 3 |
| CONSIGN21 | 14 | 15 | 5 | 13 |
| REBECCA20 | 9 | 5 | 4 | 15 |
| CORRELATE22 | 7 | 6 | NR | 9 |
AE, adverse event; HFSR, hand–foot skin reaction; NR, not reported.
AEs typically occur early in treatment, reaching the maximum grade and highest incidence during the first two cycles, and subsequently declining to a stable incidence.21,24,38 Many AEs are low grade, and regorafenib toxicity is not cumulative.39 In the CORRECT trial, the incidence of grade ⩾3 HFSR, fatigue, hypertension, and rash/desquamation typically peaked in Cycle 1 and reached a relatively stable lower incidence over later cycles, while the incidence of diarrhea remained relatively constant throughout the study.38 HFSR first occurred at a median of 15 days, with the worst grade at a median of 22 days.38 Therefore, it is important to monitor patients closely while on treatment, with proactive and frequent monitoring particularly in the first 1–2 cycles, with appropriate dose adjustment, treatment breaks, and intervention to avoid permanent discontinuation of treatment.
We recommend that patients are evaluated at least weekly for the first 4 weeks (first cycle) and then every 2 weeks during Cycle 2. Evaluation can be through face-to-face consultations or telephone calls from a trained healthcare professional.37 Specialist nurses can play an important role in the management of AEs, by educating patients about what to expect and providing support in the first few weeks of therapy. Close monitoring during the first cycle ensures that the dose can be optimized in the second cycle. Experience from clinical trials has shown that after initial dose adjustments, patients can usually tolerate long-term treatment.
A correlation has been observed between HFSR during regorafenib treatment and OS regardless of when it occurs or at what grade. This important finding should be communicated to patients experiencing HFSR to encourage them to remain on treatment. In a post hoc exploratory analysis of the CORRECT study, the median OS of patients with HFSR at any time in the study was 9.5 months versus 4.7 months for patients without HFSR (HR 0.41).40 Median OS was also numerically longer in those who experienced HFSR in Cycle 1 (7.2 versus 5.7 months in patients without HFSR in Cycle 1; HR 0.66).40 Similarly, the REBECCA and Japanese studies showed that OS was longer in patients experiencing HFSR early.20,24 A correlation has also been reported between OS and skin rash or hypothyroidism in a small retrospective analysis of 144 patients41; however, there are no prospective data documenting a link between incidence of AEs and longer survival. Therefore, it is important to educate patients before starting treatment on the potential AEs and how to promptly recognize and manage them; this, in turn, may motivate them to adhere to treatment.
Management of AEs
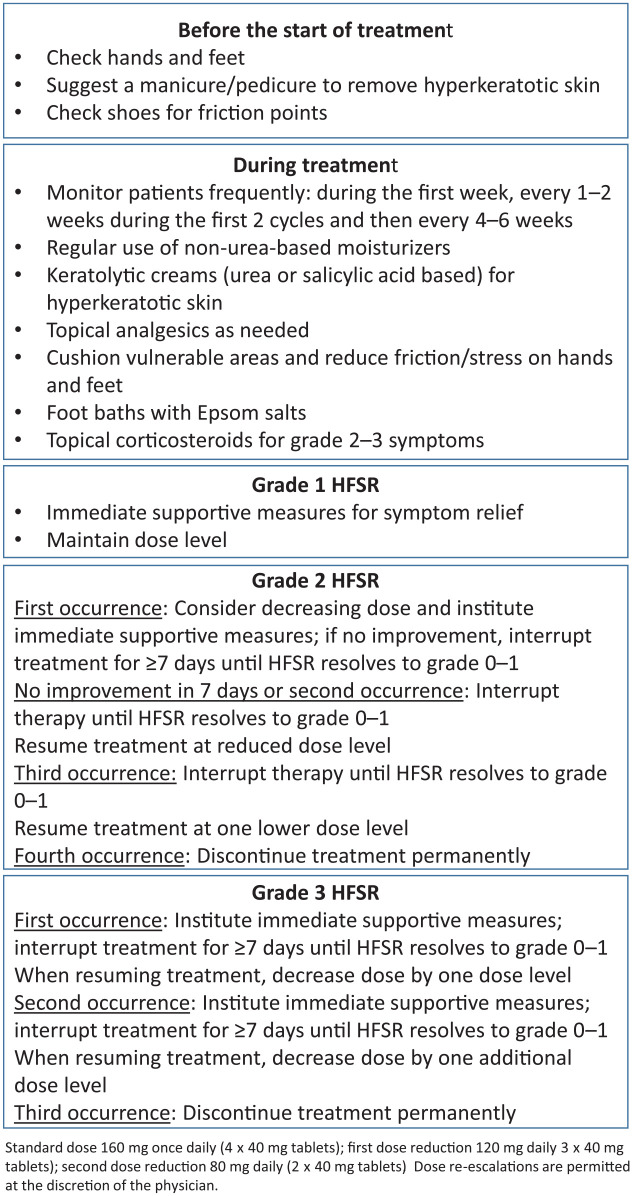
As aforementioned, appropriate AE management includes both preventive measures and symptom management, as well as dose adjustments and temporary interruption.37,42,43 Management of clinically relevant AEs (HFSR, hypertension, and fatigue) is summarized in Table 3, and Figure 1 provides detailed guidance for HFSR.
Table 3.
Management of selected AEs.
| Before starting treatment | Management | |
|---|---|---|
| HFSR | • Examination of the hands and feet • Manicure/pedicure to remove hyperkeratotic skin • Advise patients to avoid friction or stress on skin |
• Supportive measures as indicated • Regorafenib interruption and dose adjustment, in line with prescribing information (see Figure 1 for full details) |
| Hypertension | • Pre-existing hypertension should be controlled before the start of treatment | • Monitor blood pressure at least weekly • Patients should be advised to measure and record their blood pressure at home and to report elevation above a specified level • Hypertension should be controlled with appropriate antihypertensive therapy such as ACE inhibitors with sequential addition of a beta-blocker and calcium antagonist if needed. Diuretics should be avoided • Regorafenib interruption and dose adjustment in line with prescribing information |
| Fatigue | • Gentle exercise may be helpful • Thyroid replacement if hypothyroidism occurs • Prophylactic oral dexamethasone has been reported to lessen fatigue • Regorafenib interruption and dose adjustment, in line with prescribing information, may be needed for persistent fatigue |
Figure 1.
Management of HFSR. Adapted from McLellan et al.44
HFSR, hand–foot skin reaction.
HFSR can be particularly problematic for patients because it can affect their ability to undertake day-to-day activities, and should be pre-empted if possible and actively managed to allow patients to remain on active therapy for longer. Preventive strategies include skin examinations, manicures/pedicures, exfoliation of pressure points, creams, and avoidance of stress and friction on the skin.37,42,44 If HFSR occurs, management should be proactive and include reinforcement of preventive measures, topical creams, pain relief as necessary, and dose interruption/reduction (see Figure 1 and Table 3 for details).37,42,44
Hypertension occurs relatively frequently during regorafenib treatment, but it is not cumulative, and provided it is treated appropriately, it has negligible impact on patients’ QoL.43 Pre-existing hypertension should be well controlled before the start of regorafenib treatment and blood pressure should be measured frequently during treatment, at least weekly in the first two cycles and then at the start of subsequent cycles. Patients should be advised to monitor their blood pressure at home and to report specified increases.42,43 If hypertension occurs, it should be treated with appropriate antihypertensive therapy in line with usual guidelines, together with regorafenib dose interruption/reduction.
Fatigue can have a serious impact on QoL and may be difficult to manage.43 Patients should be encouraged to take gentle exercise.42 If thyroid function is impaired, appropriate replacement therapy may be needed.45 It has been reported that prophylactic oral dexamethasone lessens fatigue during regorafenib treatment.46 Dose interruption or reduction should be considered for persistent fatigue.
Diarrhea can be managed with standard anti-diarrhea medication such as loperamide. Patients should be given advice on diet modifications and be encouraged to keep a food record to help identify the cause.37,43 Dose interruption or reduction may be needed for persistent grade 3 diarrhea.
Since AEs may be an early indicator of anti-tumor activity, appropriate management to control symptoms is extremely important to minimize treatment interruption.41
Dosing and dose modifications
The standard dose of regorafenib is 160 mg (4 × 40 mg tablets) once daily for 3 weeks on followed by 1 week off therapy (3/1 schedule), which is one cycle.9,10 The dose can be adjusted in 40 mg (one tablet) weekly steps, but should not be lower than 80 mg/day; the maximum daily dose is 160 mg. Treatment modifications (dose reduction or delay) can be used in conjunction with appropriate symptomatic treatment to manage AEs. Re-escalation is an option once the toxicity has resolved, and this should be at the physician’s discretion, taking into account patient preference, as well as relevant clinical factors such as performance status and duration and severity of prior adverse reactions. The prescribing information provides guidance on treatment interruption and dose reduction for the management of AEs.9,10 Details of the dose modifications for HFSR management are given in Figure 1.44 The prescribing information also gives guidance on dose interruptions and modifications in case of liver toxicity. Dose interruptions and/or reductions may be required for other AEs based on individual safety and tolerability. In the pivotal trials (CORRECT and CONCUR), most patients were titrated to a tolerable regorafenib dose and some patients were able to return to the full dose once the toxicity had resolved.16,17 Dose and schedule modifications play a key role in managing AEs and allow patients to remain on therapy for as long as possible, thus optimizing benefit.37
Alternative starting doses
Many clinicians start regorafenib at a lower dose (80 or 120 mg/day) and escalate according to patient tolerance.1,19,37,47–49 A lower starting dose (80 or 120 mg/day) may be appropriate for certain patients, with subsequent dose escalation to reach the recommended dose of 160 mg/day subject to tolerability at the highest dose given.37 Real-life experience suggests that in patients who do not meet the entry criteria for CORRECT, regorafenib can be started at 120 mg/day and then escalated to 160 mg/day on Day 14 if tolerated.42 A randomized, phase II trial (ReDOS) evaluated the strategy of starting treatment at 80 mg/day for the first week, with weekly escalation to 120 mg/day and then to 160 mg/day if no significant drug-related toxicities or disease progression occurred; dose escalation is thus based on clinical judgement.50 This approach was superior to the standard starting dose of 160 mg/day, with more patients completing two cycles and starting the third cycle compared with those starting at the standard dose (43% versus 26%, p = 0.043) [ClinicalTrials.gov identifier: NCT02368886].50 There was a non-significant trend toward longer OS with the dose-escalation schedule, as well as improved QoL after the second week. A key challenge in a real-world clinical setting is to try and ensure patients reach their first tumor evaluation (8-week scan) and receive regorafenib long enough to potentially observe at least disease stabilization because continued durable benefit may be expected in some cases. The ReDOS study results indicate that a reduced starting dose (and thus a reduced need to actively handle AEs), followed by flexible dose escalation where tolerability allows, may be a way of achieving this in many patients who may not otherwise have been able to benefit from regorafenib treatment. The regorafenib dose-escalation schedule evaluated in ReDOS has been incorporated in the latest NCCN guidelines and may represent an important option for selected patients.19
Other trials have evaluated flexible first cycle dosing strategies. REGOCC-12 was a single-arm Japanese study in which regorafenib was administered at 120 mg/day with an option to increase to 160 mg/day from Cycle 2 onwards in the absence of significant toxicity.51 The disease control rate (primary endpoint) was 37%, and 7% of patients had stable disease lasting for ⩾6 months. It was concluded that the 120 mg starting dose appeared to have similar efficacy to the 160 mg dose.51 Another study (RESET) also evaluated a 120 mg/day starting dose of regorafenib in Japanese patients. In this study, dose escalation to 160 mg on Day 15 was achieved in only 6/70 patients and the disease control rate was lower than expected (32.4%), suggesting that efficacy may be compromised if dose escalation is not implemented.52 However, PFS was not reported, and this was a small study, which should therefore be interpreted with caution. The randomized, phase II REARRANGE study investigated different dosing approaches of induction (first cycle) treatment with regorafenib in patients with mCRC, including an assessment of safety, efficacy, and dose-related outcomes.53 While REARRANGE did not meet its primary endpoint of improved tolerability in patients who received regorafenib at a reduced or intermittent dose (120 mg/day for 3 weeks on/1 week off, or 160 mg/day for 1 week on/1 week off, respectively) compared with the standard dose, flexible dosing showed numerical improvement in relevant AEs such as fatigue, HFSR and hypertension, while maintaining efficacy, thereby supporting the results from ReDOS.50,53
Communicating with patients
Patient preference is a very important consideration, particularly in later lines of therapy, and it is essential to explain the options to the patient and involve them in treatment decisions. For example, trifluridine/tipiracil (TAS-102) is an alternative efficacious salvage option in patients who are refractory to standard therapies. Crossover treatment with both drugs improves survival, and, in one study, median OS was longer in patients who crossed over from regorafenib to TAS-102 (11.5 months) compared with those who crossed over from TAS-102 to regorafenib (7.6 months); nonetheless, decisions on their sequencing will depend on individual patient characteristics and preferences.54–56 Key points to communicate to patients are summarized in Box 1. The SHARE Communication Framework provides a basis for shared decision-making in third-line treatment of patients with mCRC. It provides a five-step structure to facilitate physician–patient interaction that covers the advantages and disadvantages of each treatment option, potential side effects, and treatment expectations.57
Box 1.
Checklist of points to cover with patients.
| • Characteristics of available third-line treatments • The importance of personalizing treatment: there are several options for third-line therapy with different characteristics and the choice is influenced by patient characteristics and preferences • Patient expectations on treatment outcomes, in terms of both efficacy (benefit through disease stabilization) and safety • Advantages and disadvantages of each option, including safety and potential side effects • Strategies for management of side effects, including preventive measures • Patient’s role in adhering to treatment and reporting AEs promptly • The association between early occurrence of HFSR and longer OS, as motivation to continue therapy • The importance of close and regular contact with the oncology team, especially in the first few weeks of treatment |
AE, adverse event; HFSR, hand–foot skin reaction; OS, overall survival.
The shift to an oral, targeted agent places greater responsibility on the patient to adhere to treatment; education about the importance of adherence and its potential impact on clinical outcomes has an important role. The drug’s mechanism of action, potential AEs, and the strategy for preventing and managing these AEs should be explained to the patient before starting treatment. The importance of close and regular communication between the patient and the oncology team, especially within the first few weeks of therapy, was described earlier. Patients should be informed about the importance of reporting any possible AEs promptly so that they can be managed appropriately, increasing the likelihood of remaining on treatment for a longer period.
Conclusion
Key take-home messages to consider when treating patients with regorafenib in the third-line setting are listed in Box 2. Appropriate patient selection and good management of AEs can allow patients to achieve maximum benefit from regorafenib, maintain good QoL, and potentially increase survival time, allowing patients to receive subsequent lines of therapy. Fourth-line therapy can include trifluridine/tipiracil, which is effective after regorafenib.58
Box 2.
Take-home messages.
| • The availability of more lines of therapy has contributed to improvements in OS • Treatment sequencing and planning is important to ensure patients have the opportunity to receive all available treatment options • Patient choice, tolerability, and QoL become more important in later lines • Regorafenib is an active drug in the third-line setting; survival benefit is achieved through disease stabilization with some patients achieving long-term benefit • Regorafenib has a consistent and generally manageable AE profile • Effective management of AEs, which may include dose modifications where appropriate, enables patients to remain on therapy and benefit from it • There is evidence that the occurrence of HFSR may be an early marker of clinical benefit • Close patient monitoring plays an important role in the proactive management of AEs: patients should be monitored at least weekly for the first 4 weeks (first cycle) and then every 2 weeks for the next cycle, and every month thereafter • A lower starting dose with weekly escalation may be considered to help certain patients to stay on treatment for a longer period, past the first tumor evaluation at 8 weeks • Good communication between patients and the oncology team is essential |
AE, adverse event; HFSR, hand–foot skin reaction; OS, overall survival; QoL, quality of life.
To optimize outcomes, consideration should be given to offering eligible patients all active agents, as later-line therapies are associated with significant OS benefit. It is expected that the treatment of patients with mCRC will continue to improve with increasing individualization of treatment as more biomarkers are identified and allow a greater understanding about which patient subgroups will or will not benefit from specific agents.
Acknowledgments
Editorial assistance in the preparation of this article was provided by Christine Drewienkiewicz of Open Health Medical Communications (Choice) with financial support from Bayer.
Footnotes
Conflicts of interest statement: FL has held consulting/advisory roles with Amgen, Bayer, and Sanofi; is a speakers bureau member for Amgen, Bayer, Roche, and Sanofi; has received research funding from Amgen, Bayer, Merck Serono, and Roche; and has received personal fees from Amgen, Merck Serono, and Roche. J-BB has received honoraria from Amgen, AstraZeneca, Bayer, Celgene, Merck Serono, Pierre Fabre, Roche, Sanofi, Servier, and Shire; has held consulting/advisory roles with Amgen, Bayer, Merck Serono, and Servier; and has received personal fees from Amgen, Merck Serono, and Roche. FP has held consulting/advisory roles with Amgen, Bayer, Lilly, Merck Serono, Roche, Sanofi and Servier. HT has received honoraria from Bayer, Chugai Pharma, Lilly Japan, Merck Serono, Taiho Pharmaceutical, Takeda, and Yakult Honsha, and has received research funding from Boehringer Ingelheim, MSD Oncology, Otsuka, and Takeda; SY has received honoraria from Bayer Yakuhin, Bristol-Myers Squibb Japan, Chugai Pharma, Lilly Japan, Merck Serono, Ono Pharmaceutical, Sanofi, Taiho Pharmaceutical, Takeda, and Yakult Honsha. TB-S has held consulting/advisory roles with Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Genentech/Roche, Glenmark, Ipsen, Lilly, Merrimack, National Comprehensive Cancer Network, and Regeneron and Research to Practice (a Multi-tumor Regional Symposia series); has received research funding from National Comprehensive Cancer Network, National Cancer Institute, and Oncolytics; has provided expert testimony for Helsinn Therapeutics; and holds other relationships with ARMO BioSciences, Exelixis, Merck, Polaris, and SillaJen. LA, F-CK, TM, R-HX, TW and SZ have no conflicts of interest to declare.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Bayer.
Contributor Information
Fotios Loupakis, Unit of Oncology 1, Department of Clinical and Experimental Oncology, Istituto Oncologico Veneto, IRCCS, Padua, Italy.
Lorenzo Antonuzzo, Medical Oncology Unit, Azienda Ospedaliero Universitaria Careggi, Florence, Italy.
Jean-Baptiste Bachet, Sorbonne Université, Service d’hépato-gastro-entérologie, Hôpital Pitié Salpêtrière – Paris 6, APHP, Paris, France.
Feng-Che Kuan, Department of Hematology and Oncology, Chang-Gung Memorial Hospital, Chiayi, Taiwan.
Teresa Macarulla, Medical Oncology Department, University Hospital of Vall d’Hebron, Barcelona, Spain.
Filippo Pietrantonio, Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy; Department of Oncology and Hemato-oncology, University of Milan, Milan, Italy.
Rui-Hua Xu, Department of Medical Oncology, Sun Yat-sen University Cancer Center, Collaborative Innovation Center for Cancer Medicine, Guangzhou, China.
Hiroya Taniguchi, Department of GI Oncology, National Cancer Center Hospital East, Kashiwa, Japan.
Thomas Winder, Department of Internal Medicine II, Hematology and Oncology, Academic Teaching Hospital Feldkirch, Feldkirch, Austria; Swiss Tumor Molecular Institute, Zürich, Switzerland.
Satoshi Yuki, Department of Gastroenterology and Hepatology, Hokkaido University Hospital, Sapporo, Japan.
Shan Zeng, Department of Oncology, Xiangya Hospital, Central South University, Changsha, Hunan, China.
Tanios Bekaii-Saab, Department of Hematology and Medical Oncology, Mayo Clinic, Phoenix, AZ, USA.
References
- 1. Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016; 27: 1386–1422. [DOI] [PubMed] [Google Scholar]
- 2. Vogel A, Hofheinz RD, Kubicka S, et al. Treatment decisions in metastatic colorectal cancer – beyond first and second line combination therapies. Cancer Treat Rev 2017; 59: 54–60. [DOI] [PubMed] [Google Scholar]
- 3. Cremolini C, Schirripa M, Antoniotti C, et al. First-line chemotherapy for mCRC–a review and evidence-based algorithm. Nat Rev Clin Oncol 2015; 12: 607–619. [DOI] [PubMed] [Google Scholar]
- 4. Elias D, Vigano L, Orsi F, et al. New perspectives in the treatment of colorectal metastases. Liver Cancer 2016; 6: 90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wild AT, Yamada Y. Treatment options in oligometastatic disease: stereotactic body radiation therapy – focus on colorectal cancer. Visc Med 2017; 33: 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arnold D, Prager GW, Quintela A, et al. Beyond second-line therapy in patients with metastatic colorectal cancer: a systematic review. Ann Oncol 2018; 29: 835–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 2011; 129: 245–255. [DOI] [PubMed] [Google Scholar]
- 8. Abou-Elkacem L, Arns S, Brix G, et al. Regorafenib inhibits growth, angiogenesis, and metastasis in a highly aggressive, orthotopic colon cancer model. Mol Cancer Ther 2013; 12: 1322–1331. [DOI] [PubMed] [Google Scholar]
- 9. STIVARGA® (regorafenib). US food and drug administration prescribing information: Bayer Healthcare Pharmaceuticals Inc, Whippany, NJ, https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/203085s007lbl.pdf. (2018, accessed 2 September 2020). [Google Scholar]
- 10. Regorafenib (Stivarga). European Medicines Agency. Summary of product characteristics: Bayer AG, Leverkusen, Germany, https://www.ema.europa.eu/en/documents/product-information/stivarga-epar-product-information_en.pdf (2018, accessed 2 September 2020). [Google Scholar]
- 11. Sun W, Patel A, Normolle D, et al. A phase 2 trial of regorafenib as a single agent in patients with chemotherapy-refractory, advanced, and metastatic biliary tract adenocarcinoma. Cancer 2019; 125: 902–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mir O, Brodowicz T, Italiano A, et al. Safety and efficacy of regorafenib in patients with advanced soft tissue sarcoma (REGOSARC): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 2016; 17: 1732–1742. [DOI] [PubMed] [Google Scholar]
- 13. Duffaud F, Mir O, Boudou-Rouquette P, et al. Efficacy and safety of regorafenib in adult patients with metastatic osteosarcoma: a non-comparative, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol 2019; 20: 120–133. [DOI] [PubMed] [Google Scholar]
- 14. Davis LE, Bolejack V, Ryan CW, et al. Randomized double-blind phase II study of regorafenib in patients with metastatic osteosarcoma. J Clin Oncol 2019; 37: 1424–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lombardi G, De Salvo GL, Brandes AA, et al. Regorafenib compared with lomustine in patients with relapsed glioblastoma (REGOMA): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol 2019; 20: 110–119. [DOI] [PubMed] [Google Scholar]
- 16. Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013; 381: 303–312. [DOI] [PubMed] [Google Scholar]
- 17. Li J, Qin S, Xu R, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2015; 16: 619–629. [DOI] [PubMed] [Google Scholar]
- 18. Røed Skårderud M, Polk A, Kjeldgaard Vistisen K, et al. Efficacy and safety of regorafenib in the treatment of metastatic colorectal cancer: a systematic review. Cancer Treat Rev 2018; 62: 61–73. [DOI] [PubMed] [Google Scholar]
- 19. National Cancer Comprehensive Network. NCCN guidelines for colon cancer, version 4. https://www.nccn.org/professionals/physician_gls/default.aspx#site (2020, accessed 2 September 2020).
- 20. Adenis A, de la, Fouchardiere C, Paule B, et al. Survival, safety, and prognostic factors for outcome with regorafenib in patients with metastatic colorectal cancer refractory to standard therapies: results from a multicenter study (REBECCA) nested within a compassionate use program. BMC Cancer 2016; 16: 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Van Cutsem E, Martinelli E, Cascinu S, et al. Regorafenib for patients with metastatic colorectal cancer who progressed after standard therapy: results of the large, single-arm, open-label phase IIIb CONSIGN study. Oncologist 2019; 24: 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ducreux M, Fiala-Buskies S, Cervantes A, et al. Safety and effectiveness of regorafenib in patients with metastatic colorectal cancer (mCRC) in routine clinical practice: final analysis from the prospective, observational CORRELATE study. Ann Oncol 2018; 29(Suppl. 5): abstract O-012. [DOI] [PubMed] [Google Scholar]
- 23. Schulz H. Clinical efficacy and safety of regorafenib (REG) in the treatment of metastatic colorectal cancer (mCRC) in daily practice in Germany: final results of the prospective multicentre non-interventional RECORA study. J Clin Oncol 2018; 36(Suppl. 4S): abstract 748. [Google Scholar]
- 24. Yamaguchi K, Komatsu Y, Satoh T, et al. Large-scale, prospective observational study of regorafenib in Japanese patients with metastatic colorectal cancer in a real-world clinical setting. Oncologist 2019; 24: e450–e457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kopeckova K, Buchler T, Bortlicek Z, et al. Regorafenib in the real-life clinical practice: data from the Czech Registry. Target Oncol 2017; 12: 89–95. [DOI] [PubMed] [Google Scholar]
- 26. Van Cutsem E, Verheul HM, Flamen P, et al. Imaging in colorectal cancer: progress and challenges for the clinicians. Cancers (Basel) 2016; 8: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ricotta R, Verrioli A, Ghezzi S, et al. Radiological imaging markers predicting clinical outcome in patients with metastatic colorectal carcinoma treated with regorafenib: post hoc analysis of the CORRECT phase III trial (RadioCORRECT study). ESMO Open 2016; 1: e000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arai H, Miyakawa K, Denda T, et al. Early morphological change for predicting outcome in metastatic colorectal cancer after regorafenib. Oncotarget 2017; 8: 110530–110539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ozaki Y, Shindoh J, Gonoi W, et al. Changes in CT morphology can be an independent response marker for patients receiving regorafenib for colorectal liver metastases: retrospective pilot study. BMC Cancer 2018; 18: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. ClinicalTrials.gov. Evaluation of treatment Response with CHOI and RECIST Criteria and CT texture analysis in patients with metastatic colorectal cancer treated with regorafenib (TEXCAN). https://clinicaltrials.gov/ct2/show/NCT02699073 (2019, accessed 15 May 2019).
- 31. Hendlisz A, Deleporte A, Vandeputte C, et al. Regorafenib assessment in refractory advanced colorectal cancer: RegARd-C study protocol. BMJ Open 2015; 5: e007189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grothey A, Falcone A, Humblet Y, et al. Characteristics of patients with metastatic colorectal cancer (mCRC) treated with regorafenib who had progression-free survival (PFS) >4 months: subgroup analysis of the phase 3 CORRECT trial. Ann Oncol 2016; 27(Suppl. 6): abstract 516P. [Google Scholar]
- 33. Kim TW, Qin S, Huang L, et al. Subgroup analysis by progression-free survival (PFS) of Asian patients treated with regorafenib in the phase 3 CONCUR trial. Ann Oncol 2017; 28(Suppl. 3): abstract P-295. [Google Scholar]
- 34. Spallanzani A, Gelsomino F, Caputo F, et al. Immunotherapy in the treatment of colorectal cancer: a new kid on the block. J Cancer Metastasis Treat 2018; 4: 28. [Google Scholar]
- 35. Grothey A, Van Cutsem E, Wagner A, et al. Characteristics and outcomes of patients enrolled in the CORRECT and CONCUR phase 3 trials of regorafenib for metastatic colorectal cancer (mCRC). Ann Oncol 2015; 26(Suppl. 4): O–011. [Google Scholar]
- 36. Shitara K, Yamanaka T, Denda T, et al. REVERCE: a randomized phase II study of regorafenib followed by cetuximab versus the reverse sequence for previously treated metastatic colorectal cancer patients. Ann Oncol 2019; 30: 259–265. [DOI] [PubMed] [Google Scholar]
- 37. Grothey A, George S, van Cutsem E, et al. Optimizing treatment outcomes with regorafenib: personalized dosing and other strategies to support patient care. Oncologist 2014; 19: 669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Grothey A. Time profile of adverse events (AEs) from regorafenib (REG) treatment for metastatic colorectal cancer (mCRC) in the phase III CORRECT study. J Clin Oncol 2013; 31(Suppl. 15): abstract 3637. [Google Scholar]
- 39. Grothey A, Van Cutsem E, Sobrero A, et al. Time course of regorafenib-associated adverse events in the phase III CORRECT study. J Clin Oncol 2013; 31(Suppl. 4): abstract 467. [Google Scholar]
- 40. Grothey A. Hand-foot skin reaction (HFSR) and outcomes in the phase 3 CORRECT trial of regorafenib for metastatic colorectal cancer (mCRC). J Clin Oncol 2017; 35(Suppl. 15): abstract 3551. [Google Scholar]
- 41. Giampieri R, Prete MD, Prochilo T, et al. Off-target effects and clinical outcome in metastatic colorectal cancer patients receiving regorafenib: the TRIBUTE analysis. Sci Rep 2017; 7: 45703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hofheinz RD, Arnold D, Kubicka S, et al. Improving patient outcomes with regorafenib for metastatic colorectal cancer – patient selection, dosing, patient education, prophylaxis, and management of adverse events. Oncol Res Treat 2015; 38: 300–308. [DOI] [PubMed] [Google Scholar]
- 43. De Wit M, Boers-Doets CB, Saettini A, et al. Prevention and management of adverse events related to regorafenib. Support Care Cancer 2014; 22: 837–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McLellan B, Ciardiello F, Lacouture ME, et al. Regorafenib-associated hand-foot skin reaction: practical advice on diagnosis, prevention, and management. Ann Oncol 2015; 26: 2017–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pani F, Massidda M, Pusceddu V, et al. Regorafenib-induced hypothyroidism and cancer-related fatigue: is there a potential link? Eur J Endocrinol 2017; 177: 85–92. [DOI] [PubMed] [Google Scholar]
- 46. Fukuoka S, Shitara K, Noguchi M, et al. Prophylactic use of oral dexamethasone to alleviate fatigue during regorafenib treatment for patients with metastatic colorectal cancer. Clin Colorectal Cancer 2017; 16: e39–e44. [DOI] [PubMed] [Google Scholar]
- 47. Calcagno F, Lenoble S, Lakkis Z, et al. Efficacy, safety and cost of regorafenib in patients with metastatic colorectal cancer in French clinical practice. Clin Med Insights Oncol 2016; 10: 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gotfrit J, Vickers M, Sud S, et al. Real-life treatment of metastatic colorectal cancer with regorafenib: a single-centre review. Curr Oncol 2017; 24: 234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Osawa H. Response to regorafenib at an initial dose of 120 mg as salvage therapy for metastatic colorectal cancer. Mol Clin Oncol 2017; 6: 365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bekaii-Saab TS, Ou FS, Ahn DH, et al. Regorafenib dose-optimisation in patients with refractory metastatic colorectal cancer (ReDOS): a randomised, multicentre, open-label, phase 2 study. Lancet Oncol 2019; 20: 1070–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kudo T, Kato T, Kagawa Y, et al. Phase II dose titration study of regorafenib for patients with unresectable metastatic colorectal cancer that progressed after standard chemotherapy. J Clin Oncol 2018; 36(Suppl. 4S): abstract 821. [Google Scholar]
- 52. Suzuki T, Sukawa Y, Imamura CK, et al. A phase II study of regorafenib with a lower starting dose in patients with metastatic colorectal cancer: exposure-toxicity analysis of unbound regorafenib and its active metabolites (RESET Trial). Clin Colorectal Cancer 2020; 19: 13–21.e3. [DOI] [PubMed] [Google Scholar]
- 53. Argiles G, Mulet Margalef N, Valladares-Ayerbes M, et al. Results of REARRANGE trial: A randomized phase 2 study comparing different dosing approaches for regorafenib (REG) during the first cycle of treatment in patients (pts) with metastatic colorectal cancer (mCRC). Ann Oncol 2019; 30(Suppl. 4): abstract O-026. [Google Scholar]
- 54. Abrahao ABK, Ko YJ, Berry S, et al. A comparison of regorafenib and TAS-102 for metastatic colorectal cancer: a systematic review and network meta-analysis. Clin Colorectal Cancer 2018; 17: 113–120. [DOI] [PubMed] [Google Scholar]
- 55. Moriwaki T, Fukuoka S, Taniguchi H, et al. Propensity score analysis of regorafenib versus trifluridine/tipiracil in patients with metastatic colorectal cancer refractory to standard chemotherapy (REGOTAS): a Japanese Society for Cancer of the Colon and Rectum multicenter observational study. Oncologist 2018; 23: 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sueda T, Sakai D, Kudo T, et al. Efficacy and safety of regorafenib or TAS-102 in patients with metastatic colorectal cancer refractory to standard therapies. Anticancer Res 2016; 36: 4299–4306. [PubMed] [Google Scholar]
- 57. The SHARE Communication Framework for shared decision-making in 3rd-line treatment of metastatic colorectal cancer (mCRC), https://giconnect.info/the-share-communication-framework-for-shared-decision-making-in-3rd-line-treatment-of-metastatic-colorectal-cancer-mcrc/
- 58. Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 2015; 372: 1909–1919. [DOI] [PubMed] [Google Scholar]