Abstract
The risk of a gonadal tumor is high in testicular disorder of sexual development (DSD) with the Y chromosome, but cases of DSD without the Y chromosome are extremely rare. We reported a gonadal tumor in a phenotypically male individual with 46, XX testicular DSD. A testicular tumor was incidentally found in a 32-year-old phenotypic male who was presented to the hospital with male infertility. A diagnosis of 46, XX testicular DSD was made by the presentation of karyotype analysis of 46, XX with the sex-determining region of the Y chromosome (SRY) positive and gonadal tissue without female gonads. Surgery was performed due to a gradually growing tumor. The partial orchidectomy was performed with the diagnosis of a benign Leydig cell tumor in frozen biopsy.
Keywords: 46, XX testicular DSD, SRY, Leydig cell tumor
The sex-determining region of the Y chromosome (SRY) gene mainly contributes to male differentiation. The SRY gene leads gonadal primordia to the formation of testis. Initially reported by de la Chapelle in 1964, 46, XX testicular disorder of sexual development (DSD) is characterized by a male phenotype despite a 46, XX karyotype (de la Chapelle et al., 1964). The incidence rate is 1:20000 (de la Chapelle, 1981). Among all cases of 46, XX testicular DSD, 80% originate from the translocation of Y chromosome material with the SRY gene during meiosis (de la Chapelle et al., 1990). The presence of the Y chromosome in DSD patients increases the risk for a gonadal tumor (Pleskacova et al., 2010). A gonadal tumor in 46, XX testicular DSD without the Y chromosome is extremely rare. This paper reports on a case of Leydig cell tumor with 46, XX SRY-positive testicular DSD.
Case Presentation
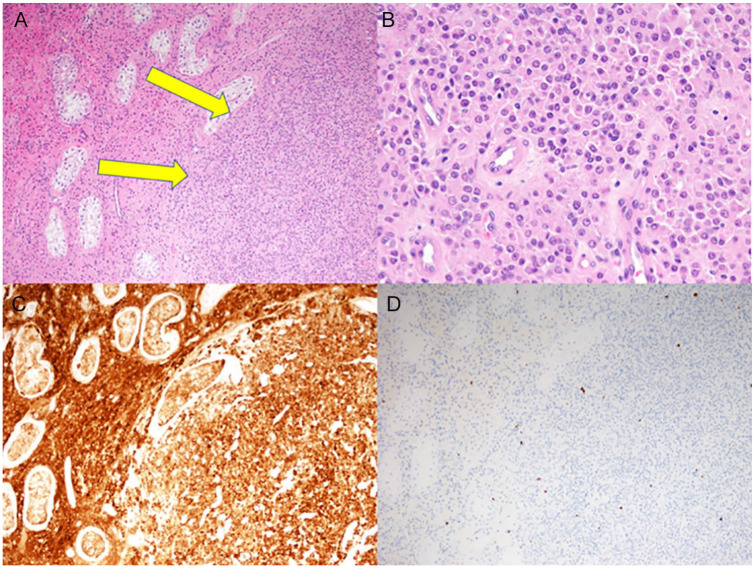
A 32-year-old phenotypic male visited a nearby clinic after experiencing infertility for several years. He was referred to our hospital for a left gradual growing testicular tumor over 3 months and azoospermia. Upon examination, his height was noted as 172 cm and his weight as 78.2 kg, both within the normal range for his age group. Neonate screening was negative. Both testes were palpable and the volume was 14 mL, slightly less than normal upon physical examination. His puberty stage was Tanner 5 and the growth of pubic and axillary hair was normal without gynecomastia. An ultrasound revealed a well circumscribed mass measuring 10 mm × 6.2 mm with markedly increased vascular flow. Serum levels of tumor markers (α-fetoprotein, intact human chorionic gonadotropin) were within the normal range. Semen analyses showed azoospermia and hormone profiles were elevated gonadotropins levels as follows: luteinizing hormone 17.5 mIU/mL, follicle stimulating hormone 26.1 mIU/mL, total testosterone 3.65 ng/mL (reference range: 1.31–8.17) measured by electrochemiluminescence method, estradiol 23.8 pg/mL, and Prolactin (RIA) 22.4 ng/mL. The karyotype analysis in 50 cells was 46, XX without mosaicism. Fluorescent in situ hybridization (FISH) analysis was performed to detect a lack or presence of the SRY gene. The SRY gene was detected in the short (p) arm of the X chromosome (Figure 1). Molecular analysis for the identification of Y chromosome deletion showed a lack of Y chromosome azoospermia factors. A pelvic MRI showed a homogeneous mass with a lower intensity than the normal testis on T2-weighted images (Figure 2). No abnormal finding was observed in the abdomen. A partial resection of the left testis was selected for the diagnosis of a benign Leydig cell tumor on frozen biopsy. Histologically, the tumor showed relatively sharply delimited growth without capsular formation. Seminiferous tubules were not involved in the tumor and the existing tubules and hyperplastic Leydig cells were shoved aside (Figure 3A). Tumor cells were polygonal with monomorphic oval nuclei and eosinophilic cytoplasm (Figure 3B). Immunohistochemically, the tumor cells were positive for Inhibin-alpha. The Ki-67 labelling index was low (<1%). The tumor was therefore diagnosed as a Leydig cell tumor. The nontumor part of the testis showed normal testicular morphology. A normal testis specimen showed that the presence of normal Leydig cells and Sertoli cells was identified without the absence of germ cells and female gonadal tissue. As a result of these findings, the patient was diagnosed as having 46, XX testicular DSD with a Leydig cell tumor. Postoperative total testosterone level was within normal range (4.09 ng/mL). Twelve months after the surgery, there was no evidence of any local recurrence or distant metastases.
Figure 1.
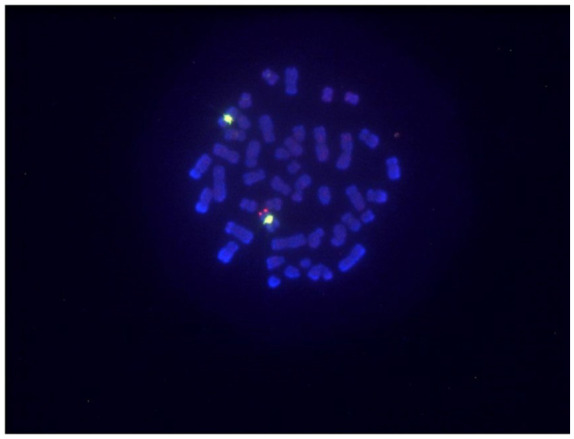
Fluorescent in situ hybridization (FISH). Note. FISH analysis was performed on both interphase nuclei and metaphase spreads of the patient. Sex determining-region Y was identified.
Figure 2.
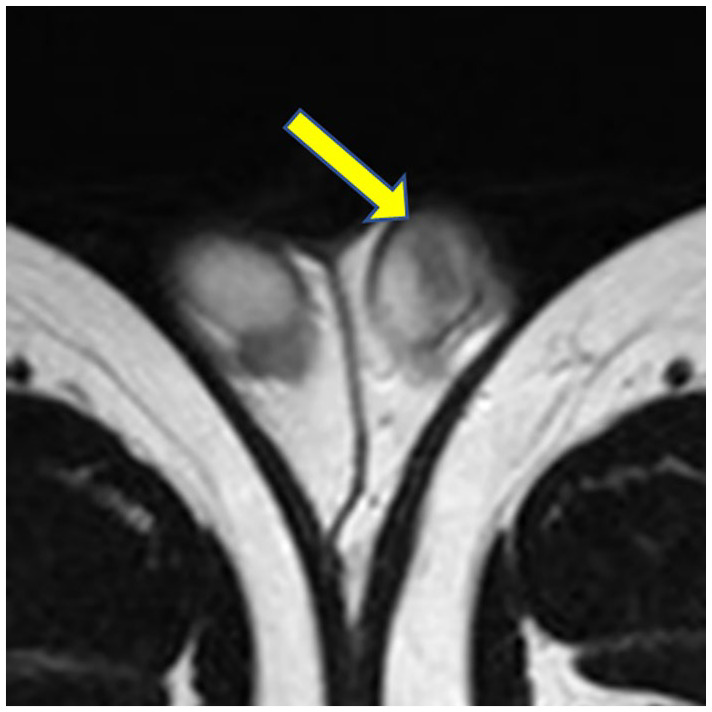
MRI T2-weighted image. Note. The tumor showed more hypointense than surrounding testicular parenchyma.
Figure 3.
Histological findings of the testicular biopsy specimen. (A) Tumor cell proliferation without capsular formation shoves aside the existing seminiferous tubules (arrows) (HE stain x10). (B) Tumor cells were polygonal with oval nuclei and eosinophilic cytoplasm (HE stain x10). (C) Immunohistochemically, the tumor cell and existing hyperplastic Leydig cells were positive for inhibin-alpha (x10). (D) A small number of tumor cells were positive for Ki-67 (x10).
Discussion
DSD is divided into three classes on the basis of the karyotype analysis: 46, XY DSD, sex chromosomal DSD and 46, XX DSD. Moreover, 46, XX DSD are classified into three groups: disorders of androgen excess, unclassified disorders, and disorders of gonadal development. Of those, disorders of gonadal development are divided into three groups: 46, XX (Ovo)testicular DSD, monogenic forms of primary ovarian insufficiency and syndromic forms (Cools et al., 2018). Most often, 46, XX testicular DSD presents with a normal phenotype and translocation of the SRY gene. Diagnosis was performed from the presentation of male hypogonadotropic hypogonadism, gynecomastia, and azoospermia with age due to a lack of symptoms and a normal phenotype at birth (Gao et al., 2013). The lack of an azoospermia factor in the presence of the Y chromosome long arm leads to male infertility in every case (Chen et al., 2019; Ergun-Longmire et al., 2005). Assisted reproductive technology using donor sperm is the only option for conceiving a child.
Differential diagnosis of 46, XX testicular DSD, which is 46, XX karyotype and phenotypic males with testes, is ovotesticular DSD (Grinspon & Rey, 2016). Early gonadectomy for one option may be considered since gonadal tumors with malignant potential occur in approximately 3% of all cases of ovotesticular DSD containing Y materials, although the recent international trend is to wait for surgery until becoming an adult (Scarpa, 2017), giving importance to an exclusion diagnosis. In comparison to 46, XX testicular DSD, ambiguous genitalia is the common manifestation in 90% of the cases and normal genitalia is in 10% of the cases (Sultan et al., 2002). Both cases that show normal genitalia with 46, XX karyotype are difficult to distinguish. Therefore, final diagnosis is based on histopathological analysis with or without ovary component of gonadal tissues. The present case was a phenotypic male showing 46, XX karyotype with SRY translocation on one of the X chromosomes and testis tissue without ovary tissue.
A testicular Leydig cell tumor was diagnosed in our patient. Generally, DSD is associated with an increased risk for a germ cell tumor. Disturbed gonadal development and microenvironment in DSD can lead to abnormal expression such as POU5F1, KITLG, and TSPY, which are associated with the development of germ cell tumor (Hersmus et al., 2017). The only previous case, one reported by Carcavilla et al., identified a testicular germ cell tumor with 46, XX testicular DSD (Carcavilla et al., 2008). In the present case, Leydig cell tumor classified as sex cord stromal tumor originated from nongerm cell tumor, which is a different origin of germ cell tumor. To the best of our knowledge, this was the first report of a testicular nongerm cell tumor (Leydig cell tumor) with 46, XX testicular DSD. The mechanisms of tumorigenesis on Leydig cell tumor have not yet been elucidated. As one known factor, chronic and permanent stimulation of LH receptor which expressed on Leydig cell induced tumorigenesis (Kent Christensen & Peacock, 1980). Chronic high gonadotropin level from birth in the present case may be related to tumorigenesis. Besides, several Leydig cell tumor cases with aneuploidies representing 47, XXY Klinefelter syndrome and mice model of Klinefelter syndrome are previously reported (Eicher et al., 1991; Maqdasy et al., 2015; Wistuba et al., 2010). But the karyotype analysis on patients with testicular tumor was neglected in most studies (Paduch, 2006; Raman et al., 2005). Karyotype analysis in patients with Leydig cell tumor is seldom conducted and incidence rate of chromosomal abnormalities such as DSD may be possibly underestimated.
Leydig cell tumor is mostly benign, and 5% have a malignant potential. Of those, a minority that is clinically malignant present with metastasis (Gheorghisan-Galateanu, 2014). Fortunately, the current case might be benign, as we found no malignancy features in the Leydig cell tumor, including hormonal production, age (≧40), tumor size (≧ 5 cm), infiltrative margins, and hemorrhage and necrosis extending beyond the testicular parenchyma (Farkas et al., 2000; Mameli et al., 2016).
The present case had several limitations. The other molecular analysis on Y chromosome such as PABY and TSPY in relation to gonadal tumor was not carried out. Endocrinological data on LH, FSH, total testosterone, and estradiol in childhood were not identified.
In conclusion, this paper reports an extremely rare case of an SRY-positive 46, XX testicular DSD with a Leydig cell tumor.
Acknowledgments
The authors would like to thank the patients participating in this study for providing clinical information and images and for signing informed consents.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Consent for Publication: Written informed consents for publication were obtained from the patients.
ORCID iDs: Akiyoshi Osaka  https://orcid.org/0000-0002-5159-8028
https://orcid.org/0000-0002-5159-8028
Kazutaka Saito  https://orcid.org/0000-0002-7261-0188
https://orcid.org/0000-0002-7261-0188
References
- Carcavilla A., Alonso M., Ezquieta B., García-Galloway E., Barrio R., Nistal M. (2008). An XX male with an intratubular undifferentiated germ cell neoplasia. Fertility and Sterility, 90(5), 2005. e3-2005. e5. [DOI] [PubMed] [Google Scholar]
- Chen T., Tian L., Wu F., Xuan X., Ma G., Tang R., Lu J. (2019). Clinical and genetic analysis in males with 46, XX disorders of sex development: A reproductive centre experience of 144 cases. Andrologia, 51(4), e13232. [DOI] [PubMed] [Google Scholar]
- Cools M., Nordenström A., Robeva R., Hall J., Westerveld P., Flück C., Köhler B., Berra M., Springer A., Schweizer K., Pasterski V., & COST Action BM1303 working group 1. (2018). Caring for individuals with a difference of sex development (DSD): A consensus statement. Nature Reviews Endocrinology, 14(7), 415–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Chapelle A. (1981). The etiology of maleness in XX men. Human Genetics, 58(1), 105–116. [DOI] [PubMed] [Google Scholar]
- de la Chapelle A., Hastbacka J., Korhonen T., Maenpaa J. (1990). The etiology of XX sex reversal. Reproduction, Nutrition, Development, Suppl 1, 39s–49s. doi: 10.1051/rnd:19900704. [DOI] [PubMed] [Google Scholar]
- de la Chapelle A., Hortling H., Niemi M., Wennström J. (1964). XX sex chromosomes in a human male: First case. Acta Medica Scandinavica, 175(Suppl 412), 25–38. [DOI] [PubMed] [Google Scholar]
- Eicher E. M., Hale D. W., Hunt P. A., Lee B. K., Tucker P. K., King T. R., Eppig J. T., Washburn L. L. (1991). The mouse Y* chromosome involves a complex rearrangement, including interstitial positioning of the pseudoautosomal region. Cytogenetic and Genome Research, 57(4), 221–230. [DOI] [PubMed] [Google Scholar]
- Ergun-Longmire B., Vinci G., Alonso L., Matthew S., Tansil S., Lin-Su K., McElreavey K., New M. I. (2005). Clinical, hormonal and cytogenetic evaluation of 46, XX males and review of the literature. Journal of Pediatric Endocrinology and Metabolism, 18(8), 739–748. [DOI] [PubMed] [Google Scholar]
- Farkas L. M., Székely J. G., Pusztai C., Baki M. (2000). High frequency of metastatic Leydig cell testicular tumours. Oncology, 59(2), 118–121. [DOI] [PubMed] [Google Scholar]
- Gao X., Chen G., Huang J., Bai Q., Zhao N., Shao M., Jiao L., Wei Y., Chang L., Li D., Yang L. (2013). Clinical, cytogenetic, and molecular analysis with 46, XX male sex reversal syndrome: Case reports. Journal of Assisted Reproduction and Genetics, 30(3), 431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheorghisan-Galateanu A. A. (2014). Leydig cell tumors of the testis: A case report. BMC Research Notes, 7(1), 656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinspon R. P., Rey R. A. (2016). Disorders of sex development with testicular differentiation in SRY-negative 46, XX individuals: Clinical and genetic aspects. Sexual Development, 10(1), 1–11. [DOI] [PubMed] [Google Scholar]
- Hersmus R., van Bever Y., Wolffenbuttel K. P., Biermann K., Cools M., Looijenga L. H. (2017). The biology of germ cell tumors in disorders of sex development. Clinical Genetics, 91(2), 292–301. [DOI] [PubMed] [Google Scholar]
- Kent Christensen A., Peacock K. C. (1980). Increase in Leydig cell number in testes of adult rats treated chronically with an excess of human chorionic gonadotropin. Biology of Reproduction, 22(2), 383–391. [DOI] [PubMed] [Google Scholar]
- Mameli C., Selvaggio G., Cerini C., Bulfamante G., Madia C., Riccipetitoni G., Zuccotti G. V. (2016). Atypical Leydig cell tumor in children: Report of 2 cases. Pediatrics, 138(5), e20160151. [DOI] [PubMed] [Google Scholar]
- Maqdasy S., Bogenmann L., Batisse-Lignier M., Roche B., Franck F., Desbiez F., Tauveron I. (2015). Leydig cell tumor in a patient with 49, XXXXY karyotype: A review of literature. Reproductive Biology and Endocrinology, 13(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paduch D. A. (2006). Testicular cancer and male infertility. Current Opinion in Urology, 16(6), 419–427. [DOI] [PubMed] [Google Scholar]
- Pleskacova J., Hersmus R., Oosterhuis J. W., Setyawati B. A., Faradz S. M., Cools M., Wolffenbuttel K. P., Lepl J., Drop S. L., Looijenga L. H. (2010). Tumor risk in disorders of sex development. Sexual Development. Genetics, Molecular Biology, Evolution, Endocrinology, Embryology, and Pathology of Sex Determination and Differentiation, 4(4–5), 259–269. [DOI] [PubMed] [Google Scholar]
- Raman J. D., Nobert C. F., Goldstein M. (2005). Increased incidence of testicular cancer in men presenting with infertility and abnormal semen analysis. The Journal of Urology, 174(5), 1819–1822. [DOI] [PubMed] [Google Scholar]
- Scarpa M. (2017). Ovotesticular differences of sex development: Surgery or not surgery? That is the question. Frontiers in Pediatrics, 5, 1–3. doi: 10.3389/fped.2017.00231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan C., Paris F., Jeandel C., Lumbroso S., Galifer R. B. (2002). Ambiguous genitalia in the newborn. Paper presented at the Seminars in Reproductive Medicine, 20(03) 181–188. [DOI] [PubMed] [Google Scholar]
- Wistuba J., Luetjens C. M., Stukenborg J., Poplinski A., Werler S., Dittmann M., Damm O. S., Hamalainen T., Simoni M., Gromoll J. (2010). Male 41, XXY* mice as a model for Klinefelter syndrome: Hyperactivation of Leydig cells. Endocrinology, 151(6), 2898–2910. [DOI] [PubMed] [Google Scholar]