Abstract
Background:
Despite its increasing use in the management of musculoskeletal conditions, questions remain regarding the preparation methods of platelet-rich plasma (PRP) and its clinical applications for intra-articular hip disorders, including femoroacetabular impingement syndrome (FAIS), labral pathology, and osteoarthritis (OA).
Purpose:
To systematically review and assess the preparation methods and clinical outcomes from randomized clinical trials (RCTs) on the use of PRP for intra-articular hip disorders.
Study Design:
Systematic review; Level of evidence, 2.
Methods:
A systematic review in accordance with the 2009 PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines was performed in September 2019. The Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, PubMed, Ovid Medline, and Embase were queried for studies regarding the use of PRP to treat intra-articular hip disorders. Qualifying articles were English-language RCTs describing the use of PRP for intra-articular hip disorders, either as standalone treatment or surgical augmentation. Two authors independently assessed article eligibility. Data pertaining to patient characteristics, indication for treatment, PRP preparation method, follow-up period, and clinical outcomes were extracted. Study results were qualitatively reported and quantitatively compared using meta-analysis when appropriate.
Results:
Seven RCTs met inclusion criteria. Four studies described the use of PRP for hip OA and 3 utilized PRP at arthroscopy for FAIS and labral tears. Outcomes after PRP for OA demonstrated improvement in validated patient-reported outcome measures for up to 1 year; however, pooled effect sizes found no statistically significant difference between PRP and hyaluronic acid (HA) regarding pain visual analog scale scores at short-term (≤2 months; P = .27), midterm (4-6 months; P = .85), or long-term (1 year; P = .42) follow-up. When injected at arthroscopy, 1 study reported improved outcomes, 1 reported no difference in outcomes, and 1 reported worse outcomes compared with controls. The meta-analysis demonstrated no statistically significant difference on the modified Harris Hip Score (mHHS) between PRP and control cohorts at a minimum 1-year follow-up. There were considerable deficiencies and heterogeneity in the reporting of PRP preparation methods for both indications.
Conclusion:
Treatment of OA with PRP demonstrated reductions in pain and improved patient-reported outcomes for up to 1 year. However, there was no statistically significant difference between PRP and HA in pain reduction. Likewise, for FAIS and labral surgery there was no statistically significant difference in mHHS outcomes between patients treated with PRP and controls. Given the limited number of studies and variability in PRP preparations, additional high-quality randomized trials are warranted.
Keywords: platelet-rich plasma, osteoarthritis, hip arthroscopy, femoroacetabular impingement, outcomes
Intra-articular hip pathologies, notably osteoarthritis (OA) and femoroacetabular impingement syndrome (FAIS), represent a major cause of disability and pain for many patients and a substantial economic burden on society.32 This has led to increased interest in disease-modifying and hip joint preservation treatments, such as orthobiologics, over the past few decades. News coverage and media reporting of outcomes after orthobiologic treatments in elite athletes have also drawn considerable attention, further increasing the demand for these therapies and potentially generating unrealistic patient expectations.2
Platelet-rich plasma (PRP) is one of the most commonly used orthobiologics. The term “PRP” broadly describes autologous preparations of peripheral blood that have undergone centrifugation to increase the platelet concentration; however, the other constituents can be quite variable, including the concentration of leukocytes.7 The clinical use of PRP aims to promote tissue regeneration through the effects of growth factors and cytokines released by activated platelets24 and has been described for the treatment of a wide spectrum of musculoskeletal conditions, such as acute muscle injuries,21,43 degenerative6,10,18,26,28,45 and traumatic9 cartilage lesions, meniscal disorders,5,39 ligamentous injuries,5,19,36,40 rotator cuff injuries,20,46 plantar fasciitis,31 lateral epicondylitis,34,38 and other tendinopathies.16 Likewise, PRP has been increasingly used to treat a variety of hip disorders, either alone or as an augmentation for hip arthroscopic procedures.8,13,41,42
Despite its increased utilization in the treatment of intra-articular hip disorders, the efficacy of PRP on the treatment of these pathologies has not been completely elucidated, and its use remains controversial. Furthermore, the influence of composition on the regenerative characteristics of PRP has yet to be determined.30,35 The primary purpose of this systematic review was to examine and provide a comprehensive review of the evidence from randomized clinical trials (RCTs) on PRP preparation methods for the treatment of intra-articular hip pathologies, including FAIS, labral pathology, and OA and clinical outcomes. The secondary aim of this review was to describe the range of PRP preparation methods. Correspondingly, it was hypothesized that there would be considerable heterogeneity in the reporting of PRP preparation methods, and that there would be a significant benefit in patients who received PRP injections, as indicated by statistically significant differences in patient-reported outcomes.
Methods
Article Identification and Selection
A systematic review of PRP application for the treatment of intra-articular hip disorders was conducted in accordance with the 2009 PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. A comprehensive literature search was performed using the Cochrane Database of Systematic Reviews, the Cochrane Central Register of Controlled Trials, PubMed (1980 to present), Medline (1980 to present), and Embase (1980 to present). Queries were performed and screened between September and November 2019. The following Boolean search terms were used: “platelet rich plasma” AND (“hip arthroscopy” OR “hip pathology” OR “femoroacetabular impingement” OR “osteoarthritis” OR “biomechanics”). The systematic review protocol was registered with the PROSPERO prospective registrar of systematic reviews (No. CRD42020159802).
Inclusion criteria were English-language literature and randomized clinical studies reporting on the use of PRP for intra-articular hip disorders, either as a standalone treatment or as an augmentation for arthroscopic hip procedures. Publications were excluded if they involved the treatment of extra-articular hip disorders, tendon and/or muscle injection of PRP, animal studies, or nonrandomized studies, or did not include at least 1 patient-reported outcome. There was no minimum duration of follow-up that was required for inclusion.
Two investigators (F.L.G. and D.B.H.) independently reviewed the abstracts from all identified articles. If necessary, full-text articles were reviewed to appropriately identify articles for inclusion. Additionally, reference lists from the included studies were reviewed to verify that all eligible articles were considered.
Data Extraction
Data were extracted from the full text of all eligible articles using standardized data collection forms and included patient characteristics, indication for treatment, PRP preparation method, control group and treatment (eg, placebo, hyaluronic acid [HA], and corticosteroid), follow-up period, and clinical outcomes. For continuous variables, the means, standard deviations, and ranges were collected when reported. Data were recorded into a custom spreadsheet using a modified information extraction table.23
Assessment of Methodological Quality
The methodologic quality of each included study was assessed with the Coleman Methodology Score (CMS).11 The CMS is a 10-item questionnaire scored from 0 to 100 points that has been used in the rating of RCTs22 and is based on the CONSORT (Consolidated Standards of Reporting Trials) statement.1 A perfect score of 100 points represents a study design that largely avoids the influence of chance, biases, and confounding factors.11
Statistical Analysis
A series of meta-analyses were conducted for outcomes reported in at least 3 studies. The meta-analyses were performed using a random-effects model with inverse variance for data pooling and weighting. Forest plots were constructed for visual presentation of the results from individual studies and pooled summary estimates. A P value <.05 was considered statistically significant. Study heterogeneity was assessed through I 2 tests. All statistical analyses were performed using Review Manager 5 (Version 5.3; The Nordic Cochrane Center).44
For the variables that did not meet the aforementioned criteria (reported in a minimum of 3 studies), a meta-analysis was considered methodologically inappropriate, and the data were qualitatively synthesized and reported in both narrative fashion and table format. Extracted data were presented as means, medians, and ranges, where appropriate.
Results
Study Characteristics
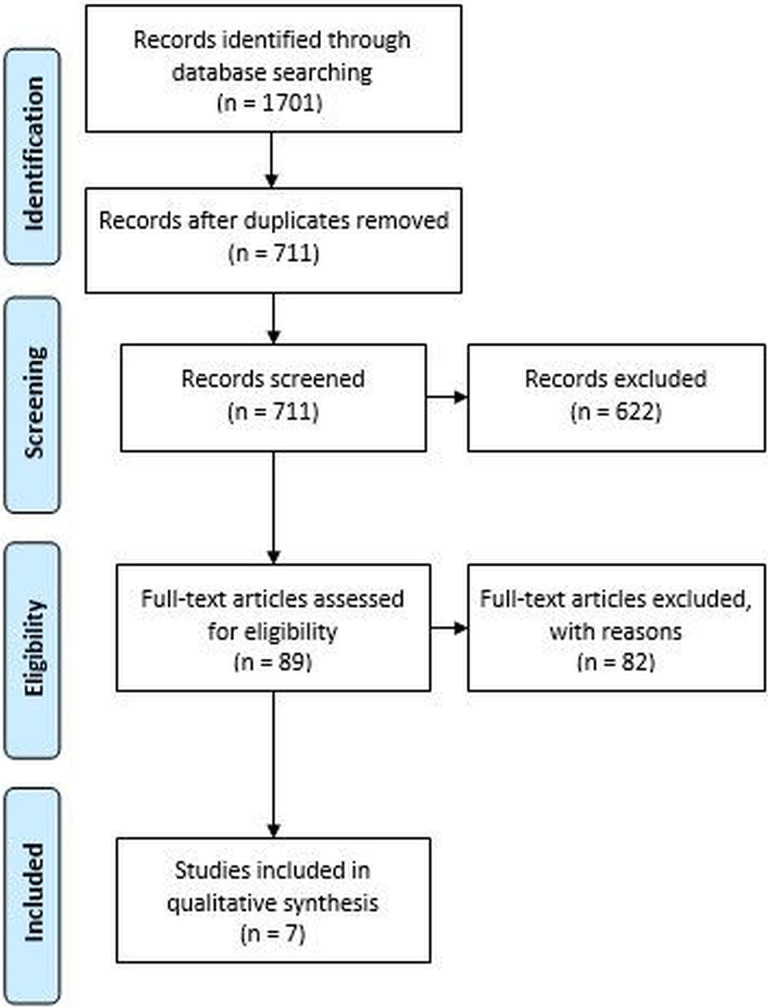
The literature search identified 1701 studies through the initial database query. After duplicates and non-English titles were removed, 711 articles were screened, with 7 articles3,13–15,29,41,42 ultimately meeting the inclusion criteria (Figure 1). The mean CMS for the 7 studies assessed in this review was 87.2 (range, 83-93).
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowchart showing application of the selection criteria to the studies identified with the search strategy.
Four studies3,13–15 described the use of PRP for hip OA treatment, and 3 studies29,41,42 reported on PRP augmentation at the time of arthroscopic FAIS and labral surgery. The weighted mean age was 45.3 years (range, 34.6-72.5 years), and the weighted mean number of PRP injections per patient was 1.9 (range, 1-3 injections). Detailed study characteristics are reported in Table 1.
Table 1.
Study Characteristicsa
| Author (Year) | Pathology/Treatment | Total No. of Cases (No. of Cases per Group) | Mean Age, y (Range) | Number of PRP Injections (Interval Between Injections) | Control Group(s): (Type and No. of Injections; Interval Between Injections) | Follow-up | Coleman Methodology Score |
|---|---|---|---|---|---|---|---|
| PRP for OA treatment | |||||||
| Battaglia (2013)3 | OA K-L grades 2-4/US-guided injections | 100 (PRP group: NR; HA group: NR) | 53 (25-76) | 3 intra-articular injections (2 wk apart) | HA (3 intra-articular injections; 2 wk apart) | 1, 3, 6, and 12 mo | 89 |
| Dallari (2016)13 | OA K-L grades 1-4/US-guided injections | 111 (PRP group: 44; PRP + HA group: 31; HA group: 36) | NR (18-65) | 3 intra-articular injections (1 wk apart) | a. PRP + HA b. HA (3 intra-articular injections; 1 wk apart) |
2, 6, and 12 mo | 93 |
| Di Sante (2016)14 | OA K-L grades 2-3/US-guided injections | 43 (PRP group: 21; HA group: 22) | 72.5 (NR) | 3 intra-articular injections (1 wk apart) | HA (3 intra-articular injections; 1 wk apart) | 1 and 4 mo | 84 |
| Doria (2017)15 | OA K-L grades 0-2/US-guided injections | 80 (PRP group: 40; HA group: 40) | 67.6 (40-72) | 3 intra-articular injections (1 wk apart) | HA (3 intra-articular injections; 1 wk apart) | 6 and 12 mo | 83 |
| PRP augmentation for FAIS and labral surgery | |||||||
| Redmond (2015)42 | FAIS + labral tear/arthroscopic labral repair or debridement with or without acetabuloplasty, femoroplasty, microfracture, and/or capsular repair | 271 (PRP group: 91; bupivacaine group: 180) | 36.2 (NR) | Single intra-articular injection at the end of surgery | 0.25% bupivacaine (single intra-articular injection at the end of surgery) | 3 and 24 mo | 86 |
| LaFrance (2015)29 | FAIS + labral tear/arthroscopic labral repair + femoral osteochondroplasty | 35 (PRP group: 20; normal saline group: 15) | 34.6 (18-63) | Single intra-articular injection at the end of surgery | 0.9% normal saline (single intra-articular injection at the end of surgery) | 1, 3, 6, and 12 mo | 83 |
| Rafols (2015)41 | FAIS + labral tear/arthroscopic labral repair + femoral osteochondroplasty | 57 (PRP group: 30; no injection group: 27) | 35.3 (16-52) | Single intra-articular injection at the end of surgery | No injection | 2 d; 3, 6, and 24 mo | 93 |
aFAIS, femoroacetabular impingement syndrome; HA, hyaluronic acid; K-L, Kellgren-Lawrence classification; NR: not reported; OA, osteoarthritis; PRP, platelet-rich plasma; US, ultrasound.
PRP Preparation and Processing Protocols
Among the included studies, there was heterogeneity in PRP preparation and processing protocols. The initial whole blood volume, centrifugation rate, and duration were reported in 5 studies3,13–15,42 (71.4%). For the first centrifugation, the median rate was 1500 rpm (range, 1480-3100 rpm) and the median duration was 6 minutes (range, 5-15 minutes). For the second centrifugation, the median rate was 3400 rpm (range, 3100-3500 rpm) and the median duration was 12.5 minutes (range, 9-15 minutes). The mean whole blood volume extracted was 94.8 mL (range, 8-150 mL). The volume of PRP injected into each hip joint was reported in all studies, with a mean injected PRP volume of 4.9 mL (range, 3-7 mL). The mean PRP platelet concentration after preparation was reported in 3 studies3,14,42 with a mean platelet concentration 3.8 times greater than that of whole blood (range, 2-7 times).
An exogenous activation method used to induce platelet degranulation and release of growth factors and cytokines into the PRP solution was reported in 3 studies (42.9%).3,13,41 Two studies used 10% calcium chloride,3,13 and 1 study used an undisclosed exogenous activator.41 Three studies14,15,29 (42.9%) did not mention whether an activator was used, and 1 study42 (14.3%) specifically reported not using an activator. Detailed PRP preparation information is reported in Table 2.
Table 2.
PRP Preparation and Composition Data for the Included Studiesa
| Author (Year) | PRP Preparation Method | Volume Blood Drawn (mL) | PRP Volume for Each Injection (mL) | Platelet Concentration in the PRP | Platelet Activation | PAW Classification |
|---|---|---|---|---|---|---|
| PRP for OA treatment | ||||||
| Battaglia (2013)3 | 2-spin method (1800 rpm for 15 min + 3500 rpm for 10 min) | 150 | 5 | 7× baseline | 10% calcium chloride | P3-x-A |
| Dallari (2016)13 | 2-spin method (1480 rpm for 6 min + 3400 rpm for 15 min) | 150 | 5 | NR | 10% calcium chloride | NA-x-NA |
| Di Sante (2016)14 | 2-spin method (3100 rpm for 9 min + 3100 rpm for 9 min) | 8 | 3 | 2× baseline | NR | P2-NA-B |
| Doria (2017)15 | 2-spin method (1480 rpm for 6 min + 3400 rpm for 15 min) | 150 | 5 | NR | NR | NA-NA-NA |
| PRP augmentation for FAIS and labral surgery | ||||||
| Redmond (2015)42 | 1-spin method (1500 rpm for 5 min) | 16 | 4-7 | 2.5× baseline | No | P2-Bβ |
| LaFrance (2015)29 | Accelerate Concentrating System (Exactech Biologics) | NR | 5 | NR | NR | P3-NA-Aα |
| Rafols (2015)41 | GPS III System (Biomet Biologics) | NR | 6 | NR | Yes (undisclosed) | P4-x-Aα |
aFAIS, femoroacetabular impingement syndrome; NR, not reported; OA, osteoarthritis; PAW, platelets, activation, and white cells; PRP, platelet-rich plasma.
PRP for OA Treatment
There were 4 studies3,13–15 that evaluated outcomes after intra-articular injection of PRP for the management of OA (Tables 1 and 3). All 4 studies, a total of 334 patients, compared the efficacy of PRP with that of hyaluronic acid (HA). The severity of symptomatic hip OA included across the studies varied considerably, ranging from grade 0 to grade 4 according to the Kellgren-Lawrence (KL) classification.27
Table 3.
Patient-Reported Outcomes After PRP Treatment for Hip Osteoarthritisa
| Follow-up | ||||
|---|---|---|---|---|
| Outcome Score | Pretreatment | Short-term (≤2 mo) | Midterm (4-6 mo) | Long-term (12 mo) |
| Battaglia (2013) 3 | ||||
| VAS | ||||
| PRP | 5.47 | 3.72 | 4.29 | 4.75 |
| HA | 5.97 | 3.58 | 4.04 | 4.59 |
| HHS | ||||
| PRP | 58.11 | 73.72 | 70.23 | 65.73 |
| HA | 62.90 | 78.02 | 75.79 | 72.55 |
| Dallari (2016) 13 | ||||
| VAS | ||||
| PRP | — | 2.3 | 2.1 | 2.4 |
| HA | — | 3.8 | 4.4 | 4.2 |
| PRP + HA | — | 3.5 | 3.5 | 3.8 |
| WOMAC | ||||
| PRP | — | 73 | 72 | — |
| HA | — | 59 | 59 | — |
| PRP + HA | — | 59 | 59 | — |
| HHS | ||||
| PRP | — | — | — | — |
| HA | — | — | — | — |
| PRP + HA | — | — | — | — |
| Di Sante (2016) 14 | ||||
| VAS | NR | |||
| PRP | 7.08 | 4.73 | 6.36 | |
| HA | 6.32 | 5.27 | 3.63 | |
| WOMAC–pain | NR | |||
| PRP | 58.8 | 44.2 | 53.4 | |
| HA | 42.3 | 29.6 | 19.9 | |
| WOMAC–stiffness | NR | |||
| PRP | 53.7 | 46.4 | 47.2 | |
| HA | 57.6 | 47.6 | 32.9 | |
| WOMAC–physical function | NR | |||
| PRP | 59.8 | 49.1 | 50.8 | |
| HA | 45.8 | 39.1 | 28.3 | |
| Doria (2017) 15 | ||||
| VAS | ||||
| PRP | 7.5 | NR | 6.3 | 6.4 |
| HA | 7.8 | NR | 6.3 | 6.1 |
| WOMAC–pain | ||||
| PRP | 23.7 | NR | 7.8 | 7.4 |
| HA | 24 | NR | 9.7 | 9 |
| WOMAC–stiffness | ||||
| PRP | 3.8 | NR | 2.1 | 2 |
| HA | 4.3 | NR | 3.1 | 3.1 |
| WOMAC–physical function | ||||
| PRP | 29.4 | NR | 12.3 | 12 |
| HA | 28.5 | NR | 11.3 | 10.9 |
| HHS | ||||
| PRP | 64 | NR | 75 | 78 |
| HA | 62 | NR | 74 | 75 |
aHA, hyaluronic acid; HHS, Harris Hip Score; NR, not reported; PRP, platelet-rich plasma; VAS, visual analog scale (scaled to 0-10); WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index; —, data were reported as box plot graphs only.
Battaglia et al3 and Dallari et al13 reported improvement in pain visual analog scale (VAS) from pretreatment to short-term (up to 2 months) and midterm (between 4 and 6 months) follow-up time points. Di Sante et al14 reported improvement in VAS from pretreatment to short-term follow-up, but not at the midterm follow-up. Doria et al15 did not evaluate patients at the short-term follow-up but reported improvement in the VAS from pretreatment to midterm and long-term (12 months) follow-ups. In 2 studies,3,13 the VAS at the long-term follow-up demonstrated increased pain compared with the short-term follow-up; however, these were still significantly improved compared with pretreatment pain scores. The remaining 2 studies did not allow such temporal comparison because the VAS was not assessed at either the short-term15 or long-term14 follow-up.
Three studies3,13,15 evaluated patients with the Harris Hip Score (HHS). Both Battaglia et al3 and Doria et al15 demonstrated statistically significant improvements in HHS after treatment. In contrast, Dallari et al did not find any significant improvement in HHS in either group. However, none of the studies reported statistically significant differences between control and treatment groups for posttreatment changes in HHS.
Three studies13–15 evaluated patients with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) questionnaire. Dallari et al13 reported significantly greater improvement in the WOMAC scores from pretreatment to short-term and midterm follow-ups for the PRP-treated group, but a loss of statistical significance compared with HA and PRP + HA at the long-term follow-up. Di Sante et al14 evaluated patients only at short-term and midterm follow-ups and found no statistically significant difference in the WOMAC scores before and after PRP treatment, while HA treatment resulted in statistically significant improvements in WOMAC scores at 4 weeks; however, these results were not sustained at 16 weeks. Finally, Doria et al15 evaluated the patients only at midterm and long-term follow-ups, reporting improvement of the WOMAC scores compared with pretreatment; however, no statistically significant difference was found between the PRP- and HA-treated groups (Table 3).
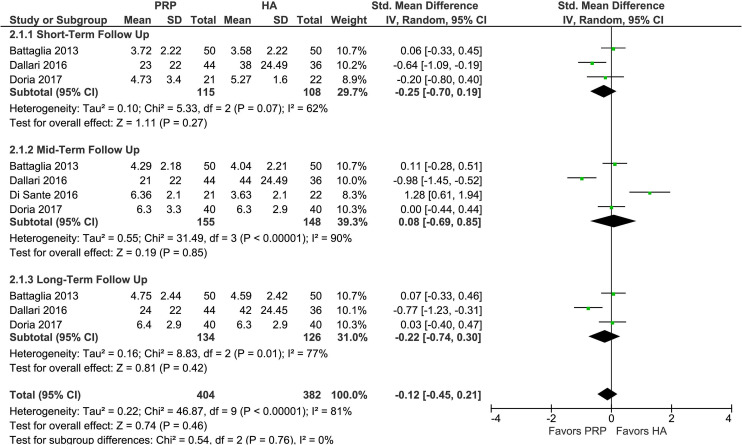
A meta-analysis was performed, and the pooled effect sizes showed no statistically significant difference between PRP and HA in terms of pain reduction as determined by the VAS, either at the short-term (P = .27), midterm (P = .85), or long-term (P = .42) follow-up (Figure 2). Heterogeneity, as evaluated by I 2 tests, demonstrated values of 62%, 90%, and 77% at the short-, mid-, and long-term follow-ups, respectively.
Figure 2.
Forest plot depicting visual analog scale (VAS) score summary estimate (center of the diamond) and 95% CI (width of diamond) comparing platelet-rich plasma (PRP) and hyaluronic acid (HA) injections for hip osteoarthritis at the short-term (up to 2 months), midterm (between 4 and 6 months), and long-term (1 year) follow-ups. Means and SDs are reported for the VAS scale. IV, inverse variance.
PRP Augmentation for FAIS and Labral Surgery
Three studies29,41,42 evaluated the efficacy of PRP injection as an augmentation for FAIS and labral surgery (Tables 1 and 4). Arthroscopic labral repair plus femoral osteochondroplasty was performed in 92 hips, and arthroscopic labral repair or debridement with or without acetabuloplasty, femoroplasty, microfracture, and/or capsular repair was done on 271 hips.
Table 4.
Patient-Reported Outcome Scores for PRP Augmentation for Femoroacetabular Impingement Syndrome and Labral Surgerya
| Author (Year) | Outcome Score | Group | Pretreatment | Short-term FU | Midterm FU | Long-term FU | Extended FU |
|---|---|---|---|---|---|---|---|
| LaFrance (2015)29 | mHHS | PRP | 51.9 | 66.6 | 78.4 | 75.9 |
NR |
| Normal saline | 50.3 | 59.6 | 83.4 | 81.3 | |||
| HOS-ADL | PRP | 59.1 | 68.4 | 79.6 | 84.1 | ||
| Normal saline | 55.7 | 58.9 | 88.3 | 85.0 | |||
| HOS-SS | PRP | 35.1 | 31.6 | 61.7 | 65.4 | ||
| Normal saline | 29.2 | 20.2 | 75.6 | 75.2 | |||
| NAHS | PRP | 54.9 | 66.3 | 81.3 | 82.0 | ||
| Normal saline | 52.6 | 59.1 | 87.6 | 80.9 | |||
| Rafols (2015)41 | VAS | PRP | 5.04 | 3.04 | 0.71 | NR | NR |
| No PRP | 4.94 | 5.20 | 0.77 | ||||
| mHHS | PRP | 70.79 | NR | 94.80 | NR | 97.10 | |
| No PRP | 71.48 | 94.00 | 94.76 | ||||
| Redmond (2015)42 | VAS | PRP | 5.64 | NR | 2.62 | NR | 3.36 |
| BUP | 5.44 | 2.58 | 2.52 | ||||
| mHHS | PRP | 62.77 | 82.09 | 78.58 | |||
| BUP | 64.40 | 80.93 | 82.63 | ||||
| HOS-ADL | PRP | 64.49 | 81.63 | 79.77 | |||
| BUP | 66.43 | 83.66 | 83.57 | ||||
| HOS-SS | PRP | 41.31 | 61.43 | 67.47 | |||
| BUP | 43.52 | 61.83 | 69.08 | ||||
| NAHS | PRP | 58.02 | 76.62 | 78.34 | |||
| BUP | 61.30 | 77.65 | 81.27 |
aPosttreatment scores are divided into short-term (up to 2 months postop), midterm (3-6 months postop), long-term (12 months postop), and extended (24 months postop) follow-up time points. BUP, bupivacaine; FU, follow-up; HOS-ADL, Hip Outcome Score Activities of Daily Living subscale; HOS-SS, Hip Outcome Score Sport-Specific subscale; mHHS, modified Harris Hip Score; NAHS: Non-Arthritic Hip Score; NR, not reported; PRP, platelet-rich plasma; VAS, visual analog scale.
LaFrance et al29 compared the efficacy of PRP with that of 0.9% normal saline injection. The authors described improvement in all patient-reported outcome measures throughout the study period; however, no statistically significant difference between the groups was observed.
Rafols et al41 compared PRP injection and no injection. The authors found some differences in outcomes between the groups, with the PRP group reporting lower VAS scores only on the second day after surgery and a lower incidence of joint effusion at the 6-month follow-up. All other outcomes showed no statistically significant differences between the groups.
Redmond et al42 evaluated the efficacy of PRP injection compared with bupivacaine injection. Surgeries included either labral repair or labral debridement, as well as other intra-articular procedures, including acetabuloplasty, femoroplasty, microfracture, and capsular repair. The authors reported that the PRP group demonstrated a lower mean modified HHS (mHHS) and a higher VAS score than the bupivacaine group 2 years after surgery (Table 4), but the reason for these findings could not be identified.
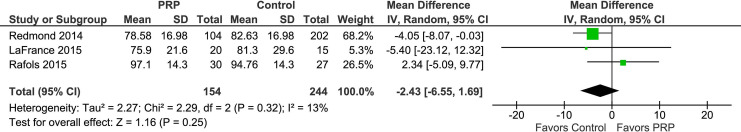
A meta-analysis was performed, and the pooled effect sizes showed no statistically significant difference between PRP and controls for mHHS at a minimum 12-month follow-up (P = .25) (Figure 3). Heterogeneity was assessed with I 2 tests, with a calculated value of 13%.
Figure 3.
Forest plot depicting modified Harris Hip Score (mHHS) summary estimate (center of the diamond) and 95% CI (width of diamond) comparing platelet-rich plasma (PRP) and controls for femoroacetabular impingement syndrome and labral surgery with a minimum 12-month follow-up. Means and SDs are reported in points on the mHHS scale. IV, inverse variance.
Discussion
The most important finding of this investigation was the absence of statistically significant differences between PRP, HA, and combined therapies in the treatment of hip OA. The treatment of hip OA with PRP accounted for the majority of relevant publications, representing 57.1% of RCTs identified by this systematic review. In this patient population, PRP injection resulted in improvements in both pain and select patient-reported outcome measures for up to 1 year after injection. However, meta-analysis and pooled effect sizes demonstrated no statistically significant difference between PRP- and HA-treated groups in terms of pain reduction, as determined by the VAS, for up to 1 year after injection in patients with hip OA.
Although the pain reduction and improvements in patient-reported outcome measures may last for up to 12 months after PRP injection for hip OA, this effect was most evident in the first 4 to 6 months after treatment and seemed to decrease after that period.3,13 Systematic reviews and meta-analyses of RCTs have similarly reported that PRP can reduce pain and improve functional status in patients affected by knee OA.12,33,45 However, indications and results from knee OA treatment with PRP cannot be immediately extrapolated to the hip, since cartilage characteristics and biomechanics are disparate among joints.4,17,37
In addition, PRP is increasingly being used as augmentation for hip arthroscopy8,13,41,42; however, the relevant literature is still limited and controversial. In this review, 3 such studies met inclusion criteria. Rafols et al41 reported lower postoperative pain scores at 48 hours and fewer joint effusions at 6 months in patients receiving PRP at the time of arthroscopic FAIS and labral surgery. Conversely, LaFrance et al29 compared the efficacy of PRP with that of a 0.9% normal saline injection and reported that PRP injection did not improve the clinical outcomes up to 1 year postoperatively in patients undergoing arthroscopic FAIS and labral surgery. Finally, Redmond et al42 compared the efficacy of PRP with that of bupivacaine injection for arthroscopic FAIS and labral surgery and reported that the PRP group had higher pain scores and lower mHHS than the bupivacaine group 2 years after surgery. Pooled effect sizes from the meta-analysis demonstrated no statistically significant difference in mHHS between patients treated with PRP and the controls, with a minimum follow-up of 1 year.
Other noteworthy findings include the heterogeneity of PRP preparation methods and the considerable deficiencies in the reporting of preparation-related factors and basic attributes of PRP preparations. Similar to the systematic review performed by Chahla et al,7 the current study found no consensus in the timing or number of injections needed, nor agreement on the formulation or standardized reporting methodology of processing. This variability in the preparation and resultant end product may influence the reported outcomes; therefore, it is important to interpret the results in light of a potentially heterogeneous PRP product and deficiencies in reporting. In order to improve the reporting and ultimately the ability to assess the impact of PRP treatment, reported metrics should include, at a minimum, the starting volume, anticoagulant utilized, detailed preparation technique (including spin rate and/or gravitational forces and number of separate centrifuge events), make and model of centrifuge, use of activating agents, and final concentrations of platelets, nucleated cells, and red blood cells.7
It should be noted that evaluating the patient-reported outcomes after PRP augmentation for arthroscopic hip surgery may be challenging. It is well-documented that statistically significant changes in patient-reported outcome instruments may in fact have little clinical significance.25 Additionally, it is difficult for these studies to differentiate between the clinical impact of PRP alone and the clinical impact of the PRP plus the associated arthroscopic techniques, which in 1 study42 included labral repair or labral debridement with or without acetabuloplasty, femoroplasty, microfracture, and/or capsular repair. These concomitant procedures may confound analysis and the identification of any clinical benefit directly attributable to the PRP injection. In addition to pathology-specific outcome scores, there is a need for sensitive and specific objective outcome tools and advanced imaging modalities to enable the accurate assessment of outcomes for musculoskeletal conditions.
The results of this study must be interpreted in the context of the following limitations. First, no attempt was made to correlate PRP preparation methods with the patient-reported outcomes. Such evaluation is currently confounded by the different time points used in individual studies, as well as the deficiencies in the reporting of PRP preparation methods and composition characteristics. Second, although there is high-quality evidence suggesting that the use of PRP is associated with the improvement of select patient-reported outcome measures, the use of different outcome instruments in the included RCTs did not allow for a quantitative effect estimate to be calculated. With the exception of the VAS for OA and the mHHS for FAIS and labral surgery patients, the results presented are largely qualitative. Furthermore, the strength of this systematic review was limited by the available literature, including both articles that were identified for inclusion and those that were potentially omitted or missed during the query. For example, search terms did not specifically include “labrum” or “labral,” which may have resulted in the potential omission of relevant studies. In the articles that were identified for inclusion, the descriptions of PRP preparation protocols in clinical studies were inconsistent, and a large proportion of the studies did not provide sufficient information to allow the protocol to be reproduced. The current reporting of PRP preparation and composition does not enable comparison of the PRP products being delivered to patients. Last, the data describing the role of PRP as augmentation for hip arthroscopy are unclear, with a limited number of studies and controversial results, requiring additional high-quality RCTs.
Conclusion
The treatment of OA with PRP demonstrated reductions in pain and improved patient-reported outcomes for up to 1 year. However, there was no statistically significant difference between PRP and HA in terms of pain reduction. Likewise, for FAIS and labral surgery, there was no statistically significant difference in mHHS between patients treated with PRP and controls. Given the limited number of studies and variability in PRP preparations, additional high-quality randomized trials are warranted.
Footnotes
Final revision submitted April 1, 2020; accepted April 16, 2020.
One or more of the authors has declared the following potential conflict of interest or source of funding: B.U.N. has received educational support from Smith & Nephew and hospitality payments from Stryker, Wright Medical, and Zimmer Biomet. S.J.N. has received research support from Allosource, Arthrex, Athletico, DJO, Linvatec, Miomed, Smith & Nephew, and Stryker; educational support from Elite Orthopedics; consulting fees from Ossur and Stryker; and royalties from Ossur and Springer. J.C. has received educational support from Arthrex and Smith & Nephew and consulting fees from Arthrex, ConMed Linvatec, and Smith & Nephew. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Altman DG, Schulz KF, Moher D, et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med. 2001;134(8):663–694. [DOI] [PubMed] [Google Scholar]
- 2. Andia I, Maffulli N. A contemporary view of platelet-rich plasma therapies: moving toward refined clinical protocols and precise indications. Regen Med. 2018;13(6):717–728. [DOI] [PubMed] [Google Scholar]
- 3. Battaglia M, Guaraldi F, Vannini F, et al. Efficacy of ultrasound-guided intra-articular injections of platelet-rich plasma versus hyaluronic acid for hip osteoarthritis. Orthopedics. 2013;36(12):e1501–e1508. [DOI] [PubMed] [Google Scholar]
- 4. Bedi A, Kelly BT. Femoroacetabular impingement. J Bone Joint Surg Am. 2013;95(1):82–92. [DOI] [PubMed] [Google Scholar]
- 5. Braun HJ, Wasterlain AS, Dragoo JL. The use of PRP in ligament and meniscal healing. Sports Med Arthrosc Rev. 2013;21(4):206–212. [DOI] [PubMed] [Google Scholar]
- 6. Campbell KA, Saltzman BM, Mascarenhas R, et al. Does intra-articular platelet-rich plasma injection provide clinically superior outcomes compared with other therapies in the treatment of knee osteoarthritis? A systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(11):2213–2221. [DOI] [PubMed] [Google Scholar]
- 7. Chahla J, Cinque ME, Piuzzi NS, et al. A call for standardization in platelet-rich plasma preparation protocols and composition reporting: a systematic review of the clinical orthopaedic literature. J Bone Joint Surg Am. 2017;99(20):1769–1779. [DOI] [PubMed] [Google Scholar]
- 8. Chahla J, LaPrade RF, Mardones R, et al. Biological therapies for cartilage lesions in the hip: a new horizon. Orthopedics. 2016;39(4):e715–e723. [DOI] [PubMed] [Google Scholar]
- 9. Chahla J, Stone J, Mandelbaum BR. How to manage cartilage injuries? Arthroscopy. 2019;35(10):2771–2773. [DOI] [PubMed] [Google Scholar]
- 10. Cole BJ, Karas V, Hussey K, Pilz K, Fortier LA. Hyaluronic acid versus platelet-rich plasma: a prospective, double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am J Sports Med. 2017;45(2):339–346. [DOI] [PubMed] [Google Scholar]
- 11. Coleman BD, Khan KM, Maffulli N, Cook JL, Wark JD. Studies of surgical outcome after patellar tendinopathy: clinical significance of methodological deficiencies and guidelines for future studies. Victorian Institute of Sport Tendon Study Group. Scand J Med Sci Sports. 2000;10(1):2–11. [DOI] [PubMed] [Google Scholar]
- 12. Dai WL, Zhou AG, Zhang H, Zhang J. Efficacy of platelet-rich plasma in the treatment of knee osteoarthritis: a meta-analysis of randomized controlled trials. Arthroscopy. 2017;33(3):659–670. [DOI] [PubMed] [Google Scholar]
- 13. Dallari D, Stagni C, Rani N, et al. Ultrasound-guided injection of platelet-rich plasma and hyaluronic acid, separately and in combination, for hip osteoarthritis. Am J Sports Med. 2016;44(3):664–671. [DOI] [PubMed] [Google Scholar]
- 14. Di Sante L, Villani C, Santilli V, et al. Intra-articular hyaluronic acid vs platelet-rich plasma in the treatment of hip osteoarthritis. Med Ultrason. 2016;18(4):463–468. [DOI] [PubMed] [Google Scholar]
- 15. Doria C, Mosele GR, Caggiari G, Puddu L, Ciurlia E. Treatment of early hip osteoarthritis: ultrasound-guided platelet rich plasma versus hyaluronic acid injections in a randomized clinical trial. Joints. 2017;5(3):152–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dragoo JL, Wasterlain AS, Braun HJ, Nead KT. Platelet-rich plasma as a treatment for patellar tendinopathy: a double-blind, randomized controlled trial. Am J Sports Med. 2014;42(3):610–618. [DOI] [PubMed] [Google Scholar]
- 17. El Bitar YF, Lindner D, Jackson TJ, Domb BG. Joint-preserving surgical options for management of chondral injuries of the hip. J Am Acad Orthop Surg. 2014;22(1):46–56. [DOI] [PubMed] [Google Scholar]
- 18. Filardo G, Di Matteo B, Di Martino A, et al. Platelet-rich plasma intra-articular knee injections show no superiority versus viscosupplementation: a randomized controlled trial. Am J Sports Med. 2015;43(7):1575–1582. [DOI] [PubMed] [Google Scholar]
- 19. Fleming BC, Proffen BL, Vavken P, Shalvoy MR, Machan JT, Murray MM. Increased platelet concentration does not improve functional graft healing in bio-enhanced ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1161–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gwinner C, Gerhardt C, Haneveld H, Scheibel M. Two-staged application of PRP in arthroscopic rotator cuff repair: a matched-pair analysis. Arch Orthop Trauma Surg. 2016;136(8):1165–1171. [DOI] [PubMed] [Google Scholar]
- 21. Hamilton B, Tol JL, Almusa E, et al. Platelet-rich plasma does not enhance return to play in hamstring injuries: a randomised controlled trial. Br J Sports Med. 2015;49(14):943–950. [DOI] [PubMed] [Google Scholar]
- 22. Harris JD, Erickson BJ, Abrams GD, et al. Methodologic quality of knee articular cartilage studies. Arthroscopy. 2013;29(7):1243–1252. [DOI] [PubMed] [Google Scholar]
- 23. Harris JD, Quatman CE, Manring MM, Siston RA, Flanigan DC. How to write a systematic review. Am J Sports Med. 2014;42(11):2761–2768. [DOI] [PubMed] [Google Scholar]
- 24. Hsu WK, Mishra A, Rodeo SR, et al. Platelet-rich plasma in orthopaedic applications: evidence-based recommendations for treatment. J Am Acad Orthop Surg. 2013;21(12):739–748. [DOI] [PubMed] [Google Scholar]
- 25. Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10(4):407–415. [DOI] [PubMed] [Google Scholar]
- 26. Koh YG, Kwon OR, Kim YS, Choi YJ. Comparative outcomes of open-wedge high tibial osteotomy with platelet-rich plasma alone or in combination with mesenchymal stem cell treatment: a prospective study. Arthroscopy. 2014;30(11):1453–1460. [DOI] [PubMed] [Google Scholar]
- 27. Kohn MD, Sassoon AA, Fernando ND. Classifications in brief: Kellgren-Lawrence classification of osteoarthritis. Clin Orthop Relat Res. 2016;474(8):1886–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kon E, Mandelbaum B, Buda R, et al. Platelet-rich plasma intra-articular injection versus hyaluronic acid viscosupplementation as treatments for cartilage pathology: from early degeneration to osteoarthritis. Arthroscopy. 2011;27(11):1490–1501. [DOI] [PubMed] [Google Scholar]
- 29. LaFrance R, Kenney R, Giordano B, Mohr K, Cabrera J, Snibbe J. The effect of platelet enriched plasma on clinical outcomes in patients with femoroacetabular impingement following arthroscopic labral repair and femoral neck osteoplasty. J Hip Preserv Surg. 2015;2(2):158–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. LaPrade RF, Goodrich LR, Phillips J. Use of platelet-rich plasma immediately after an injury did not improve ligament healing, and increasing platelet concentrations was detrimental in an in vivo animal model. Am J Sports Med. 2018;46(3):702–712. [DOI] [PubMed] [Google Scholar]
- 31. Martinelli N, Marinozzi A, Carnì S, Trovato U, Bianchi A, Denaro V. Platelet-rich plasma injections for chronic plantar fasciitis. Int Orthop. 2013;37(5):839–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mather RC III, Nho SJ, Federer A, et al. Effects of arthroscopy for femoroacetabular impingement syndrome on quality of life and economic outcomes. Am J Sports Med. 2018;46(5):1205–1213. [DOI] [PubMed] [Google Scholar]
- 33. Meheux CJ, McCulloch PC, Lintner DM, Varner KE, Harris JD. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy. 2016;32(3):495–505. [DOI] [PubMed] [Google Scholar]
- 34. Merolla G, Dellabiancia F, Ricci A, et al. Arthroscopic debridement versus platelet-rich plasma injection: a prospective, randomized, comparative study of chronic lateral epicondylitis with a nearly 2-year follow-up. Arthroscopy. 2017;33(7):1320–1329. [DOI] [PubMed] [Google Scholar]
- 35. Murray IR, LaPrade RF. Platelet-rich plasma: renewed scientific understanding must guide appropriate use. Bone Joint Res. 2016;5(3):92–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Murray MM, Palmer M, Abreu E, Spindler KP, Zurakowski D, Fleming BC. Platelet-rich plasma alone is not sufficient to enhance suture repair of the ACL in skeletally immature animals: an in vivo study. J Orthop Res. 2009;27(5):639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Novakofski KD, Berg LC, Bronzini I, et al. Joint-dependent response to impact and implications for post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2015;23(7):1130–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Palacio EP, Schiavetti RR, Kanematsu M, Ikeda TM, Mizobuchi RR, Galbiatti JA. Effects of platelet-rich plasma on lateral epicondylitis of the elbow: prospective randomized controlled trial. Rev Bras Ortop. 2016;51(1):90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pujol N, Salle De Chou E, Boisrenoult P, Beaufils P. Platelet-rich plasma for open meniscal repair in young patients: any benefit? Knee Surg Sports Traumatol Arthrosc. 2015;23(1):51–58. [DOI] [PubMed] [Google Scholar]
- 40. Radice F, Yánez R, Gutiérrez V, Rosales J, Pinedo M, Coda S. Comparison of magnetic resonance imaging findings in anterior cruciate ligament grafts with and without autologous platelet-derived growth factors. Arthroscopy. 2010;26(1):50–57. [DOI] [PubMed] [Google Scholar]
- 41. Rafols C, Monckeberg JE, Numair J, Botello J, Rosales J. Platelet-rich plasma augmentation of arthroscopic hip surgery for femoroacetabular impingement: a prospective study with 24-month follow-up. Arthroscopy. 2015;31(10):1886–1892. [DOI] [PubMed] [Google Scholar]
- 42. Redmond JM, Gupta A, Stake CE, Hammarstedt JE, Finch NA, Domb BG. Clinical results of hip arthroscopy for labral tears: a comparison between intraoperative platelet-rich plasma and bupivacaine injection. Arthroscopy. 2015;31(3):445–453. [DOI] [PubMed] [Google Scholar]
- 43. Reurink G, Goudswaard GJ, Moen MH, et al. Platelet-rich plasma injections in acute muscle injury. N Engl J Med. 2014;370(26):2546–2547. [DOI] [PubMed] [Google Scholar]
- 44. Review Manager (RevMan). Version 5.3 The Nordic Cochrane Centre, The Cochrane Collaboration; 2014.
- 45. Riboh JC, Saltzman BM, Yanke AB, Fortier L, Cole BJ. Effect of leukocyte concentration on the efficacy of platelet-rich plasma in the treatment of knee osteoarthritis. Am J Sports Med. 2016;44(3):792–800. [DOI] [PubMed] [Google Scholar]
- 46. Vavken P, Sadoghi P, Palmer M, et al. Platelet-rich plasma reduces retear rates after arthroscopic repair of small- and medium-sized rotator cuff tears but is not cost-effective. Am J Sports Med. 2015;43(12):3071–3076. [DOI] [PubMed] [Google Scholar]