Abstract
Objective:
This study aimed to investigate the serum inflammatory cytokines levels in patients with COPD, pneumonia and lung cancer, and assess the correlation between the levels of inflammatory cytokines levels and development of these diseases.
Methods:
Two hundred thirty-two patients including 114 patients with pneumonia, 76 patients with chronic obstructive pulmonary disease (COPD) and 42 patients with lung cancer, and 62 age-matched healthy volunteers as controls were enrolled. The pro-inflammatory cytokine IL-6, IL-2, IFN-γ, TNF-α, anti-inflammatory cytokines IL-4 and IL-10 in serum were analyzed by flow cytometry microsphere array (CBA).
Results:
We found that the levels of TNF-α and IL-10 in patients with lung cancer, COPD and pneumonia were significantly higher than control group. The IL-6 in the lung cancer group were significantly increased compared with the controls and COPD group, pneumonia group. IFN-γ and IL-2 levels were lower in lung cancer compared with controls and COPD group, pneumonia group. TNF-α, IL-4 and IL-10 levels were increased in patients with COPD and pneumonia compared with controls. In addition, the concentrations of IFN-γ and IL-6 were increased in acute exacerbation COPD (AECOPD) group compared with stable COPD group.
Conclusion:
In conclusion, elevated TNF-α and IL-10 levels in serum may be related with lung diseases including lung cancer, COPD and pneumonia. Additionally, IFN-γ and IL-6 might be potential biomarkers for the further deterioration of lung disease patients. The increased concentrations of IFN-γ and IL-6 might be used to predict the exacerbation of COPD.
Keywords: cytokines, lung cancer, pneumonia, COPD, AECOPD, biomarker
Introduction
Lung cancer is the most frequently diagnosed cancer and the leading cause of cancer-related deaths worldwide,1 with approximately 10-20% 5-year survival rate.2 Many studies showed that chronic obstructive pulmonary disease (COPD) is an important risk factor of lung cancer.3 COPD is a common lung disease that includes chronic bronchitis and emphysema, exerts a high morbidity in the middle-aged and elderly population.4 The International Lung Cancer Collaboration Group reported that emphysema and the chronic bronchitis increased the occurrence rate of lung cancer by 2.44 and 1.47 times, respectively.5 Anthonisen et al found that lung cancer is one of the predominant causes in the death of patients with COPD according to 14.5 years of follow-up.6,7 Smoking is usually recognized as a causative factor of COPD.8 With the increase of the number of smoking population and the aggravation of environmental pollution, the morbidity and mortality of COPD are increased year by year. The Global Burden of Disease Study speculates that there are 328 million patients with COPD around the world.9 According to report from the World Health Organization, COPD will become the third leading cause of death worldwide by 2030.10 Until the end of 2017, years lived with disability (YLD) of the COPD has become the fourth disease in China.11 Patients with acute exacerbation COPD (AECOPD) usually shows a sudden deterioration in lung function and ultimately death.12,13 Infections, tobacco, toxic particle inhalation and air pollution have all been reported to induce the excessive secretion of cytokines and chemotactic factors that can destroy immune system. Studies have reported that inflammatory damage is the main causative factor of COPD progression.14 Pneumonia is the lung disease with the inflammation of terminal airway, alveoli and pulmonary interstitium. Inflammation has been reported to enhance tumor development.15,16 However the mechanisms of inflammation-induced tumor development remain unclearly. Several studies also found that the levels of serum inflammatory cytokines in lung cancer patients can monitor the tumor development and prognosis.17,18 Currently, the cancer treatment targeting inflammation and tumor microenvironment has been considered as a novel therapy direction19,20
There is evidence supporting the hypothesis that inflammatory conditions in solid tumor tissues promote cancer development including tumor initiation, promotion, progression, and metastasis. Some pro-inflammatory cytokines such as interleukin (IL)-6 in tumor microenvironment are related to tumor development.20 IL-6 favors lung cancer growth by inhibiting the host anti-tumor immune system.21-23 Additionally, in some cancers including lung cancer, secretion of anti-inflammatory cytokines IL-4 and IL-10 by tumor cells help to drive and sustain pro-tumorigenic inflammatory loops.24,25 However, the pro-inflammatory cytokines tumor necrosis factor alpha (TNF-α), interferon gamma (IFN-γ) and IL-2 mediate antitumor effects.26-28 IL-1, TNF-α and IL-6 play an important role in the acute phase response of COPD.29-35 Previous studies have shown that increase of the IL-10 and TNF-α were significantly associated with COPD progression.36,37 During exacerbations of COPD, the levels of IL-6, IL-1β, TNF-α and IL-8 were found to be increased in sputum.38 Research has shown that the balance between IL-6 and IL-10 serum levels showed to be a more discriminative marker for severity definition and evaluation of recovery in patients with pneumonia.39 The levels of IL-1β, IL-6 and TNF-α are usually elevated in patients with community-acquired pneumonia.40-42
Therefore, we speculated the concentration of serum inflammatory cytokines might be related with the occurrence and development of lung diseases such as lung cancer, COPD and pneumonia. To our knowledge, no previous studies have compared the difference of serum inflammatory cytokines levels among COPD, AECOPD and lung cancer. In this study, we compared the levels of inflammatory cytokines among COPD, pneumonia and lung cancer. Additionally, we also assessed the levels of cytokines between stable COPD and AECOPD patients. Altogether, our study provided theoretical basis for developing a clinical strategy for COPD, pneumonia and lung cancer diagnosis.
Methods
Study Subjects
From November 2018 to March 2019, 114 patients with pneumonia who were admitted to the Affiliated Longyan First Hospital of Fujian Medical University for respiratory medicine, 76 patients with COPD, 42 patients with non-small cell lung cancer (NSCLC) and 62 healthy participants as control at the same time were enrolled. Diagnostic criteria for these diseases were referred to the Chinese Medical Association Respiratory Diseases Committee for the diagnosis and treatment of chronic obstructive pulmonary disease, guidelines for the diagnosis and treatment of community-acquired pneumonia, and the National Comprehensive Cancer Network (NCCN) NSCLC diagnosis, respectively.
All subjects were excluded from diabetes, hypertension, connective tissue disease, and other neoplastic diseases. Among them, there were 52 males and 24 females in the COPD group, the age ranged from 52 to 87 (70.3 ± 10.8) years. There were 72 males and 42 females in the pneumonia group, aged 18-82 (58.1 ± 20.8) years old, 29 males and 13 females in the lung cancer group, age 32-84 (68.2 ± 11.2) years old. Another 62 medical center health volunteers were selected as the control group, including 36 males and 26 females, aged 40-69 (55.67 ± 7.6) years old. There was no significant difference in gender ratio and age between COPD, pneumonia, lung cancer group and control group (P > 0.05). This study was approved by the human research ethics committee of the Affiliated Longyan First Hospital of Fujian Medical University (2018-025). And consent forms were obtained from each patient prior to sample collection.
Flow Cytometry
Peripheral venous blood samples (5 mL) were collected from each patient when they were enrolled in the investigation. The blood was centrifuged at 800×g for 20 mins to separate the serum, which was stored at 2-8°C before testing and complete detection within 24 hours after sampling. Levels of IL-2, IFN-γ, IL-4, IL-10, IL-6, TNF-α were analyzed by Human Th1/Th2 phenotyping Kit (Hangzhou Cellgene Biotech Co., Ltd, Zhejiang, China) on a DxFLEX clinical flow cytometer (Beckman Coulter, USA).
Statistical Analysis
All data were analyzed using SPSS 19.0 (USA), and the data were presented as the mean ± SD (standard deviation, SD). Comparisons of cytokines were assessed by One-way ANOVA, P < 0.05 was considered to indicate a statistically significant difference.
Results
Subject Characteristics
A summary of patient demographics and clinical characteristics was shown in Table 1. No significant difference of age and BMI were found in all patients with COPD, pneumonia or lung cancer and control group. However, the numbers of smokers were obviously increased in the patients with COPD and lung cancer groups compared with control group (Table 1). There were 43 (37.7%), 42 (55.3%), 24 (57.1%), 21 (33.8%) subjects with a smoking history in pneumonia, COPD, lung cancer and control group, respectively. It also indirectly suggested that smoking was a key pathogenic factor of lung cancer and COPD.
Table 1.
Subject Characteristics.
| Variable | Group | |||
|---|---|---|---|---|
| Pneumonia (n = 114) | COPD (n = 76) | Lung cancer (n = 42) | Control (n = 62) | |
| Age (years), mean ± SD | 58.1 ± 20.8 | 70.3 ± 10.8 | 68.2 ± 11.2 | 55.67 ± 7.6 |
| Male/Female | 72/42 | 52/24 | 29/13 | 36/26 |
| Smokers (%) | 43 (37.7%) | 42 (55.3%)* | 24 (57.1%)* | 21 (33.8%) |
| Obesity (BMI > 25%) (%) | 22 (19.2%) | 17 (22.3%) | 12 (28.6%) | 16 (25.8%) |
BMI, body mass index. *, P < 0.05; comparison with the control group.
The COPD group was further divided into stable COPD and AECOPD according to the degree of disease. There was no significantly difference in age between control group, stable COPD group and AECOPD group (Table 2). Lung function in COPD patients was assessed using Global Initiative for Chronic Obstructive Lung Disease (GOLD), COPD assessment test (CAT) and modified Medical Research Council (mMRC). As shown in Table 2, pulmonary function was significantly deteriorated in AECOPD patients compared with stable COPD patients (P < 0.05).
Table 2.
Comparison of Pulmonary Function in AECOPD and Stable COPD.
| Variable | Stable COPD (n = 41) | AECOPD (n = 35) | P-value | ||
|---|---|---|---|---|---|
| Subjects | Ratio (%) | Subjects | Ratio (%) | ||
| GOLD (grade) | <0.05 | ||||
| 1-2 | 22 | 53.7% | 10 | 28.6% | |
| 3-4 | 19 | 46.3% | 25 | 71.4% | |
| CAT(Score) | <0.05 | ||||
| 0-20 | 20 | 48.8% | 16 | 45.7% | |
| 21-40 | 21 | 51.2% | 19 | 54.3% | |
| mMRC | <0.05 | ||||
| 0-1 | 22 | 53.7% | 13 | 37.1% | |
| ≥2 | 19 | 46.3% | 22 | 62.9% | |
| Pulmonary function | <0.05 | ||||
| A-B | 24 | 58.5% | 12 | 34.2% | |
| C-D | 17 | 41.5% | 23 | 65.8% | |
AECOPD, acute exacerbation of chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; CAT, COPD assessment test; mMRC, modified Medical Research Council. A, B, C and D rank the lung function in patients with COPD.
The Level of Cytokines in the Patients With Lung Cancer, COPD and Pneumonia
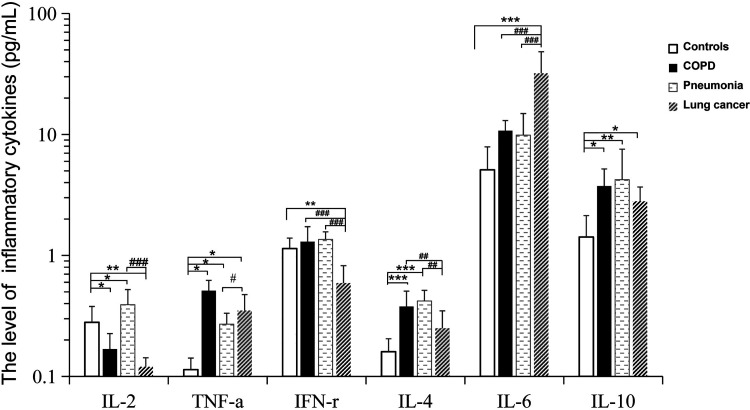
The levels of IL-2, TNF-α, IFN-γ, IL-4, IL-6 and IL-10 were detected by flow cytometry. As shown in Table 3 and Figure 1, the IL-2 (0.12 ± 0.07 pg/mL) and IFN-γ (0.59 ± 0.26 pg/mL) concentrations in the peripheral blood of the lung cancer group were significantly lower compared with IL-2 (0.28 ± 0.11 pg/mL) and IFN-γ (1.14 ± 0.28 pg/mL) levels in the control group, respectively. And then to compare with TNF-α (0.11 ± 0.02pg/mL), IL-6 (5.10 ± 2.79 pg/mL) and IL-10 (1.42 ± 0.71 pg/mL) levels in the control group, the concentrations of TNF-α (0.35 ± 0.13 pg/mL), IL-6 (31.98 ± 16.29 pg/mL) and IL-10 (2.80 ± 0.88 pg/mL) in the lung cancer group were significantly increased. The IL-2 (0.39 ± 0.15 pg/mL), TNF-α (0.27 ± 0.07 pg/mL), IL-4 (0.41 ± 0.12 pg/mL) and IL-10 (4.23 ± 3.33 pg/mL) levels in the pneumonia group were higher than control group. Additionally, the levels of IL-2, IFN-γ(1.36 ± 0.21pg/mL) and IL-4 (0.41 ± 0.12 pg/mL) in patients with pneumonia were increased compared with lung cancer group. In the COPD patients, our results showed that the levels of TNF-α (0.51 ± 0.12pg/mL), IL-4 (0.37 ± 0.13pg/mL) and IL-10 (3.74 ± 1.59 pg/mL) were higher than control group. Then the IL-2 (0.17 ± 0.07pg/mL) was decreased compared with controls. Compared with lung cancer group, the concentrations of IFN-γ and IL-4 were significantly increased and the IL-6 was decreased in both patients with COPD and pneumonia. In addition, the level of IL-2 was higher in pneumonia group than lung cancer group and COPD group. And the levels of TNF-α and IL-10 in patients with lung cancer, COPD and pneumonia were significantly higher than control group. These results suggested that the imbalance of inflammatory cytokines occurred in patients with lung cancer, COPD and pneumonia. It revealed that inflammatory cytokines play an important role in the development of these lung diseases including lung cancer, COPD and pneumonia.
Table 3.
The Level of Cytokines in the Patients With COPD, Pneumonia and Lung Cancer.
| Groups | IL-2 (pg/mL) | TNF-α (pg/mL) | IFN-γ (pg/mL) | IL-4 (pg/mL) | IL-6 (pg/mL) | IL-10 (pg/mL) |
|---|---|---|---|---|---|---|
| Controls | 0.28 ± 0.11 | 0.11 ± 0.02 | 1.14 ± 0.28 | 0.16 ± 0.05 | 5.10 ± 2.79 | 1.42 ± 0.71 |
| COPD | 0.17 ± 0.07* | 0.51 ± 0.12* | 1.29 ± 0.44### | 0.37 ± 0.13***## | 10.75 ± 4.43### | 3.74 ± 1.59* |
| Pneumonia | 0.39 ± 0.15*### | 0.27 ± 0.07*# | 1.36 ± 0.21### | 0.41 ± 0.12***## | 9.88 ± 5.08### | 4.23 ± 3.33** |
| Lung cancer | 0.12 ± 0.07** | 0.35 ± 0.13* | 0.59 ± 0.26** | 0.25 ± 0.11 | 31.98 ± 16.29*** | 2.80 ± 0.88* |
*, P < 0.05; **, P < 0.01; ***, P < 0.001; comparison with the control group; #, P < 0.05; ###, P < 0.001; comparison with the Lung cancer group, +++, P < 0.001; comparison with the pneumonia group.
Figure 1.
The level of cytokines in the patients with COPD, pneumonia and lung cancer. the level of IL-2, TNF-α, IFN-r, IL-4, IL-6, IL-10 in serum of patients with pneumonia (n=114), COPD (n=76), lung cancer (n=42), healthy control (n=62) were analyzed by flow cytometry microsphere array (CBA). Data represent mean ± SD.*P < 0.05; **P < 0.01; ***P < 0.001, controls versus COPD or pneumonia or lung cancer group. ## P < 0.01; ### P < 0.001, lung cancer group versus COPD or pneumonia.
The Level of Cytokines in the Stable COPD and AECOPD Patients
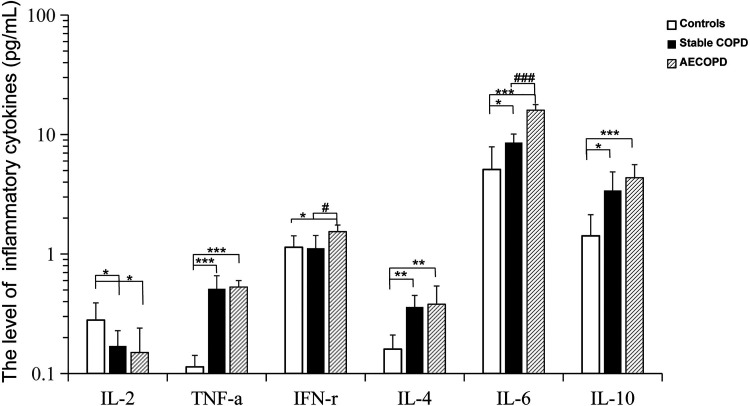
Patients with COPD were further divided into stable COPD and AECOPD according to the severity of the disease. There was significant difference in lung function between COPD and AECOPD group (Table 2). As shown in Table 4 and Figure 2, the concentration of TNF-α (0.53 ± 0.07 pg/mL), IL-6 (15.99 ± 1.81 pg/mL) and IL-10 (4.35 ± 1.25 pg/mL) in AECOPD group were sharply increased compared with control group. The levels of IL-4 (0.36 ± 0.09 pg/mL), IL-6 (8.56 ± 1.55 pg/mL) and IL-10 (3.40 ± 1.47 pg/mL) in stable groups were higher than controls. And the TNF-α (0.51 ± 0.15pg/mL) in stable-COPD group was significantly increased relative to controls. Besides, levels of IL-2 in stable-COPD group and AECOPD group were lower compared with control group, relatively. The results showed that serum inflammatory cytokines have changed in patients with stable COPD and AECOPD. Furthermore, the levels of IFN-γ and IL-6 of AECOPD patients were prominently higher than stable COPD groups. We speculated that IFN-γ and IL-6 might help to evaluate the severity of COPD and predict the clinical outcomes.
Table 4.
Comparsion of the Levels of Cytokines between COPD and AECOPD Patient.
| Groups | n | IL-2 (pg/mL) | TNF-α (pg/mL) | IFN-γ (pg/mL) | IL-4 (pg/mL) | IL-6 (pg/mL) | IL-10 (pg/mL) |
|---|---|---|---|---|---|---|---|
| Controls | 62 | 0.28 ± 0.11 | 0.11 ± 0.02 | 1.14 ± 0.28 | 0.16 ± 0.05 | 5.10 ± 2.79 | 1.42 ± 0.71 |
| Stable COPD | 41 | 0.17 ± 0.06* | 0.51 ± 0.15*** | 1.12 ± 0.31# | 0.36 ± 0.09** | 8.56 ± 1.55*### | 3.40 ± 1.47* |
| AECOPD | 35 | 0.15 ± 0.09* | 0.53 ± 0.07*** | 1.54 ± 0.21* | 0.38 ± 0.16** | 15.99 ± 1.81*** | 4.35 ± 1.25*** |
*, P < 0.05; **, P < 0.01; ***, P < 0.001; comparison with the control group; #, P < 0.05; ###, P < 0.001; comparison with the AECOPD group.
Figure 2.
Comparsion of the levels of cytokines between COPD and AECOPD patient. The level of IL-2, TNF-α, IFN-r, IL-4, IL-6, IL-10 in serum of patients with stable- COPD (n = 41), AECOPD (n = 35) and healthy control (n = 62) were analyzed by flow cytometry microsphere array (CBA). Data represent mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001, controls versus stable-COPD, AECOPD group. # P < 0.05; ### P < 0.001, stable-COPD versus AECOPD.
Discussion
In this study, our results showed that the levels of TNF-α, IL-6 and IL-10 in patients with lung cancer were significantly higher than healthy controls, while the levels of IL-2 and IFN-γ in patients with lung cancer were significantly lower than controls. IFN-γ can induce T cell proliferation, differentiation, maturation, and promote T cell immune function.42,43 In addition, IFN-γ also enhances natural killer cells (NK), mononuclear macrophages and cytotoxic T cells (CTL) killing activity and lymphocyte infiltration inflammatory response, and then induces delayed type hypersensitivity.44 Thus, IFN-γ has a strong anti-tumor immunological effects.45 Here, we found that IFN-γ in lung cancer group was significantly lower than that other groups. This implied that low IFN-γ levels in lung cancer patients failed to prevent lung tumor development. However, this could not exclude a possibility that lung cancer cells decreased the IFN-γ secrete by inhibiting the activity of immune cells. Previous study showed that serum IFN-γ levels are significantly higher in AECOPD than healthy control group, suggesting that IFN-γ is a better diagnostic value for AECOPD.46 Our results were consistent with this report. Compared with stable COPD and heathy control group, the concentration of the IFN-γ in the AECOPD group were higher.
Moreover, we showed that serum TNF-α, IL-6 and IL-10 levels were significantly increased in patients with lung cancer. It suggested that TNF-α, IL-6 and IL-10 might be important biomarkers for distinguishing patients with the lung cancer from healthy subjects. Previous study reported that the increase of only IL-6 levels in patients’ serum can be used to assist in the diagnosis of sepsis.47 Here, we thought the increase of above 3 markers TNF-α, IL-6 and IL-10 might be used to distinguish patients with the lung cancer from sepsis. It also hinted that the 3 factors in combination might be used to distinguish lung cancer from other lung diseases, such as sepsis or bacterial/viral infection.48 TNF-α, IL-4 and IL-10 levels were increased in patients with COPD and pneumonia, suggesting TNF-α, IL-4 and IL-10 could be used to assist in the diagnosis of COPD and pneumonia from sepsis or lung diseases with bacterial or viral infection. In addition, the serum concentrations of Th17-related cytokines such as IL-17 and IL-23 were associated with M.intracellulare lung disease.49 The levels of both IL-6 and IL-8 in cord blood are useful predictors of onset of chronic lung disease.50 The results of our study showed that multiple cytokines were changed in the occurrence and development of pneumonia, COPD and lung cancer groups. Many studies have shown that the occurrence and development of lung diseases are caused by a variety of factors.13,14,26 The diagnostic and predictive role of cytokines in lung diseases still needed more in-depth discussion and analysis.
What is more, the concentrations of TNF-α, IL-4, IL-6 and IL-10 were increased in stable COPD and AECOPD group relative to health controls. Previous studies have reported that fibroblasts, endothelial and epithelial cells could secrete IL-6, and thus serum IL-6 levels are increased in COPD.51,52 IL-4 and IL-10 have been reported to be increased in AECOPD group relative to health controls.43 Moreover, IL-10 combined with “kidney injury” biomarker NGAL exerts a better diagnostic value for AECOPD with acute kidney injury.46 Our results were consistent with these reports. Compared with stable group, the concentration of the IL-10 and IL-6 in the AECOPD group were higher. Lung function deterioration could increase the levels of inflammatory cytokines including IFN-γ and IL-6.53-55 Therefore, the increase of serum IFN-γ and IL-6 levels in patients with AECOPD suggested the deterioration of lung function were more serious than these ones with COPD. Prospective studies will need to confirm whether measuring these inflammatory cytokines can identify the risk of lung disease.
Altogether, our study provided theoretical basis for developing a clinical strategy to aid diagnosis. however, this study has limitations which relatively small size affected the power to detect associations between systemic markers and clinical parameters. Perhaps, there will be more discoveries as we expand the sample size. Next, we will expand the sample size to further study the subject.
Acknowledgments
Authors thank Doctor Cao Xiaoming and Doctor Li Youtang for their support in collecting clinical samples.
Authors’ Note: Study conception and design: Jian Chen and Qingfu Dai; data analysis: Jian Chen and Xincai Li; sample collection and experiment: Jian Chen, Xincai Li, ChaoLin Huang and Ying Lin; manuscript drafting: Jian Chen; manuscript revising: Qingfu Dai, Xincai Li. All authors reviewed and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Qingfu Dai  https://orcid.org/0000-0001-8859-7208
https://orcid.org/0000-0001-8859-7208
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Ahmedin J. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. [DOI] [PubMed] [Google Scholar]
- 2. Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sanchez-Salcedo P, Berto J, De-Torres JP, et al. Lung cancer screening: fourteen year experience of the Pamplona Early Detection Program (P-IELCAP). Arch Bronconeumol. 2015;51(4):169–176. [DOI] [PubMed] [Google Scholar]
- 4. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. Gold executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. [DOI] [PubMed] [Google Scholar]
- 5. Brenner DR, Boffetta P, Duell EJ, et al. Previous lung diseases and lung cancer risk: a pooled analysis from the international lung cancer consortium. Am J Epidemiol. 2012;176(7):573–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bombassei GJ. The effects of a smoking cessation intervention on 14.5-year mortality. Ann Intern Med. 2005;143(8):615; author reply 615. [DOI] [PubMed] [Google Scholar]
- 7. Anthonisen NR, Manfreda J, Warren CP, et al. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106(2):196–204. [DOI] [PubMed] [Google Scholar]
- 8. Spilling CA, Bajaj MK, Burrage DR, et al. Contributions of cardiovascular risk and smoking to chronic obstructive pulmonary disease (COPD)-related changes in brain structure and function. Int J Chron Obstruct Pulmon Dis. 2019;14:1855–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferkol T, Schraufnagel D. The global burden of respiratory disease. Ann Am Thorac Soc. 2014;11(3):404–406. [DOI] [PubMed] [Google Scholar]
- 10. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2163–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lewer D, Tweed EJ, Aldridge RW, Morley KI. Causes of hospital admission and mortality among 6683 people who use heroin: a cohort study comparing relative and absolute risks. Drug Alcohol Depend. 2019;204:107–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen H, Wang Y, Bai C, Wang X. Alterations of plasma inflammatory biomarkers in the healthy and chronic obstructive pulmonary disease patients with or without acute exacerbation. J Proteomics. 2012;75(10):2835–2843. [DOI] [PubMed] [Google Scholar]
- 13. Thurlbeck WM. Pathophysiology of chronic obstructive pulmonary disease. Clin Chest Med. 1990;11(3):389–403. [PubMed] [Google Scholar]
- 14. Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2016;138(1):16–27. [DOI] [PubMed] [Google Scholar]
- 15. Elinav E, Nowarski R, Thaiss CA, et al. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13(11):759–771. [DOI] [PubMed] [Google Scholar]
- 16. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brenner DR, Fanidi A, Grankvist K, et al. Inflammatory cytokines and lung cancer risk in 3 prospective studies. Am J Epidemiol. 2017;185(2):86–95. [DOI] [PubMed] [Google Scholar]
- 18. Marrugal A, Ojeda L, Paz-Ares L, Molina-Pinelo S, Ferrer I. Proteomic-based approaches for the study of cytokines in lung cancer. Dis Markers. 2016;2016:2138627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hsieh HL, Tsai MM. Tumor progression-dependent angiogenesis in gastric cancer and its potential application. World J Gastrointest Oncol. 2019;11(9):686–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim MY, Oskarsson T, Acharyya S, et al. Tumor self-seeding by circulating cancer cells. Cell. 2009;139(7):1315–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rose-John S. IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int J Biol Sci. 2012;8(9):1237–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gao SP, Mark KG, Leslie K, et al. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest. 2007;117(12):3846–3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sansone P, Storci G, Tavolari S, et al. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. 2007;117(12):3988–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Angelo LS, Talpaz M, Kurzrock R. Autocrine interleukin-6 production in renal cell carcinoma: evidence for the involvement of p53. Cancer Res. 2002;62(3):932–940. [PubMed] [Google Scholar]
- 25. Stanic B, van de Veen W, Wirz OF, et al. IL-10-overexpressing B cells regulate innate and adaptive immune responses. J Allergy Clin. Immunol. 2015;135(3):771–780. [DOI] [PubMed] [Google Scholar]
- 26. Carswell EA, Old LJ, Kassel RL, et al. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975;72(9):3666–3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alvarez M, Bouchlaka MN, Sckisel GD, et al. Increased antitumor effects using IL-2 with anti-TGF-β reveals competition between mouse NK and CD8 T cells. J Immunol. 2014;193(4):1709–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Floros T, Tarhini AA. Anticancer cytokines: biology and clinical effects of interferon-alpha2, interleukin (IL)-2, IL-15, IL-21, and IL-12. Semin Oncol. 2015;42(4):539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pasquali S, Mocellin S. The anticancer face of interferon alpha (IFN-alpha): from biology to clinical results, with a focus on melanoma. Curr Med Chem. 2010;17(29):3327–3336. [DOI] [PubMed] [Google Scholar]
- 30. Endeman H, Meijvis SC, Rijkers GT, et al. Systemic cytokine response in patients with community-acquired pneumonia. Eur Respir J. 2011;37(6):1431–1438. [DOI] [PubMed] [Google Scholar]
- 31. Antunes G, Evans SA, Lordan JL, Frew AJ. Systemic cytokine levels in community-acquired pneumonia and their association with disease severity. Eur Respir J. 2002;20(4):990–995. [DOI] [PubMed] [Google Scholar]
- 32. Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Fred S. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374(Pt 1):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gallagher PM, Lowe G, Fitzgerald T, et al. Association of IL-10 polymorphism with severity of illness in community acquired pneumonia. Thorax. 2003;58(2):154–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Waterer GW. Airway defense mechanisms. Clin Chest Med. 2012;33(2):199–209. [DOI] [PubMed] [Google Scholar]
- 35. Naugler WE, Karin M. The wolf in sheep’s clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med. 2008;14(3):109–119. [DOI] [PubMed] [Google Scholar]
- 36. Silva B, Lira FS, Ramos D, et al. Severity of COPD and its relationship with IL-10. Cytokine. 2018;106:95–100. [DOI] [PubMed] [Google Scholar]
- 37. Eagan TM, Gabazza EC, D’Alessandro-Gabazza C, et al. TNF-alpha is associated with loss of lean body mass only in already cachectic COPD patients. Respir Res. 2012;13(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chung KF. Cytokines in chronic obstructive pulmonary disease. Eur Respir J Suppl. 2001;34:50S–59S. [PubMed] [Google Scholar]
- 39. de Brito RC, Lucena-Silva N, Torres LC, et al. The balance between the serum levels of IL-6 and IL-10 cytokines discriminates mild and severe acute pneumonia. Bmc Pulm Med. 2016;16(1):170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Puren AJ, Feldman C, Savage N, Becker PJ, Smith C. Patterns of cytokine expression in community-acquired pneumonia. Chest. 1995;107(5):1342–1349. [DOI] [PubMed] [Google Scholar]
- 41. Glynn P, Coakley R, Kilgallen I, Murphy N, O’Neill S. Circulating interleukin 6 and interleukin 10 in community acquired pneumonia. Thorax. 1999;54(1):51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hosking MP, Flynn CT, Whitton JL. Antigen-specific naive CD8+ T cells produce a single pulse of IFN-gamma in vivo within hours of infection, but without antiviral effect. J Immunol. 2014;193(4):1873–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Salerno F, Guislain A, Cansever D, Wolkers MC. TLR-mediated innate production of IFN-gamma by CD8+ T Cells Is independent of glycolysis. J Immunol. 2016;196(9):3695–3705. [DOI] [PubMed] [Google Scholar]
- 44. Bradley LM, Dalton DK, Croft M. A direct role for IFN-gamma in regulation of Th1 cell development. J Immunol. 1996;157(4):1350–1358. [PubMed] [Google Scholar]
- 45. Potzl J, Roser D, Bankel L, et al. Reversal of tumor acidosis by systemic buffering reactivates NK cells to express IFN-gamma and induces NK cell-dependent lymphoma control without other immunotherapies. Int J Cancer. 2017;140(9):2125–2133. [DOI] [PubMed] [Google Scholar]
- 46. Wei B, Tian T, Liu YG. IL-10 Combined with NGAL has diagnostic value for AECOPD combined with AKI. Int J Chron Obstruct Pulmon Dis. 2020;15:637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hou T, Huang D, Zeng R, Zhiming Y, Zhang Y. Accuracy of serum interleukin (IL)-6 in sepsis diagnosis: a systematic review and meta-analysis. Int J Clin Exp Med. 2015;8(9):15238–15245. [PMC free article] [PubMed] [Google Scholar]
- 48. Jiang M, Guan WJ, Fang ZF, et al. A critical review of the quality of cough clinical practice guidelines. Chest. 2016;150(4):777–788. [DOI] [PubMed] [Google Scholar]
- 49. Kim SY, Koh WJ, Park HY, et al. Changes in serum immunomolecules during antibiotic therapy for mycobacterium avium complex lung disease. Clin Exp Immunol. 2014;176(1):93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Takao D, Ibara S, Tokuhisa T, et al. Predicting onset of chronic lung disease using cord blood cytokines. Pediatr Int. 2014;56(4):566–570. [DOI] [PubMed] [Google Scholar]
- 51. Butler T, Ho M, Acharya G, Tiwari M, Gallati H. Interleukin-6, gamma interferon, and tumor necrosis factor receptors in typhoid fever related to outcome of antimicrobial therapy. Antimicrob Agents Chemother. 1993;37(11):2418–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Singh S, Verma SK, Kumar S, et al. Correlation of severity of chronic obstructive pulmonary disease with potential biomarkers. Immunol Lett. 2018;196:1–10. [DOI] [PubMed] [Google Scholar]
- 53. Yazici O, Gulen ST, Yenisey C, Eryilmaz U, Abas BI, Polatli M. Comparison of inflammation biomarkers among chronic obstructive pulmonary disease groups: a cross sectional study. Niger J Clin Pract. 2020;23(6):817–824. [DOI] [PubMed] [Google Scholar]
- 54. Wolbeling F, Munder A, Kerber-Momot T, et al. Lung function and inflammation during murine Pseudomonas aeruginosa airway infection. Immunobiology. 2011;216(8):901–908. [DOI] [PubMed] [Google Scholar]
- 55. Palmberg L, Sundblad BM, Ji J, Karen J, Larsson K. Cholinergic mechanisms in an organic dust model simulating an acute exacerbation in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:3611–3624. [DOI] [PMC free article] [PubMed] [Google Scholar]