Abstract
PURPOSE.
To evaluate the cost-effectiveness of axillary observation versus sentinel lymph node biopsy (SLNB) after negative axillary ultrasound (AUS).
In patients with clinical T1-T2 N0 breast cancer and negative AUS, SLNB is the current standard of care for axillary staging. However, SLNB is costly, invasive, decreasing in importance for medical decision-making, and is not considered therapeutic. Observation alone is currently being evaluated in randomized clinical trials, and is thought to be noninferior to SLNB.
METHODS.
We performed cost-effectiveness analyses of observation versus SLNB after negative AUS in post-menopausal women with clinical T1-T2 N0, HR+/HER2− breast cancer. Costs at the 2016 price level were evaluated from a third-party commercial payer perspective using the MarketScan® Database. We compared cost, quality-adjusted life years (QALYs), and net monetary benefit (NMB). Multiple sensitivity analyses varying baseline probabilities, costs, utilities, and willingness-to-pay thresholds were performed.
RESULTS.
Observation was superior to SLNB for patients with N0 and N1 disease, and for the entire patient population (NMB in US$: $655,659 for observation versus $641,778 for SLNB for the entire patient population). In the N0 and N1 groups, observation incurred lower cost and was associated with greater QALYs. SLNB was superior for patients with >3 positive lymph nodes, representing approximately 5% of the population. Sensitivity analyses consistently demonstrated that observation is the optimal strategy for AUS-negative patients.
CONCLUSION.
Considering both cost and effectiveness, observation is superior to SLNB in post-menopausal women with cT1-T2 N0, HR+/HER2− breast cancer and negative AUS.
Keywords: Breast cancer, staging, axilla, ultrasound, sentinel lymph node biopsy, cost-effectiveness
1. INTRODUCTION
Sentinel lymph node biopsy (SLNB) is currently the standard of care for axillary staging in patients with cT1-T2 N0 breast cancer. However, the role of SLNB has been questioned for multiple reasons. First, in patients with clinically-negative axilla, there is no survival benefit associated with surgical clearance of the axilla, even for patients with positive SLNB. The ACOSOG Z0011 trial randomized patients with 1–2 positive lymph nodes to either axillary lymph node dissection (ALND) or no further axillary intervention. No significant difference in the rates of axillary recurrence or survival was observed despite the presence of macrometastases (>2 mm) in non-sentinel nodes in 27.3% of patients in the ALND group.1 Second, although SLNB offers information about the extent of disease, tumor biology and patient factors are more informative for selection of systemic adjuvant therapy.2 Gene expression profiles, such as Oncotype DX, are increasingly used in both node-negative and node-positive patients to predict a patient’s likelihood of recurrence and their response to treatment.3–9 Third, although SLNB is associated with less morbidity than ALND, risks of the procedure include lymphedema (in approximately 5–10% of patients), dye reactions, nerve damage, range of motion deficits, as well as acute complications such as infection and seroma.10–20
Axillary ultrasound (AUS) has been proposed as a non-invasive alternative to SLNB for axillary staging (Figure 1), because it has a high negative predictive value.21–26 In one study, AUS had a sensitivity of 76% and negative predictive value (NPV) of 89% for detection of macrometastases (>2 mm).27 Interim results from the Intergroup-Sentinel-Mamma (INSEMA) trial demonstrated a negative predictive value of 98.4% for ≥3 positive lymph nodes in 755 cT1–2 N0 women with negative AUS undergoing SLNB.28 Although the most appropriate management algorithm following positive AUS (image-guided biopsy versus SLNB versus ALND) remains a topic of debate, we and others have proposed that observation is superior to SLNB in AUS-negative patients.
Figure 1:
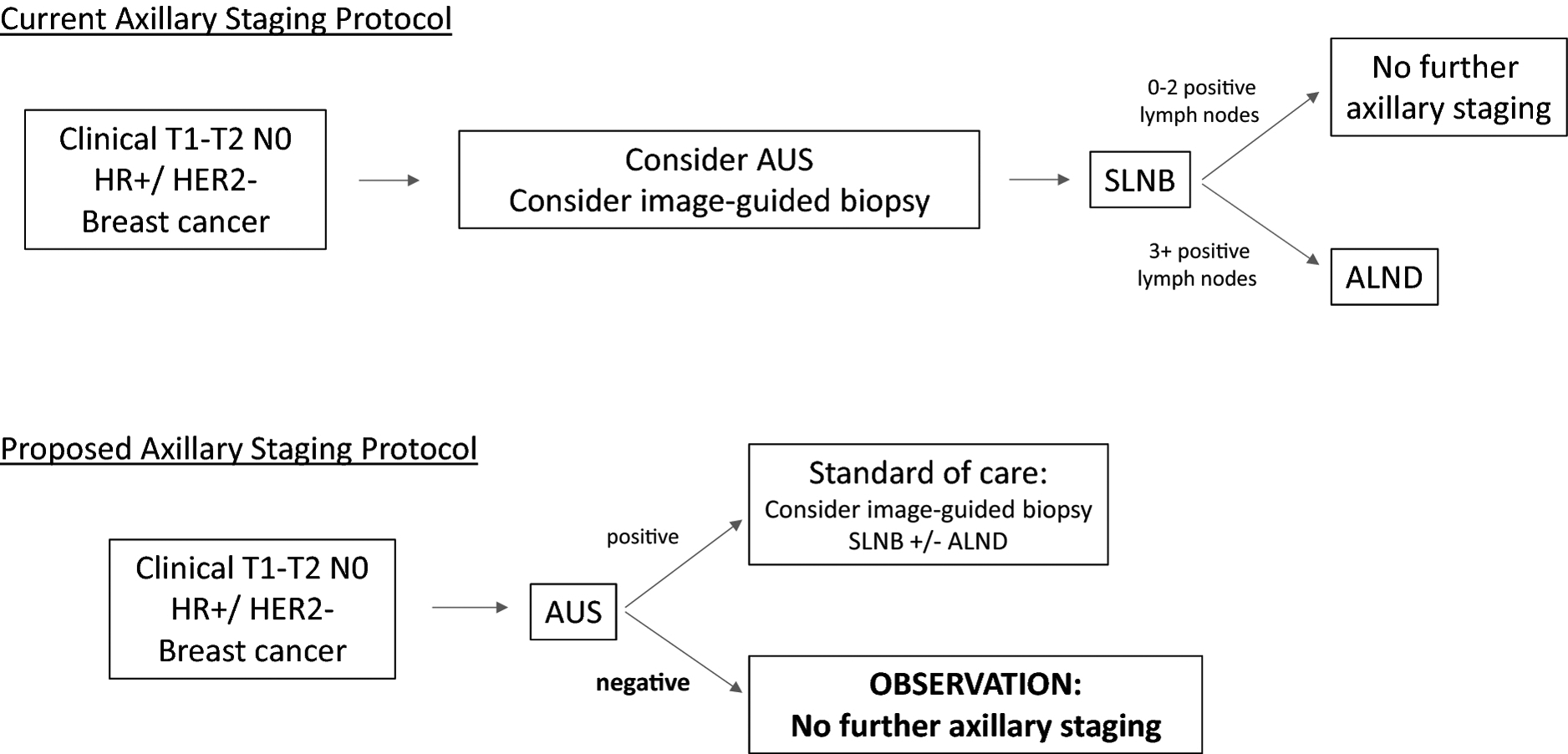
Current versus Proposed Axillary Staging Protocols
Currently, SLNB is the standard of care for staging of the axilla for patients with early stage breast cancer. However, surgery does not improve survival in patients without clinically apparent disease, and may lead to complications including lymphedema and surgical site infections. In the proposed staging algorithm, negative AUS can be used to exclude disease in the axilla, and identify those patients who can forego SLNB.
AUS: axillary ultrasound, SLNB: sentinel lymph node biopsy, ALND: axillary lymph node dissection.
The differences in quality-of-life outcomes (especially related to potential complications) and costs between observation and SLNB are substantial.29–31 There are two ongoing randomized controlled trials (NCT02167490 and NCT02466737) comparing observation to SLNB in AUS-negative patients.28, 32 These trials will not consider critical information related to cost and quality-of-life, and may not provide detailed analyses for the HR+/HER2− subtype in which observation is likely most appropriate. To date, cost-effectiveness analyses have focused on the role of AUS-guided biopsy in AUS-positive patients.30, 33 To our knowledge, there have been no cost-effectiveness analyses comparing observation to SLNB in AUS-negative patients. Providers are likely unaware of the potential economic burden patients incur due to SLNB given the lack of accessible, easily interpretable, and detailed healthcare cost data in the U.S. The costs of SLNB may not be justified considering possible quality of life detriments,31 and the minimal impact of axillary staging information on recurrence and survival for most post-menopausal, HR+/HER2− patients. cT1-T2N0, post-menopausal, HR+/HER2− patients represent a very large and low-risk subset of breast cancer patients who are unlikely to develop recurrence or progressive disease.34 HR+/HER2− is the most common subtype of breast cancer, and post-menopausal women are the most common age group.35, 36 This group is also the most well-represented in the patient population studied in clinical trials which have supplied data for the decision analytic model.1, 4, 28 In these patients, a direct comparison of disease outcomes, quality-of-life, and financial costs associated with observation and SLNB is needed. Therefore, we have performed cost-effectiveness analyses comparing observation to SLNB in cT1-T2N0, post-menopausal, HR+/HER2−, breast cancer patients with negative AUS.
2. METHODS
2.1. Target population.
The study population was post-menopausal women with cT1-T2 N0, HR+/HER2− invasive breast cancer and negative AUS. This population represents the largest subset of breast cancer patients35, 36, and potentially the best candidates for non-invasive staging for multiple reasons. First, post-menopausal women with HR+/HER2− disease have very low risks of disease recurrence or progression compared to pre-menopausal women and those with HR− disease.34 Furthermore, in this subset, medical decision making is less dependent on surgical staging, and radiation therapy has not demonstrated significant benefit. We stratified these patients into six subpopulations: pT1a N0, pT1a N1, pT1a N2+, pT1b-T2 N0, pT1b-T2 N1, and pT1b-T2 N2+ (Figure 2) to best accommodate stage-specific standards of care. Due to the inconsistency between the Z0011 definition of low nodal burden (1–2 nodes) and the 8th edition AJCC definition (N1=1–3 nodes), we defined women with 1–2 or 1–3 positive nodes as N1, and women with >3 positive nodes as N2+ (pN2-pN3).37
Figure 2:
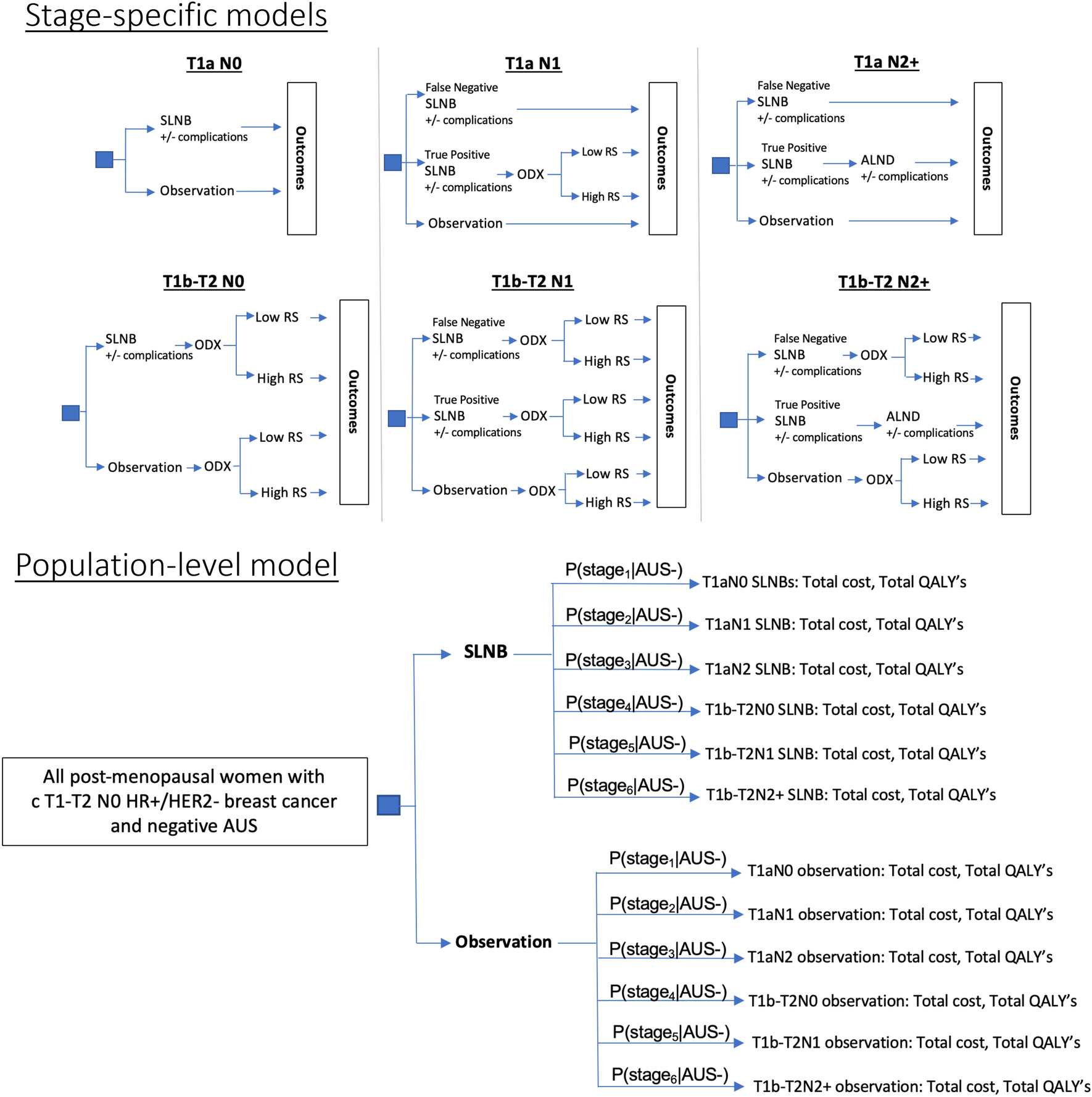
Decision Analytic Model Structures
All models featured the same initial decision node: SLNB versus observation after negative AUS. T1b-T2N0 and N1 patients received Oncotype DX testing. N2+ patients whose axillary disease was correctly identified by SLNB proceeded to ALND. N1 and N2+ patients whose axillary disease was not correctly identified by AUS or SLNB (false negatives) were treated as if they were N0. Treatment was modeled based on Oncotype Dx recurrence score and observed stage, in accordance with NCCN guidelines (S-III).
Stage-specific cost-effectiveness analysis results (including total cost and QALYs) were weighted by their respective probabilities (based on stage prevalence and likelihood of negative AUS), and integrated into a population-level model to simulate real-world outcomes. For j = 1, …, 6,
where stage1 = T1aN0, stage2 = T1aN1, stage3 = T1aN2, stage4 = T1b-T2N0, stage5 = T1b-T2N1, and stage6 = T1b-T2N2.
SLNB: Sentinel lymph node biopsy, AUS: Axillary ultrasound, ODX: Oncotype Dx test, RS: Oncotype DX Recurrence Score, ALND: axillary lymph node dissection.
2.2. Decision analytic model.
We constructed decision analytic models to compare observation versus SLNB for each of the six subpopulations (Figure 2). Observation was defined as the standard of care and surveillance that breast cancer patients receive after initial diagnostic work-up as recommended by the 2019 NCCN breast cancer guidelines.35, 38 Markov models were used to simulate potential events and outcomes patients could experience for 10 years (each cycle length = 1 year) after breast cancer diagnosis and initial treatment, which would be poorly represented accurately modeled using simple decision trees.39 The Markov models consisted of five mutually exclusive and collectively exhaustive health states: healthy (no recurrence), local recurrence, regional recurrence, distant recurrence, and deceased (Table 1, Figure 3, S-VI).
Table 1:
Key Input parameters
| Variable Type | Variable Description | Value | Range used in sensitivity analyses |
|---|---|---|---|
| Probability | False negative SLNB | 0.07 65 | 0.01–0.10 |
| Probability | Lymphedema after SLNB | 0.05 10, 15 | 0.005–0.10 |
| Probability | Surgical site infection after SLNB | 0.11 10 | 0.005–0.20 |
| Probability | Lymphedema after ALND | 0.15 10 | 0.005–0.20 |
| Probability | Surgical site infection after ALND | 0.15 10 | 0.005 – 0.20 |
| Probability | AUS true negative result given N0 disease | 0.87 36 | 0.5–1.0 |
| Probability | AUS false negative result given N1 disease (l-2LNs) | 0.59 36 | 0.1 – 0.8 |
| Probability | AUS false negative result given N2+ disease (3+LNs) | 0.25 36 | 0.1 – 0.6 |
| Cost | ALND | $7,367 54 | $l,500-$46,546 |
| Cost | SLNB | $6,430 54 | $13-$96,593 |
| Cost | Oncotype Dx | $3,912 54 | $1-$4481 |
| Cost | AUS | $164 54 | - |
| Utility | Health (post-breast cancer) | 0.88 60–69 | 0.5–1.0 |
| Utility | Local recurrence | 0.71 70,71 | 0.2–0.9 |
| Utility | Regional recurrence | 0.60 70 | 0.2–0.9 |
| Utility | Metastatic recurrence | 0.57 65, 71, 72 | 0.2–0.9 |
| Utility | Death | 0** | - |
| Utility | Lymphedema | 0.82 73 | 0.4–0.99 |
| Utility | Surgical site infection | 0.52 72, 74, 75 | 0.4–0.99 |
| Probability | Stage Tla N0 within cohort of T1-T2 patients with negative AUS | 0.084 35, 36 | 0–0.2 |
| Probability | Stage Tla N1 within cohort of T1-T2 patients with negative AUS | 0.003 35, 36 | 0–0.15 |
| Probability | Stage Tla N2 within cohort of T1-T2 patients with negative AUS | 0.004 35, 36 | 0–0.15 |
| Probability | Stage Tlb-T2 N0 within cohort of T1-T2 patients with negative AUS | 0.720 35, 36 | 0.5–0.99 |
| Probability | Stage Tlb-T2 N1 within cohort of T1-T2 patients with negative AUS | 0.144 35, 36 | 0.05–0.25 |
| Probability | Stage Tlb-T2 N2 within cohort of T1-T2 patients with negative AUS | 0.045 35, 36 | 0–0.15 |
| Annual Probability | Regional recurrence | 0.003–0.005* 62, 63 | 0.001 – 0.20 |
| Annual Probability | Metastatic recurrence | 0.008–0.044* 4, 9, 62 | 0.001 – 0.20 |
| Annual Probability | Metastatic progression after regional recurrence | 0.13 55, 57, 58 | 0.001 – 0.20 |
| Annual Probability | Death after regional recurrence | 0.08 56, 57 | 0.001–0.20 |
| Multiplier for probability | Regional recurrence in patients with unrecognized N1 disease | 1 1, 38, 46, 47 | 1–20 |
| Multiplier for probability | Regional recurrence in patients with unrecognized N2+disease | 5 38, 39, 46, 47 | 1–20 |
| Multiplier for probability | Metastatic recurrence in patients with unrecognized N1 disease | 1 1, 38, 47 | 1–20 |
| Multiplier for probability | Metastatic recurrence in patients with unrecognized N2+ disease | 5 38, 39, 47 | 1–20 |
Assumption
Stage-specific values were used from the stated range of values.
SLNB: sentinel lymph node biopsy, ALND: axillary lymph node dissection, AUS: axillary ultrasound, LNs: lymph nodes, $: US dollars
Figure 3:
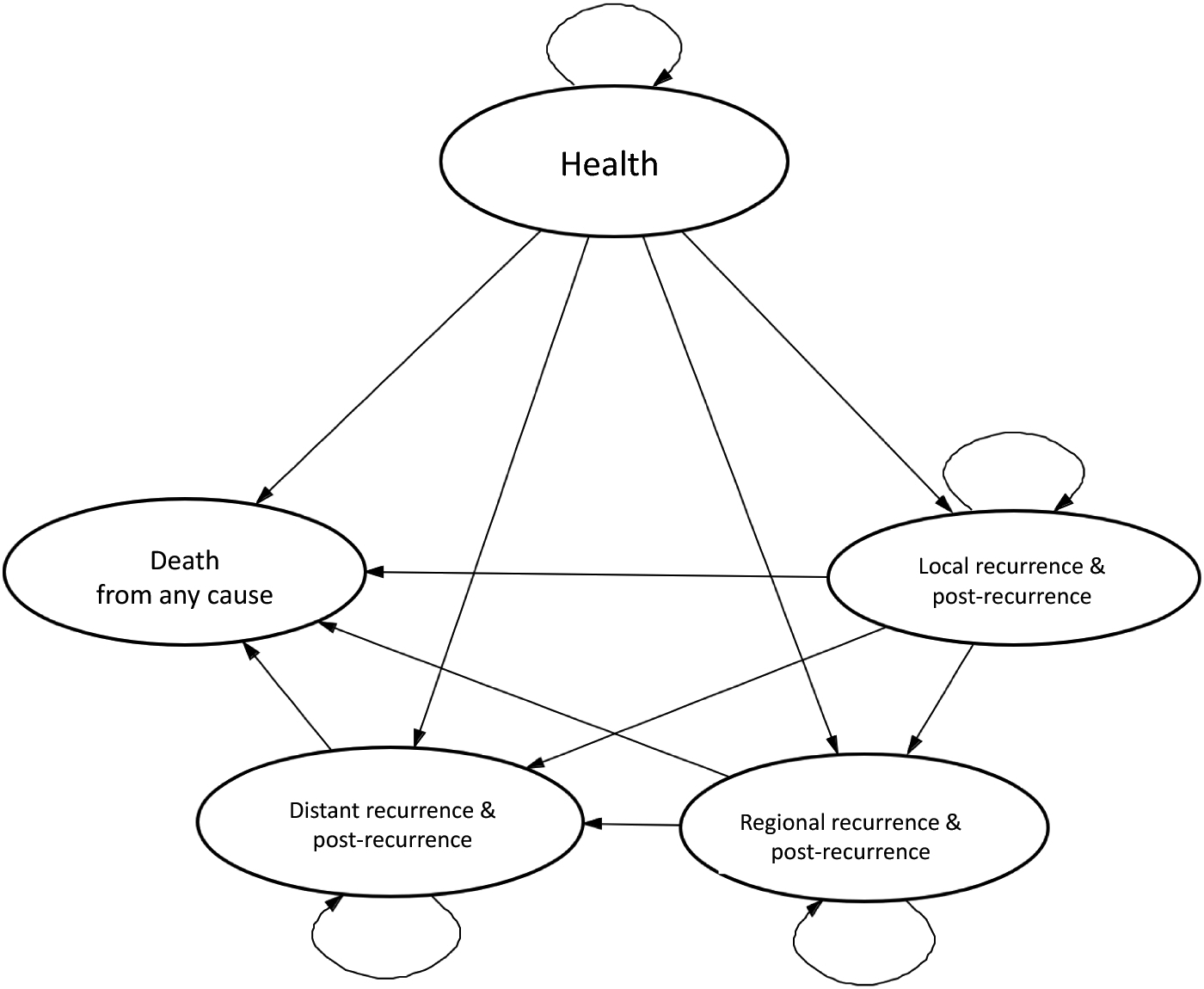
Markov Model State Transition Diagram
Recurrence states include diagnosis of recurrence, recurrence treatment, and subsequent surveillance. Simultaneous recurrence types were classified based on the most severe type (i.e. concurrent local and distant recurrences were considered a distant recurrence).
2.2.1. Input parameters: probabilities.
To populate parameters in the decision analytic models, literature reviews were conducted using PubMed, Ovid Medline, Embase, Scopus, Clinicaltrials.gov, and Cochrane Database of Systematic Reviews. A meta-analysis was also performed to assess the performance characteristics of AUS, particularly related to the false negative rate associated with a high burden of nodal disease (N2+ or ≥3 positive lymph nodes).40 This included 14,383 patients in 14 studies from various settings (academic medical centers, population-based studies, and international studies). Whenever available, we used probability values specific to patient age, T-stage, N-stage, recurrence score, and hormonal sub-type (exceptions outlined in S-II). When >1 data sources were available, the data from the best evidence (better study design, larger sample size) was used.39
In the Markov models, transition probabilities were derived from relevant survival analyses in the literature (Table 1, S-VI). Patients with false-negative AUS may be undertreated due to their unrecognized axillary disease. To account for the fact that patients with false-negative AUS results may have a higher likelihood of regional and/or distant recurrence than patients with the same stage identified as positive by SLNB, we applied a “multiplier” to the related transition probabilities. These multipliers were estimated based on the risk ratios of recurrence associated with the appropriate systemic therapy and/or radiation therapy for the stage. For example, hazard ratios for recurrence from a recent randomized clinical trial by Whelan et al, and relative risks from a meta-analysis by the Early Breast Cancer Trialists’ Collaborative Group were used to inform the multiplier estimates for the transition probability from the healthy state to the distant recurrence state.41, 42 These multipliers were calibrated and validated by comparing our predicted survival curves to survival analyses from clinical trials. We also examined values within a wide range for each of these multipliers in the sensitivity analyses (see Section 2.4).
2.2.2. Input parameters: costs.
Healthcare costs from the payer perspective were derived from the IBM® MarketScan® Commercial Database. Women at least 52 years of age coded with an International Classification of Diseases (ICD)-9/10 diagnosis code(s) for invasive breast cancer on a pathologist’s claim(s) were identified, and Current Procedural Terminology, 4th edition (CPT) codes for procedures of interested were captured within 180 days of diagnosis. From our cost analysis, the costs of AUS, Oncotype DX testing, SLNB, and ALND were: $164; $3,912; $6,430; and $7,367 respectively (S-IV). Costs were adjusted to 2016 U.S. dollars using the medical care cost component of the consumer price index, and were each at 3% as recommended by the US Panel on Cost-Effectiveness in Health and Medicine.43, 44
2.2.3. Input parameters: utilities.
Utility values for each health state of the Markov models were obtained from published data. When multiple values were available, arithmetic averages were used.
2.3. Cost-effectiveness analyses.
2.3.1. Subpopulation analyses.
Cost-effectiveness analyses were conducted in each of the six models to compare observation to SLNB after negative AUS. Based on the baseline probabilities, costs, and utilities, expected values were computed as the model outputs, including total cost and quality-adjusted life years (QALYs). A half-cycle correction was applied to the Markov models. Future costs were discounted to the present value at an annual rate of 3%. Cost-effectiveness was evaluated by comparing net monetary benefit (NMB), calculated as (QALYs × willingness-to-pay threshold) – cost, between observation and SLNB. NMB was used, as opposed to the incremental cost-effectiveness ratio (ICER), because NMB allows for assessment of interventions which are less costly and more effective.45, 46 Willingness-to-pay threshold of $100,000/QALY was used ($50,000-$150,000 in sensitivity analyses).47 The strategy with higher NMB was the superior option.
2.3.2. Population-level analysis.
Given that a patient’s pathologic nodal stage is not known at presentation, we combined the outputs from the six subpopulations using Bayes’ theorem based on (1) the prevalence of each stage (derived from the 2011–2016 SEER Cancer Registry from patients with T1-T2 HR+/HER2− breast cancer35, S-V); and (2) AUS performance (derived via systematic review of the literature and meta-analysis35, 40).
2.4. Sensitivity analyses.
Multiple one-way sensitivity analyses were performed to evaluate the effect of uncertainty from the following variables on our model conclusion, including: false negative rate of AUS, false negative rate of SLNB, probability and cost of complications from SLNB/ALND, stage prevalence, probability of disease outcomes (recurrence, death) especially given unrecognized nodal disease, and willingness-to-pay thresholds. See Table 1 for the ranges used for these parameters.
3. RESULTS
3.1. Subpopulation analyses.
Over the 10-year time horizon, N0 and N1 patients in the observation arm incurred lower costs and higher QALYs than SLNB patients. N2+ patients incurred higher cost but higher QALYs with SLNB (Table 2). Using a willingness-to-pay threshold of $100,000, NMB (calculated using both cost and quality-of-life measures) for observation was larger than that for SLNB. Thus observation was superior in N0 and N1 patients. However, in N2+ patients, NMB for observation was smaller than NMB for SLNB, and thus SLNB was superior.
Table 2:
Results of Cost-Effectiveness Analyses: subpopulation- and population-level
| T-stage | N-stage | Axillary Staging Strategy | Cost (US$) | QALYs | NMB | Favored Strategy | Calculated % of target population |
|---|---|---|---|---|---|---|---|
| T1a | N0 | Observation | $108,660 | 8.07 | $698,153 | Observation | 8.4% |
| T1a | N0 | SLNB | $115,931 | 7.97 | $681,270 | ||
| T1a | N1 | Observation | $111,807 | 7.70 | $657,991 | Observation | 0.3% |
| T1a | N1 | SLNB | $136,921 | 7.61 | $623,625 | ||
| T1a | N2 | Observation | $136,538 | 4.92 | $349,014 | SLNB | 0.4% |
| T1a | N2 | SLNB | $232,668 | 6.88 | $447,057 | ||
| T1b-T2 | N0 | Observation | $113,069 | 8.03 | $689,782 | Observation | 72.0% |
| T1b-T2 | N0 | SLNB | $120,295 | 7.93 | $672,973 | ||
| T1b-T2 | N1 | Observation | $131,345 | 7.07 | $576,124 | Observation | 14.4% |
| T1b-T2 | N1 | SLNB | $138,569 | 6.99 | $560,412 | ||
| T1b-T2 | N2 | Observation | $149,068 | 5.35 | $385,576 | SLNB | 4.5% |
| T1b-T2 | N2 | SLNB | $234,282 | 6.65 | $431,145 | ||
| All Stages (Population-level model) | Observation | $120,403 | 7.76 | $655,659 | Observation | ||
| SLNB | $131,721 | 7.73 | $641,778 | ||||
Cost-effectiveness was assessed by comparing the net monetary benefit (NMB) between strategies which was calculated by: (QALYs gained x willingness-to-pay threshold) - cost. The strategy with higher NMB is the superior option (WTP = $100,000). Axillary observation was favored over SLNB in the N0 and N1 stage-specific models (representing >95% of the target population), and in the aggregate model.
SLNB: sentinel lymph node biopsy, AUS: axillary ultrasound, QALY: quality-adjusted life-year
3.2. Population-level analysis.
Our systematic review of the AUS literature resulted in a pooled false negative rate of 59% for low axillary burden (N1 or 1–2 positive LNs), and 25% for high axillary burden (N2 or ≥3 positive LNs).48 T1b-T2 N0 was the largest subpopulation based on prevalence data (~60% of the target population).35 Observation after negative AUS was the optimal strategy for patients with N0 and N1 disease, representing approximately 95% of the study population (Table 2). Therefore, combining the prevalence of the subpopulations with the probabilities of false negative AUS (Figure 2), observation resulted in lower cost (observation $120,403 versus SLNB $131,721), higher QALYs (observation 7.76 versus SLNB 7.73), and higher NMB (observation $655,659 versus SLNB $641,778). As a result, observation was favored over SLNB.
3.3. Sensitivity analyses.
For patients with N0 and N1 disease, observation was the superior strategy despite significant variation across input variables tested via sensitivity analyses (Table 3). Only the N2+ models consistently favored SLNB, despite wide variation in input parameters. The node-positive models demonstrated sensitivity to variation in the multiplier we used to estimate the recurrence risk in the setting of unrecognized nodal disease. For example, for T1b-T2 N2+ patients with unrecognized nodal disease, SLNB was favored if the annual risk of distant recurrence was multiplied by a factor of ≥2.3 (resulting annual probability ≥0.1012). When a lower willingness-to-pay threshold was used ($50,000) the qualitative results did not change, except within N2+ subpopulations. In the population-level model, significant variation in the sensitivity of AUS did not result in a qualitative change of conclusion. Observation was favored even when the AUS false-negative rate for N2+ disease was increased to 50%, and for N1 disease to 80%.
Table 3:
Results of Key One-way Sensitivity Analyses
| Variable Description | Preferred strategy | T1a N0 | T1a N1 | T1a N2 | T1b-T2 N0 | T1b-T2 N1 | T1b-T2 N2 | Population-level model |
|---|---|---|---|---|---|---|---|---|
| SLNB | $13-$96,593 | $13-$52,000 | NA2 | |||||
| SLNB | 0.005–0.10 | 0.005–0.10 | NA2 | |||||
| SLNB | 0.4–0.99 | 0.5–0.99 | NA2 | |||||
| SLNB | 0.001 −0.20 | 0.001 −0.20 | NA2 | |||||
| SLNB | NA1 | 6.0–20 | 1–20 | NA1 | 4.4–20 | 7.7–20 | NA2 | |
| SLNB | NA1 | 1.7–20 | 2.9–20 | NA1 | 1.4–20 | 2.4–20 | NA2 | |
| SLNB | 0.2–0.9 | NA2 | ||||||
| SLNB | 0.2–0.9 | NA2 | ||||||
| SLNB | NA3 | NA3 | NA3 | NA3 | NA3 | NA3 | ||
| SLNB | NA3 | NA3 | NA3 | NA3 | NA3 | NA3 | ||
| SLNB | $50,000-$150,000 | $65,000-$150,000 |
Key variables were presented based on their clinical relevance and notable impact on the model (variables not included in the figure did not have a significant impact on model results). Results of key 1-way sensitivity analyses shown above: each input variable varied within the range of values specified in Table 1, keeping all other variables at their baseline value. In the green cells, all input values listed resulted in NMB observation > NMB SLNB, i.e., observation was superior to SLNB. In the red cells, all input values listed resulted in NMB observation < NMB SLNB, i.e., SLNB was superior to observation. For a majority of variables, the conclusion was consistent: observation after negative AUS is the optimal strategy for axillary staging.
NA1: Multipliers for breast cancer recurrence given unrecognized nodal disease (false-negative AUS or SLNB) were not applicable to N0 models.
NA2: Variables in the stage-specific models were not used in the population-level model, and therefore not applicable for sensitivity analyses at the population level.
NA3: The false-negative rates of axillary ultrasound were used only in the population-level model, and therefore not applicable for sensitivity analyses in the stage-specific models.
NMB: Net monetary benefit, SLNB: sentinel lymph node biopsy, AUS: axillary ultrasound, $: US dollars
 NMB Observation > NMB SLNB (Observation preferred) for the range of values listed
NMB Observation > NMB SLNB (Observation preferred) for the range of values listed
 NMB Observation < NMB SLNB (SLNB preferred) for the range of values listed
NMB Observation < NMB SLNB (SLNB preferred) for the range of values listed
4. DISCUSSION
This study compared the cost-effectiveness of observation versus SLNB after negative AUS in post-menopausal women with clinical T1-T2 N0, HR+/HER2− breast cancer. We found that in patients with N0 and N1 disease, observation was associated with lower costs and greater QALYs. This finding aligns with clinical intuition and NCCN recommendations as there is no therapeutic benefit associated with surgery, and biomarker and gene expression profile drive clinical decision-making in these patients. The low prevalence of AUS-negative patients with N2+ disease, and the fact that observation was clearly the superior strategy for patients with N0 and N1 disease lead to the conclusion that observation is the superior strategy for the entire patient population.
Sensitivity analyses were performed for many input parameters, including two variables of particular interest: (1) the false negative rate of AUS for the detection of high versus low nodal burden, and (2) the regional and distant recurrence rates in the setting of unrecognized nodal disease. To determine the false negative rate of AUS, we combined high quality evidence from multiple sources in a robust systematic review, and derived a reliable estimate of AUS performance for the exclusion of high and low nodal burden. The results of this systematic review confirmed that AUS is highly sensitive for the detection of N2+ disease. To account for AUS variability (for example, from operator dependence) our sensitivity analysis evaluated the impact of a very wide range of false negative rates on cost effectiveness: 10–80% for N1 disease and 10–60% for N2+ disease. The conclusion that AUS is superior to SLNB was remarkably robust, even with false negative rate of 80% for N1 disease and 60% for N2+ disease. To assess the risk of recurrence in the setting of unrecognized disease, we used a combination of relevant literature and expert opinion to estimate a risk multiplier for regional and distant recurrence. This multiplier allowed us to use known values of recurrence risk in the setting of accurately diagnosed nodal disease, and measure the effect of variation in this risk due to potential undertreatment of unrecognized nodal disease. We took into consideration risk reduction estimates for disease progression related to both systemic therapy and extended field radiation therapy. Current guidelines and a study of clinical practice suggest that physicians strongly consider gene expression profile, rather than nodal burden, when selecting systemic therapies.38, 49 We also considered that extended field radiation therapy might be omitted for patients with unrecognized nodal disease.38, 41, 42, 50, 51 For example, in a randomized trial by Whelan et al., nodal irradiation marginally benefitted N1 patients (small decrease in regional recurrence, but no overall survival benefit). However, subset analysis demonstrated no benefit for patients with HR+ disease.41, 52 Therefore, a multiplier of 1.0 for regional and/or distant recurrence in AUS false-negative cN0/pN1 patients within our target population of relatively low-risk breast cancer is justified. To account for uncertainty in these variables, we used a wide range of values in our sensitivity analyses, and the conclusion remained consistent: US is superior to SLNB in this population.
This study provides a new perspective on the benefit of observation/noninvasive axillary staging in the context of mounting literature supporting more judicious application of surgical staging. Z0011 was not the first study in breast oncology to generate evidence that surgical axillary clearance is not always associated with survival benefit.53 One of the key practice changes from Z0011 was omitting ALND for patients with <3 positive nodes. Our study demonstrates the survival, quality of life, and financial cost benefits of using a negative AUS to identify patients who can forego axillary surgery. The presence of undetected nodal disease in 27% of Z0011 patients, without survival decrement, underscores the potential that systemic therapy is effective in treating some nodal disease. This critical result suggests that, in the 5% of patients who may have unrecognized N2+ disease, the impact of undertreatment may be mitigated by the fact that all patients are treated with systemic therapy.
A considerable body of evidence suggests that complications and costs associated with SLNB significantly impact patients’ quality of life.54 Out-of-pocket costs and productivity losses contribute to a heavy burden for breast cancer survivors who suffer from lymphedema which is most often iatrogenic from axillary surgery.31 Avoidance of unnecessary surgery will decrease the financial burden and eliminate potentially long-term physical, psychological, and emotional challenges presented by surgery itself and surgical complications such as lymphedema.55 Although the psychological and emotional effects of lymphedema are not easily quantified in a cost-effectiveness analysis, they are critically important to consider when weighing the costs and benefits of axillary staging procedures with individual patients, and are avoidable when axillary observation is an appropriate alternative.
This study has several strengths. We utilized cost estimates from novel cost analyses for diagnostic procedures and complications related to lymphedema. Costs which we did not derive, were taken from sources which used the same or very similar source data.56, 57 The consistency of cost data sources enhances the validity of this study. Furthermore, we conducted extensive literature reviews related to SLNB performance and complications, Oncotype DX testing, ALND complications, health state utilities, as well as long-term recurrence and survival outcomes. When sufficient published data were available, e.g., AUS performance by nodal burden, we conducted a meta-analysis to synthesize the data. These reviews provided evidence for the values of our base models, a reference range to ensure adequate sensitivity analyses, and contributed to the formation of a realistic and reproducible model.
Of note, our model design did not include the cost, effectiveness, and complications related to bisphosphonate therapy or endocrine therapy. This is appropriate because we do not expect that the use of bisphosphonate and/or endocrine therapies differs between our two modeled strategies. Our model also did not include the potential complications and disutility associated with chemotherapy or radiotherapy. SLNB would likely result in more chemotherapy and radiotherapy use (given the recognition of nodal disease). Therefore, including these complications would only emphasize observation as the superior strategy. Similarly, we only modeled SLNB and ALND complications related to lymphedema and surgical site infection. Doing so allowed us to account for both acute and chronic complications, although we did not include all potential complications. In literature review, we found that lymphedema and surgical site infections were well-documented in large trials and data sets which generated reliable probabilities. In contrast, the costs associated with dye reactions, seroma, paresthesias and range of motion deficits were less reliable/available. Importantly, inclusion of these additional complications would only emphasize observation as the superior strategy.
A limitation of our study is the fact that only a subset of breast cancer patients (post-menopausal, HR+/HER2−) were included. However, the selected population represents the majority of breast cancer patients: HR+/HER2− subtype represents approximately 73% of breast cancer cases in the US, 90% of HR+/HER2− patients have T1-T2 tumors, and 81% of HR+/HER2− patients are > 50 years old.36 Another limitation is that we assumed patients were managed with standard regimens based on clinical guidelines, which included Oncotype DX testing for T1b-T2N0 and N1 patients. In real-world practice, there is variation in management given patient preference and providers’ clinical judgment. However, our models were based on NCCN guidelines, and informed by studies which have demonstrated increasing use of recurrence scores to guide treatment decisions.49 The proposed approach may be particularly well-suited for low-income countries in which surgery and optimal therapy for complications are even less accessible. Observation after negative AUS would not require any changes in standard-of-care surveillance as recommended by NCCN guidelines. Therefore, we would expect no difference in patient adherence to follow-up between the two examined strategies: observation after negative AUS versus SLNB.
The concern related to observation versus SLNB is that some patients may be undertreated. Patients with unrecognized nodal disease may not receive appropriate therapy such as chemotherapy and/or extended field radiation. There are important counterpoints to consider. First, SLNB is associated with false negative rates ranging from 5 to 22.9%.17 Second, observation includes appropriate therapy for the primary tumor, including surgery, radiation therapy, and systemic endocrine therapy. It is likely that systemic therapy contributes to control of nodal disease, although this is an area that requires further investigation. Third, unrecognized nodal disease would likely be identified during surveillance and successfully treated at the time of discovery, suggesting that observation may only delay definitive therapy. Current paradigms in cancer biology suggest that lymph node disease is unlikely to spread to other organs. Fourth, using SEER data and AUS performance statistics, we calculated that approximately 5% of patients in our study population would have unrecognized N2+ disease, and most of those patients would receive Oncotype DX testing and appropriate systemic therapy. The ethical implications of potential undertreatment versus decreased quality of life from potential surgical complications must be considered. In our analysis, observation is clearly supported by the ethical principle of “first, do no harm.”
In conclusion, for post-menopausal woman with cT1-T2 N0, HR+/HER2− breast cancer, observation after negative AUS results in lower costs and higher QALYs compared to SLNB. Our study provides unique and reassuring evidence for observation in low-risk, post-menopausal women with cT1-T2, HR+/HER− breast cancer after negative axillary ultrasound and avoid unnecessary surgery. Cost and quality of life outcomes will be important complements to the data generated by ongoing randomized clinical trials (SOUND, INSEMA), and altogether, will provide evidence for future guidelines. Our study clearly demonstrates that observation is a reasonable alternative to SLNB in an important subset of breast cancer patients where adjuvant therapy decision making is less dependent on axillary staging.
Supplementary Material
Acknowledgements
We thank Laura Simon and the Washington University, St. Louis Bernard Becker Library for systematic review assistance. We thank the Siteman Cancer Center for supporting breast oncology investigation.
Research reported in this publication was supported by the Washington University Institute of Clinical and Translational Sciences grant UL1TR002345 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health, and National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number TL1TR002344. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The Center for Administrative Data Research is supported in part by the Washington University Institute of Clinical and Translational Sciences grant UL1TR002345 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) and Grant Number R24 HS19455 through the Agency for Healthcare Research and Quality (AHRQ).
Funding: This study was funded by the National Center for Advancing Translational Sciences via grant #UL1TR002345 supporting Dr. Steven Poplack, and grant #TL1TR002344 supporting Aubriana McEvoy.
- UL1TR002345: National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health
-TL1TR002344: National Center for Advancing Translational Sciences of the National Institutes of Health
- This study was not supported, in any way, by a pharmaceutical company.
Footnotes
Conflict of Interest: All authors (Aubriana McEvoy, Steven Poplack, Katelin Nickel, Margaret Olsen, Foluso Ademuyiwa, Imran Zoberi, Elizabeth Odom, Jennifer Yu, Su-Hsin Chang, and William Gillanders) have no conflict of interest.
Ethical approval: This article does not contain any studies with human participants or animals performed by any of the authors.
REFERENCES
- 1.Giuliano AE, Ballman KV, McCall L, et al. Effect of Axillary Dissection vs No Axillary Dissection on 10-Year Overall Survival Among Women With Invasive Breast Cancer and Sentinel Node Metastasis: The ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA 2017; 318(10):918–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin PP, Allison DC, Wainstock J, et al. Impact of axillary lymph node dissection on the therapy of breast cancer patients. J Clin Oncol 1993; 11(8):1536–44. [DOI] [PubMed] [Google Scholar]
- 3.Ramsey SD, Barlow WE, Gonzalez-Angulo AM, et al. Integrating comparative effectiveness design elements and endpoints into a phase III, randomized clinical trial (SWOG S1007) evaluating oncotypeDX-guided management for women with breast cancer involving lymph nodes. Contemp Clin Trials 2013; 34(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sparano JA, Gray RJ, Makower DF, et al. Adjuvant Chemotherapy Guided by a 21-Gene Expression Assay in Breast Cancer. N Engl J Med 2018; 379(2):111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zujewski JA, Kamin L. Trial assessing individualized options for treatment for breast cancer: the TAILORx trial. Future Oncol 2008; 4(5):603–10. [DOI] [PubMed] [Google Scholar]
- 6.Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 2010; 11(1):55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004; 351(27):2817–26. [DOI] [PubMed] [Google Scholar]
- 8.Stemmer SM, Steiner M, Rizel S, et al. Clinical outcomes in ER+ HER2 -node-positive breast cancer patients who were treated according to the Recurrence Score results: evidence from a large prospectively designed registry. NPJ Breast Cancer 2017; 3:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nitz U, Gluz O, Christgen M, et al. Reducing chemotherapy use in clinically high-risk, genomically low-risk pN0 and pN1 early breast cancer patients: five-year data from the prospective, randomised phase 3 West German Study Group (WSG) PlanB trial. Breast Cancer Res Treat 2017; 165(3):573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst 2006; 98(9):599–609. [DOI] [PubMed] [Google Scholar]
- 11.Ashikaga T, Krag DN, Land SR, et al. Morbidity results from the NSABP B-32 trial comparing sentinel lymph node dissection versus axillary dissection. J Surg Oncol 2010; 102(2):111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langer I, Guller U, Berclaz G, et al. Morbidity of sentinel lymph node biopsy (SLN) alone versus SLN and completion axillary lymph node dissection after breast cancer surgery: a prospective Swiss multicenter study on 659 patients. Ann Surg 2007; 245(3):452–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dabakuyo TS, Fraisse J, Causeret S, et al. A multicenter cohort study to compare quality of life in breast cancer patients according to sentinel lymph node biopsy or axillary lymph node dissection. Ann Oncol 2009; 20(8):1352–61. [DOI] [PubMed] [Google Scholar]
- 14.Rao R, Euhus D, Mayo HG, et al. Axillary node interventions in breast cancer: a systematic review. JAMA 2013; 310(13):1385–94. [DOI] [PubMed] [Google Scholar]
- 15.McLaughlin SA, Wright MJ, Morris KT, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol 2008; 26(32):5213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shih YC, Xu Y, Cormier JN, et al. Incidence, treatment costs, and complications of lymphedema after breast cancer among women of working age: a 2-year follow-up study. J Clin Oncol 2009; 27(12):2007–14. [DOI] [PubMed] [Google Scholar]
- 17.Reimer T, Hartmann S, Stachs A, et al. Local treatment of the axilla in early breast cancer: concepts from the national surgical adjuvant breast and bowel project B-04 to the planned intergroup sentinel mamma trial. Breast Care (Basel) 2014; 9(2):87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Killelea BK, Long JB, Dang W, et al. Associations Between Sentinel Lymph Node Biopsy and Complications for Patients with Ductal Carcinoma In Situ. Ann Surg Oncol 2018; 25(6):1521–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldberg JI, Wiechmann LI, Riedel ER, et al. Morbidity of sentinel node biopsy in breast cancer: the relationship between the number of excised lymph nodes and lymphedema. Ann Surg Oncol 2010; 17(12):3278–86. [DOI] [PubMed] [Google Scholar]
- 20.Bezu C, Coutant C, Salengro A, et al. Anaphylactic response to blue dye during sentinel lymph node biopsy. Surg Oncol 2011; 20(1):e55–9. [DOI] [PubMed] [Google Scholar]
- 21.Houssami N, Ciatto S, Turner RM, et al. Preoperative ultrasound-guided needle biopsy of axillary nodes in invasive breast cancer: meta-analysis of its accuracy and utility in staging the axilla. Ann Surg 2011; 254(2):243–51. [DOI] [PubMed] [Google Scholar]
- 22.Hieken TJ, Trull BC, Boughey JC, et al. Preoperative axillary imaging with percutaneous lymph node biopsy is valuable in the contemporary management of patients with breast cancer. Surgery 2013; 154(4):831–8; discussion 838–40. [DOI] [PubMed] [Google Scholar]
- 23.Alvarez S, Anorbe E, Alcorta P, et al. Role of sonography in the diagnosis of axillary lymph node metastases in breast cancer: a systematic review. AJR Am J Roentgenol 2006; 186(5):1342–8. [DOI] [PubMed] [Google Scholar]
- 24.Del Riego J, Diaz-Ruiz MJ, Teixido M, et al. The impact of axillary ultrasound with biopsy in overtreatment of early breast cancer. Eur J Radiol 2018; 98:158–164. [DOI] [PubMed] [Google Scholar]
- 25.Caudle AS, Kuerer HM, Le-Petross HT, et al. Predicting the extent of nodal disease in early-stage breast cancer. Ann Surg Oncol 2014; 21(11):3440–7. [DOI] [PubMed] [Google Scholar]
- 26.Gipponi M, Fregatti P, Garlaschi A, et al. Axillary ultrasound and Fine-Needle Aspiration Cytology in the preoperative staging of axillary node metastasis in breast cancer patients. Breast 2016; 30:146–150. [DOI] [PubMed] [Google Scholar]
- 27.Tucker NS, Cyr AE, Ademuyiwa FO, et al. Axillary Ultrasound Accurately Excludes Clinically Significant Lymph Node Disease in Patients With Early Stage Breast Cancer. Ann Surg 2016; 264(6):1098–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reimer T, Stachs A, Nekljudova V, et al. Restricted Axillary Staging in Clinically and Sonographically Node-Negative Early Invasive Breast Cancer (c/iT1–2) in the Context of Breast Conserving Therapy: First Results Following Commencement of the Intergroup-Sentinel-Mamma (INSEMA) Trial. Geburtshilfe Frauenheilkd 2017; 77(2):149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verry H, Lord SJ, Martin A, et al. Effectiveness and cost-effectiveness of sentinel lymph node biopsy compared with axillary node dissection in patients with early-stage breast cancer: a decision model analysis. Br J Cancer 2012; 106(6):1045–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henry-Tillman R, Glover-Collins K, Preston M, et al. The SAVE review: sonographic analysis versus excision for axillary staging in breast cancer. J Am Coll Surg 2015; 220(4):560–7. [DOI] [PubMed] [Google Scholar]
- 31.Dean LT, Moss SL, Ransome Y, et al. “It still affects our economic situation”: long-term economic burden of breast cancer and lymphedema. Support Care Cancer 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gentilini O, Veronesi U. Abandoning sentinel lymph node biopsy in early breast cancer? A new trial in progress at the European Institute of Oncology of Milan (SOUND: Sentinel node vs Observation after axillary UltraSouND). Breast 2012; 21(5):678–81. [DOI] [PubMed] [Google Scholar]
- 33.Boughey JC, Moriarty JP, Degnim AC, et al. Cost modeling of preoperative axillary ultrasound and fine-needle aspiration to guide surgery for invasive breast cancer. Ann Surg Oncol 2010; 17(4):953–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voduc KD, Cheang MC, Tyldesley S, et al. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol 2010; 28(10):1684–91. [DOI] [PubMed] [Google Scholar]
- 35.SEER*Stat Database: Incidence - SEER 18 Regs Research Data. In Surveillance E, and End Results (SEER) Program, ed. www.seer.cancer.gov, 1975–2016.
- 36.Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst 2014; 106(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amin MB, Edge SB, American Joint Committee on Cancer AJCC cancer staging manual. Eighth edition ed. Switzerland: Springer, 2017. [Google Scholar]
- 38.(NCCN) NCCN. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. 2019(Version 1.2019). [Google Scholar]
- 39.Hunink MGM. Decision making in health and medicine : integrating evidence and values. Second edition ed. Cambridge, United Kingdom: Cambridge University Press, 2014. [Google Scholar]
- 40.Mcevoy AM CS, Gillanders W, Poplack SP. Systematic Review and Meta-Analysis: Diagnostic accuracy and clinical utility of axillary ultrasound in invasive breast cancer. PROSPERO 2019. [Google Scholar]
- 41.Whelan TJ, Olivotto IA, Levine MN. Regional Nodal Irradiation in Early-Stage Breast Cancer. N Engl J Med 2015; 373(19):1878–9. [DOI] [PubMed] [Google Scholar]
- 42.McGale P, Taylor C, Correa C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014; 383(9935):2127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Consumer Price Index Summary 2019. Available at: www.bls.gov/cpi. 2019.
- 44.Weinstein MC, Siegel JE, Gold MR, et al. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA 1996; 276(15):1253–8. [PubMed] [Google Scholar]
- 45.McFarlane PA, Bayoumi AM. Acceptance and rejection: cost-effectiveness and the working nephrologist. Kidney Int 2004; 66(5):1735–41. [DOI] [PubMed] [Google Scholar]
- 46.Trippoli S Incremental cost-effectiveness ratio and net monetary benefit: Current use in pharmacoeconomics and future perspectives. Eur J Intern Med 2017; 43:e36. [DOI] [PubMed] [Google Scholar]
- 47.Marseille E, Larson B, Kazi DS, et al. Thresholds for the cost-effectiveness of interventions: alternative approaches. Bull World Health Organ 2015; 93(2):118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mcevoy AM CS, Gillanders W, Poplack SP. A systematic review of axillary ultrasound for regional staging of invasive breast cancer: assessing exam performance by nodal burden, imaging criteria, and tumor characteristics. Washington University, St. Louis, 2019. [Google Scholar]
- 49.Hassett MJ, Silver SM, Hughes ME, et al. Adoption of gene expression profile testing and association with use of chemotherapy among women with breast cancer. J Clin Oncol 2012; 30(18):2218–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vicini FA, Horwitz EM, Lacerna MD, et al. The role of regional nodal irradiation in the management of patients with early-stage breast cancer treated with breast-conserving therapy. Int J Radiat Oncol Biol Phys 1997; 39(5):1069–76. [DOI] [PubMed] [Google Scholar]
- 51.Hirata K, Yoshimura M, Inoue M, et al. Regional recurrence in breast cancer patients with one to three positive axillary lymph nodes treated with breast-conserving surgery and whole breast irradiation. J Radiat Res 2017; 58(1):79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies--improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 2015; 26(8):1533–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fisher B, Jeong JH, Anderson S, et al. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med 2002; 347(8):567–75. [DOI] [PubMed] [Google Scholar]
- 54.Hsu T, Ennis M, Hood N, et al. Quality of life in long-term breast cancer survivors. J Clin Oncol 2013; 31(28):3540–8. [DOI] [PubMed] [Google Scholar]
- 55.Taghian NR, Miller CL, Jammallo LS, et al. Lymphedema following breast cancer treatment and impact on quality of life: a review. Crit Rev Oncol Hematol 2014; 92(3):227–34. [DOI] [PubMed] [Google Scholar]
- 56.Blumen H, Fitch K, Polkus V. Comparison of Treatment Costs for Breast Cancer, by Tumor Stage and Type of Service. Am Health Drug Benefits 2016; 9(1):23–32. [PMC free article] [PubMed] [Google Scholar]
- 57.Olsen MA, Chu-Ongsakul S, Brandt KE, et al. Hospital-associated costs due to surgical site infection after breast surgery. Arch Surg 2008; 143(1):53–60; discussion 61. [DOI] [PubMed] [Google Scholar]
- 58.Mcevoy AM CS, Nickel K, Olsen MA, Gillanders W, Poplack SP. MarketScan® Commercial Database In IBM, ed.: Center for Administrative Data Research, 2019. [Google Scholar]
- 59.Botteri E, Bagnardi V, Rotmensz N, et al. Analysis of local and regional recurrences in breast cancer after conservative surgery. Ann Oncol 2010; 21(4):723–8. [DOI] [PubMed] [Google Scholar]
- 60.Geurts YM, Witteveen A, Bretveld R, et al. Patterns and predictors of first and subsequent recurrence in women with early breast cancer. Breast Cancer Res Treat 2017; 165(3):709–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harris EE, Hwang WT, Seyednejad F, et al. Prognosis after regional lymph node recurrence in patients with stage I-II breast carcinoma treated with breast conservation therapy. Cancer 2003; 98(10):2144–51. [DOI] [PubMed] [Google Scholar]
- 62.Nielsen HM, Overgaard M, Grau C, et al. Loco-regional recurrence after mastectomy in high-risk breast cancer--risk and prognosis. An analysis of patients from the DBCG 82 b&c randomization trials. Radiother Oncol 2006; 79(2):147–55. [DOI] [PubMed] [Google Scholar]
- 63.Foukakis T, Fornander T, Lekberg T, et al. Age-specific trends of survival in metastatic breast cancer: 26 years longitudinal data from a population-based cancer registry in Stockholm, Sweden. Breast Cancer Res Treat 2011; 130(2):553–60. [DOI] [PubMed] [Google Scholar]
- 64.Schleinitz MD, DePalo D, Blume J, et al. Can differences in breast cancer utilities explain disparities in breast cancer care? J Gen Intern Med 2006; 21(12):1253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Administration SS. Actuarial Life Table 2016. Available at: https://www.ssa.gov/oact/STATS/table4c6.html. 2019.
- 66.Pan H, Gray R, Braybrooke J, et al. 20-Year Risks of Breast-Cancer Recurrence after Stopping Endocrine Therapy at 5 Years. N Engl J Med 2017; 377(19):1836–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Maaren MC, de Munck L, Strobbe LJA, et al. Ten-year recurrence rates for breast cancer subtypes in the Netherlands: A large population-based study. Int J Cancer 2019; 144(2):263–272. [DOI] [PubMed] [Google Scholar]
- 68.Stokes ME, Thompson D, Montoya EL, et al. Ten-year survival and cost following breast cancer recurrence: estimates from SEER-medicare data. Value Health 2008; 11(2):213–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


