Abstract
To understand links between early experience and biomarkers of peripheral physiology in adulthood, this study examined associations between quality of early caregiving and markers of sympathetic activation and chronic inflammation in a sample of 52 low-income mothers and their preschool-aged children. Mothers reported on levels of positive caregiving experienced during childhood using the Structural Analysis of Social Behavior-Intrex. Mother and child sympathetic activation was indexed via pre-ejection period (PEP) at rest, during a dyadic social engagement task, and for children, while interacting with an unfamiliar adult. C-reactive protein (CRP) was collected using whole blood spots to assess levels of low-grade chronic inflammation. Results showed that mothers who reported experiencing more warm guidance and support for autonomy in early childhood displayed lower resting sympathetic nervous system activation (i.e., longer PEP) and lower chronic inflammation (i.e., CRP levels). Further, lower maternal chronic inflammation levels were associated with lower sympathetic activation (i.e., longer PEP) in their children at rest, and during social interactions with mother and a female stranger.
Keywords: autonomy support, C-reactive protein, early experience, inflammation, parenting, pre-ejection period, stress, two-generational, warm guidance
1 ∣. INTRODUCTION
In recent decades, research has established that early exposure to adversity is associated with a host of negative physical health outcomes in adulthood (e.g., Miller, Chen, & Parker, 2011; Repetti, Taylor, & Seeman, 2002; Shonkoff, Boyce, & McEwen, 2009). Emerging evidence has begun to elucidate the link between early stress and biological functioning (Nusslock & Miller, 2016; Shonkoff et al., 2012), bringing new understanding to the multisystemic process of neurobiological disruption that underlies stress-related disease. There is growing recognition that quality of the early caregiving environment is critical to physical health outcomes (e.g., Lehman, Taylor, Kief, & Seeman, 2005, 2009; McLaughlin et al., 2015; Miller & Chen, 2010; Shonkoff et al., 2009). Robust links have been uncovered between exposure to family violence, child abuse and neglect, and other maladaptive experiences in childhood, and elevated risk for adulthood cardiovascular and autoimmune disorders (e.g., Adverse Childhood Experiences Study; Anda et al., 2009; Dube et al., 2003, 2009). Further, early experiences appear capable of shaping later biology, and interventions that successfully target parental sensitivity show promise in normalizing patterns of stress activation in children (Fisher & Stoolmiller, 2008). For example, foster children whose caregivers participated in relational interventions (Attachment and biobehavioral catch-up [ABC]) showed normalized patterns of cortisol responding following the intervention, suggesting that sensitive parenting behaviors may improve biomarkers of stress in children (Dozier, Peloso, Lewis, Laurenceau, & Levine, 2008). However, gaps remain regarding the link between parental sensitivity experienced during childhood and markers of adaptive biological functioning in adulthood. Further, few studies have focused on specific caregiving behaviors that may relate to biological health in adulthood, and there is a lack of consensus regarding patterns of activity that may be observed across physiological systems. In the current study, we examined associations between parents’ recollections of specific, positive caregiving behaviors experienced in early childhood and current biomarkers of parent and child peripheral physiology (i.e., sympathetic nervous system and immune system) at rest and during social interaction.
1.1 ∣. Markers of sympathetic and immune system functioning
The adaptive functioning of the body's biological systems is essential in regulating physical and psychological health across the lifespan (Blair et al., 2011; El-Sheikh et al., 2009). The caregiving environment is an important contributing factor in a child's physiological functioning, and research indicates that quality of social relatedness in parent-child dyads is a critical influence on physiological regulation. The presence of secure, supportive relationships with family members has been shown to exert positive, buffering effects on the regulation of physiological systems in children and adults (e.g., Gunnar & Quevedo, 2007; Uchino, Cacioppo, & Kiecolt-Glaser, 1996; Willemen, Schuengel, & Koot, 2009). For example, children's attachment security modulates cortisol regulation in response to distressing events (Gunnar & Donzella, 2002), and substantial differences in patterns of physiological responding have been observed in children whose caregivers are sensitive versus neglectful (Esposito, Koss, Donzella, & Gunnar, 2016).
Quality of early caregiving has been linked to healthy development in several physiological systems, including the sympathetic nervous system (SNS) and the inflammatory arm of the immune system (Kuhlman, Chiang, Horn, & Bower, 2017; Slopen, Kubzansky, McLaughlin, & Koenen, 2013), two main components of the sympathetic-adrenomedullary (SAM) system that contribute to number of chronic diseases. In situations of acute threat, the SAM system activates a “fight or flight” stress response, where the body releases epinephrine (adrenaline) and norepinephrine to facilitate rapid mobilization (Cannon, 1929; Gunnar & Quevedo, 2007). This hormonal cascade signals the activation of immune gene transcription (Gunnar & Quevedo, 2007; Irwin & Cole, 2011) leading to the production and release of pro-inflammatory cytokines (Kuhlman et al., 2017; Miller et al., 2011), which helps to facilitate an increase in acute inflammation. An acute inflammatory response is adaptive and essential to survival; however, chronic inflammation resulting from repeated, stressful life events (Repetti et al., 2002; Shonkoff et al., 2009) or in response to insensitive caregiving experienced in early childhood (Miller et al., 2011; Riis et al., 2016) is highly problematic and has been shown to be a key driver in pathogenesis of chronic diseases, including diabetes (Bertoni et al., 2010; Dandona, Aljada, Chaudhuri, Mohanty, & Garg, 2005), coronary heart disease (Lawlor, Smith, Rumley, Low, & Ebrahim, 2005; Taylor, Lehman, Kiefe, & Seeman, 2006), autoimmune disorders (Abou-Raya & Abou-Raya, 2006) and some cancers (Antoni et al., 2006; Mantovani, Allavena, Sica, & Balkwill, 2008). One marker of chronic inflammation is C-reactive protein (CRP) which, if detected at levels of minor elevation, serves as a marker of stress-related immune dysregulation (Miller et al., 2011; Ridker, 2003). In contrast to the significant CRP elevations that are seen in acute infection, CRP levels signaling chronic low-grade inflammation have been robustly associated with psychosocial stress (Danese et al., 2011; Pikhart et al., 2009), and are linked to increased vulnerability to multiple chronic diseases in adulthood (Bertoni et al., 2010; Sesso et al., 2003). Studies have also consistently shown strong associations between early environmental stress and elevated CRP levels that appear as early as age 10 (e.g., Alley et al., 2006; Danese et al., 2009; Danese, Pariante, Caspi, Taylor, & Poulton, 2007; Slopen et al., 2013) and persist into adulthood (Byrne et al., 2013; McLaughlin et al., 2015; Taylor et al., 2006). While elevated CRP in adulthood appears sensitive to disruptions in early life, there are relatively few studies exploring links between specific caregiving behaviors experienced during childhood and later CRP in adulthood. We explored these associations in the current study—specifically whether experiences of early caregiving were related to later adult inflammation.
The activity of the SAM system can also be measured through pre-ejection period (PEP), a marker of SNS influence on heart rate. More specifically, PEP captures the period of time between the beginning of a heartbeat and the ejection of blood into the aorta (Berntson et al., 1994; Esposito et al., 2016), with shorter PEP indicating greater sympathetic activation and longer PEP signifying less SNS activation (Berntson et al., 1994). While minor changes in PEP represent healthy responses to meeting basic demands, research has also evidenced alterations in PEP activity in individuals who experienced early life adversity. Further, expected patterns of PEP responding differ in emotion-eliciting and reward-sensitive cognitive tasks (Beauchaine, Gatzke-Kopp, & Mead, 2007; Gatzke-Kopp & Ram, 2018; Kalvin, Bierman, & Gatzke-Kopp, 2016). In the current study, we measured PEP during a social interaction task that required physically close, prosocial engagement with one another. Although PEP is commonly measured during tasks that elicit social evaluative stress (Gunnar, Frenn, Wewerka, & Van Ryzin, 2009; McLaughlin et al., 2015),we sought to capture SNS activity during prosocial exchanges to examine patterns of healthy, adaptive SNS responding in children and parents that remain unexplored in the current literature.
1.2 ∣. Warm, autonomy-supportive parenting and sympathetic activity
Parenting constitutes a significant environmental influence on child development (Luecken & Lemery, 2004), and autonomy-supportive parenting and warm, protective guidance have emerged as highly protective for children's behavioral and physiological development (e.g., Belsky et al., 2008; Bindman, Pomerantz, & Roisman, 2015; Noll, Clark, & Skowron, 2015). According to Deci and Ryan's Self-Determination Theory ()1985, 2012, autonomy-supportive parenting is characterized by allowing children to explore their environment, solve problems on their own, and practice independence within a safe, secure parent–child relationship (Grolnick & Pomerantz, 2009; Grolnick & Ryan, 1989). Parents who support their child's basic need for autonomous exploration allow children to approach challenging tasks and experience manageable successes and failures (Bindman et al., 2015; Grolnick, Deci, & Ryan, 1997). Several studies have indicated that autonomy-supportive parenting during early childhood is associated with higher adulthood executive functioning (Bernier, Carlson, & Whipple, 2010; Bindman et al., 2015), achievement (Grolnick, et al., 1997; Pomerantz, Grolnick, & Price, 2005), and physiological self-regulation (Noll et al., 2015). Similar favorable outcomes have been observed for parental use of warm guidance and protection, in that warm parental guidance has been suggested to facilitate self-regulation development (Moilanen, Shaw, Dishion, Gardner, & Wilson, 2010). Whereas research has established that autonomy supportive parenting and warm, protective guidance are specific parenting behaviors that promote positive child psychosocial development, much less is known about how these parenting behaviors experienced early in childhood may relate to biomarkers of adult SAM-system activity, namely SNS activation and chronic inflammation.
In the current study, we examine associations between parents’ recollections of their own early caregiving experiences of autonomy support and warm, protective guidance, and biomarkers of parent and child SNS activation and chronic inflammation, to elucidate linkages between early experience and physiological functioning. We hypothesized that adults who report experiencing caregiving in early childhood characterized by warm, protective guidance and more support for their autonomy would display lower levels of chronic inflammation (i.e., lower CRP), and lower SNS activation (i.e., longer PEP intervals) at rest and during social interactions with their own child. A second aim of this study was to explore two-generational associations between parent and child SNS activation and chronic inflammation. Though CRP elevations have been observed in late childhood (ages 8–10 years) and early adolescence (age 13 years: Slopen et al., 2013; Byrne et al., 2013, respectively), it is unclear whether evidence of low-grade chronic inflammation begins to manifest earlier in childhood (ages 3–5 years old). It is possible that these measures of SAM-system activity do not emerge until later childhood or adolescence. Further, although child SNS activation has frequently been examined using salivary cortisol as a biomarker index stress activity (Gunnar et al., 2009; Gunner & Quevedo, 2007), there is still a relative lack of information about PEP activity in high-risk young children, or how children's PEP may relate to biomarkers of their mothers’ physiological activity. Thus, we explored these questions in the current study, with a sample of low-income mothers and their preschool-aged children.
2 ∣. METHOD
2.1 ∣. Participants
Participants were 52 low-income, mother–child dyads recruited from Head Start and early Head Start agencies in the Pacific Northwest. Mothers were an average age of 30.81 years (SD = 3.53) and identified as Caucasian (69.2%), Bi- or Multi-Racial (19.2%), Hispanic-Latino (7.7%), Black or African American (1.9%), and Native American (1.9%). A majority of mothers were either married (46.2%) or living with a romantic partner (19.2%). Participating children ranged in age from 3 to 5 years (M = 4.27, SD = 0.88) and were 50% female. Children were Caucasian (55.8%), Hispanic-Latino (7.7%), or Bi- or Multi-Racial (36.5%). Most mothers in the study reported obtaining a high school education or less (61.5%), and a monthly household income that ranged from $300 to $5,833 per month (M = $2,146.99, Mdn = $2,000.00, SD = $1,140.91), with 52.6% of study families living below the U.S. federal poverty guidelines at the time of their visit. Families were characterized at moderate risk per a cumulative risk scale (M = 3.00, SD = 1.91, range = 0–7) adapted from Evans and Kim (2007).
2.2 ∣. Procedures
Data were collected as part of a larger pilot study examining stress physiology markers in mother-preschooler dyads. The study was approved by the (name withheld for blind review) Institutional Review Board (IRB Protocol Number: 04252013.029). Participating mothers and their children completed a 2.5-hr laboratory visit. After mothers had provided written informed consent for themselves and for their child, both mother and child were fitted with disposable electrodes to monitor cardiac data, using a modified Lead II placement (i.e., right clavicle, lower left rib cage and lower right abdomen) and a tetrapolar configuration of electrode pairs on either side of the neck and the sternum to record cardiac impedance. Continuous ECG data was transmitted via a Biopac MP-150 wireless system (BioNomadix, Biopac) at a sampling rate of 1,000 Hz. A research assistant monitored physiological signals from a separate room throughout the session. Mother-child dyads were seated separately in a dimly-lit room and encouraged to relax while they watched a 5-min neutral video to provide resting baseline measures for RSA and PEP. Following baseline recordings, dyads engaged in a scripted social interaction task, comprised of two joint episodes of identical format—child with mother and child with unknown female researcher—administered in counterbalanced order across families (Wismer Fries, Zigler, Kurian, Jacoris, & Pollak, 2005). For each joint episode, task instructions were presented on a computer monitor, in which each member of the dyad was instructed to count each other's fingers, point to parts of each other's faces (i.e., nose, hair, and ears), and whisper a story to each other. All instructions were presented for fixed time intervals and the story told by the research assistant was always the same. Children were seated on mother's or female experimenter's lap in front of a computer monitor for the duration of the interaction task.
Next, mothers reported on the quality of their early caregiving experiences with their own mothers using the Structural Analysis of Social Behavior (SASB) Intrex questionnaire. At the end of the visit, research staff wearing disposable non-latex gloves collected whole blood spots from a single finger-prick sample (yielding five spots total), and applied to standard collection Whatman cards. Mothers completed the procedure while their children observed, then verbally-assenting children completed blood spot collection. Cards were allowed to dry, transferred to a freezer within 24 hr, then stored at −80°C until they were assayed. The protocol is consistent with that used in a number of epidemiologic studies involving blood spot CRP measures (Blackwell, Snodgrass, Madimenos, & Sugiyama, 2010; McDade, Hawkley, & Cacioppo, 2006; Snodgrass et al., 2007). Mothers were paid $50 upon completion of the visit, reimbursed for transportation, and children received stickers and toys as compensation for their participation in the study.
2.3 ∣. Measures
2.3.1 ∣. Recollected early caregiving
The structural analysis of Social Behavior is a model for characterizing dyadic interpersonal behavior and intrapsychic representations that comprise three circumplex surfaces, each defined by the orthogonal dimensions of affiliation and interdependence. Affiliation describes communications on a continuum ranging from loving to hostile, whereas Interdependence describes communications on a continuum ranging from differentiated (i.e., autonomy-granting) to enmeshed (i.e., controlling). Figure 1 shows the eight behavioral blends of affiliation and interdependence on the three surfaces of the simplified SASB model (Benjamin, 1996). The SASB Intrex questionnaires (SASB-Intrex short form; Benjamin, 1974, 1996) were employed to assess quality of mothers’ representations of her early caregivers, specifically, mother-rated quality of perceived caregiving by their own mothers in early childhood. Study mothers rated each of 16-items on a scale ranging from 0 (never/not at all) to 100 (always/perfectly) in increments of 10, indicating how well each statement described their own mother's caregiving behaviors toward them in childhood (i.e., ages 5–10). Of focus in the current study mother's retrospective ratings of SASB Cluster 11 autonomy-support and SASB Cluster 14 warm guidance/protection in early childhood. SASB Cluster 11 autonomy-support includes items such as “…gave me the freedom to do things on my own,” whereas SASB Cluster 14 warm guidance/protection includes items such as “…helped, guided, showed me how to do things.” Higher scores indicate more support of child autonomy and more warm, protective guidance, respectively.
FIGURE 1.
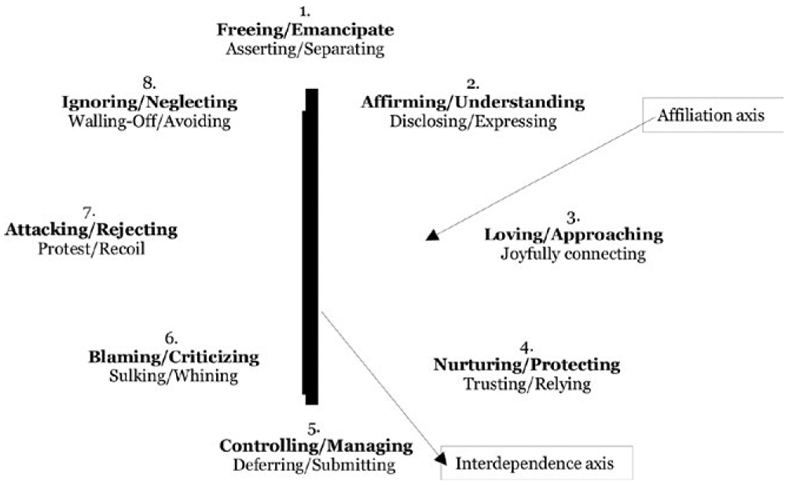
SASB simplified cluster model. The affiliation axis is the x-axis and the interdependence axis is the y-axis. Labels in bold print describe proto-typical parenting behaviors directed toward another person (i.e., child) and are the focus in the present study. Labels in underline print describe proto-typically child-like actions in response to the other (intransitive)
Chronic low-grade Inflammation
We assayed whole-blood spots for CRP using high-sensitivity enzyme-linked immunosorbent assays (ELISA). A 3.2-mm circular punch from each participant's dried blood spot (DBS) card was eluted overnight in 250 μL assay buffer. CRP levels were then assessed by ELISA according to the protocol for DBS validated in McDade, Burhop, and Dohnal (2004). CRP remains stable in dried blood spots for at least 5 days at room temperature or 14 days at 4°C and stable for years at −80°C. Next, serum equivalents were calculated using the following algorithm based on the serum-blood spot regression: serum (high-sensitivity CRP) = 1.38 * (blood spot CRP value) – 0.97 (McDade et al., 2006). Observations with values above 10 mg/L indicate frank infection (e.g., Snodgrass et al., 2007) requiring removal from statistical analysis, whereas values below 10 mg/L have been shown to index chronic low-grade inflammation associated with cardiovascular and metabolic risk (Pearson, et al., 2003). CRP values from mothers ranged from 0.09 to 9.34 mg/L (M = 2.23, SD = 2.33), whereas children's CRP values ranged from 0.09 to 8.26 mg/L (M = 0.91, SD = 1.93). No observations exceeded the threshold for frank infection.
2.3.2 ∣. SNS activation
Pre-ejection period
Complete cleaning and processing of cardiac impedance data was performed offline using MindWare 3.10 software (MindWare Technologies, Ltd., Gahanna, OH). Trained research assistants visually inspected all data to ensure that R spikes were conclusively identified. Values for PEP were calculated by superimposing the ECG on the 30 s ensemble average of the impedance wave (derivative of the Z0 wave) and calculating the time between the Q point on the ECG and the B point on the impedance wave (Lozano et al., 2007). Trained research assistants entered the electrode distance and visually inspected the data to ensure accurately that the Z and R wave points identified by MindWare software did not deviate substantially over the course of successive intervals and that calculated PEP was within a feasible range (80–130 s). PEP values were assessed in 30-s epochs and averaged for the 5-min resting baseline, 12-min maternal-child joint interaction, and 12-min examiner-child joint interaction tasks, respectively. For children, complete PEP data were available for 87% of participants during the resting baseline, 79% during the mother-child portion of the social engagement task, and 77% during the researcher-child portion of the social engagement task. Complete PEP data were available for 81% of mothers during the resting baseline and 58% of mothers during the mother-child portion of the social engagement task. Remaining data were unable to be scored due to movement artifact, inability to calculate PEP, or equipment failure. Child PEP scores during social engagement were averaged across joint task conditions with their mother and with an unfamiliar female research assistant in order to create a single child joint task score.
2.3.3 ∣. Sociodemographics
Mothers completed a comprehensive demographic interview including questions regarding socio-demographic indicators, tobacco use, and depressive symptoms. Number of cigarettes smoked per day was measured using a single self-report item: “In general, how many cigarettes do you smoke per day?” Depression symptoms were assessed using the depression subscale of the brief symptom inventory (BSI; Derogatis & Melisaratos, 1983), wherein participants rated how much depression-related problems and complaints had bothered them in the past week on a 5-point Likert scale ranging from 0 (not at all) to 4 (very much). A t-score was calculated for the seven items assessing depression to create a standardized depression symptom score for each participant. Subjective socio-economic status rating was collected using a single item in which participants identified a rung on an image of a 10-rung ladder reflecting how well-off they considered themselves in relation to others in the United States. Mother and child height (in cm) were measured using a stadiometer and their weight (in kg) was measured using a digital scale. Body mass index (BMI) was calculated for mothers and children using kg/m2.
3 ∣. RESULTS
3.1 ∣. Analytic plan & preliminary analyses
Descriptive statistics for key study variables are presented in Table 1, including ranges and possible score ranges, where applicable. We first examined relationships between sociodemographics and key study variables (see Table 2). We planned to include only variables that were statistically significantly correlated (i.e., p < 0.05) in subsequent analyses to test our main study hypotheses. Mothers’ CRP and PEP were not related to socioeconomic status, mothers’ depression symptoms, or cigarette use (r's range from −0.01 to −0.24; see Table 2). A statistically significant positive correlation was observed between mothers’ BMI, mother CRP levels, and mothers’ SASB Intrex ratings of early caregiving. Higher maternal CRP levels correlated with higher mother BMI and retrospective reports of less autonomy-granting and less warm guidance/protection from mother's own caregiver in childhood. Subjective higher SES was statistically significantly related to lower maternal depression symptoms and greater reported warm guidance/protection in mother's childhood. Although there were few significant associations between sociodemographic variables and the main study variables, we included mothers’ number of cigarettes smoked per day, depression symptoms, BMI, and SES as covariates in the primary analyses given strong evidence for their associations with chronic inflammation (Ghanim et al., 2004; Haapakoski, Mathieu, Ebmeier, Alenius, & Kivimäki, 2015; Johnson, Abbasi, & Master, 2013; Pikhart et al., 2009; Rawson et al., 2003; Taheri et al., 2007; Valkanova & Ebmeier, 2013; Yanbaeva, Dentener, Creutzberg, Wesseling, & Wouters, 2007). Within the total sample, 67% of mothers reported they were non-smokers. Because the smoking data were positively skewed, we performed a logarithm transformation before including it in subsequent analyses. BMI was in the normal weight range for 56% of mothers (i.e., 20–30); 19% of mothers had BMI in the overweight range (i.e., 30–34), and 22% fell in the obese range (i.e., 35 or above).
TABLE 1.
Descriptive statistics for mothers’ early recollections of caregiving, mother and child CRP Levels, and mother and child PEP
| Variable | n | M, SD | Range |
|---|---|---|---|
| Early autonomy support | 47 | 51.02 (33.38) | 0–100 (0–100) |
| Early warm guidance/protection | 47 | 67.72 (35.01) | 0–100 (0–100) |
| Mother depression symptom score | 52 | 54.54 (9.23) | 42–78 (0–100) |
| Mother cigarettes per day | 52 | 3.19 (5.23) | 0-–20 |
| Socioeconomic status rating | 52 | 3.75 (1.81) | 1–8 (1–10) |
| Mother BMI | 50 | 30.35 (7.26) | 20.34–53.03 |
| Mother CRP | 48 | 2.23 (2.33) | 0.09–9.34 |
| Mother Baseline PEP | 42 | 116.25 (10.81) | 90–139.20 |
| Mother PEP with Child | 30 | 109.53 (11.89) | 77.14–129.55 |
| Child CRP | 42 | 0.91 (1.93) | 0.09–8.26 |
| Child Baseline PEP | 45 | 92.38 (9.15) | 75-–111 |
| Child PEP in Social Engagement | 42 | 92.46 (9.08) | 73.40–111.85 |
| Child BMI | 51 | 15.86 (1.39) | 13.36–20.43 |
Notes. CRP: C-reactive protein; PEP: pre-ejection period.
Standard deviations are displayed parenthetically. Possible score ranges are displayed parenthetically where applicable.
TABLE 2.
Correlations between mothers’ recollections of early caregiving, mother and child CRP Levels, and mother and child PEP
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Early autonomy support | — | |||||||||||||||
| 2. Early warm guidance | 0.37** | — | ||||||||||||||
| 3. Mother CRP | −0.36* | −0.30* | — | |||||||||||||
| 4. Mother PEP baseline | 0.38* | 0.14 | −0.23 | — | ||||||||||||
| 5. Mother joint task PEP with child | 0.07 | 0.01 | 0.04 | 0.81*** | — | |||||||||||
| 6. Mother BMI | −0.20 | −0.10 | 0.57*** | −0.28 | −0.13 | — | ||||||||||
| 7. Mother BSI-depression | −0.13 | −0.21 | −0.04 | −0.24 | 0.08 | 0.05 | — | |||||||||
| 8. Mother cigarettes per day | 0.07 | −0.19 | −0.01 | 0.20 | −0.23 | −0.16 | −0.09 | — | ||||||||
| 9. SES | 0.11 | 0.30* | −0.16 | −0.01 | −0.14 | −0.20 | −0.47*** | −0.04 | — | |||||||
| 10. Child CRP | 0.02 | 0.10 | −0.17 | −0.22 | 0.36 | −0.07 | 0.10 | −0.06 | −0.19 | — | ||||||
| 11. Child PEP baseline | 0.26 | 0.24 | 0.53*** | 0.13 | −0.13 | −0.21 | −0.01 | 0.01 | 0.24 | 0.13 | — | |||||
| 12. Joint task PEP | 0.19 | 0.26 | −0.53** | 0.04 | −0.10 | −0.14 | 0.12 | −0.01 | 0.16 | 0.21 | 0.96** | — | ||||
| 13. Child BMI | −0.03 | −0.17 | 0.09 | −0.17 | −0.45* | 0.46*** | 0.04 | −0.13 | −0.11 | −0.33* | −0.09 | −0.08 | −0.08 | — | ||
| 14. Child age | −0.05 | 0.10 | 0.10 | −0.03 | 0.07 | 0.10 | −0.27 | 0.23 | −0.01 | 0.19 | 0.05 | 0.07 | 0.08 | −0.17 | — | |
| 15. Child gender | 0.28 | 0.08 | −0.17 | 0.18 | −0.06 | −0.05 | −0.21 | 0.23 | −0.09 | 0.12 | −0.06 | −0.16 | −0.16 | 0.19 | 0.25 | — |
Notes. BMI: body mass index; SES: socioeconomic status; CRP: C-reactive protein; PEP: pre-ejection period.
Higher SES scores represent higher subjective socioeconomic status ratings.
p ≤ 0.05.
p ≤ 0.01.
p ≤ 0.001.
With respect to the main study variables, we next calculated zero-order correlations among maternal and child PEP and CRP, and mothers’ early caregiving. Mothers’ resting PEP scores were statistically significantly correlated with their report of childhood experienced autonomy-granting caregiving, such that mothers who recalled greater autonomy support from their own caregivers displayed longer PEP at rest, or lower resting SNS arousal. Maternal PEP scores during the joint task with her child were related only to child BMI, such that shorter maternal PEP (i.e., greater SNS arousal) during interaction was associated with higher child BMI, consistent with other studies of maternal self-reported stress and higher child BMI (Stenhammer et al., 2010). Given that maternal joint task PEP scores were not statistically significantly related to key variables of interest in the current sample, maternal PEP scores were dropped from subsequent analyses.
Regarding children's physiological indicators, neither child CRP levels nor child PEP scores at rest or during social interaction were associated with child age, family SES, or maternal depression, cigarette use, or maternal BMI, maternal PEP scores, or mother's retrospective reports of her own early caregiving (r's range from 0.004 to 0.26; see Table 2). We observed a statistically significant negative association between child CRP and child BMI, such that children with higher BMI scores displayed lower chronic inflammation. This runs counter to findings from previous studies using large, nationally representative samples, which have documented elevated CRP levels among children with higher BMI (e.g., Ford et al., 2001), and we reason this association may reflect the restricted range in child BMI observed in the sample. Given the lack of statistically significant and meaningful bivariate correlations with key variables, child CRP was dropped from subsequent analyses. Finally, children's PEP scores at rest and during social interactions with mother and female researcher were positively correlated with mothers’ CRP levels, such that greater child SNS activation at rest and during social interactions was associated with higher maternal CRP scores, that is, greater chronic inflammation in their mothers.
3.2 ∣. Maternal physiology and retrospective reports of early caregiving
To test our main study hypotheses, we conducted hierarchical linear regressions (HLRs) modeling associations between maternal physiology (i.e., CRP, resting PEP) and her recollections of positive caregiving during her own childhood.
3.2.1 ∣. CRP
First, we conducted HLRs to model associations between mothers’ current CRP levels and each SASB early caregiving score (i.e., Autonomy-support [SASB Cluster 11] and guidance/protection [SASB Cluster 14]). Step 1 entry of mothers’ cigarette use, BMI, BSI depression scores, and SES together, were significantly related to her CRP levels, F(4,40) = 5.10, (p < 0.01), R2 = 0.34. Mothers with higher BMI and who reported greater cigarette use, higher depression symptoms, and lower SES, tended to display greater chronic inflammation, and these variables accounted for approximately 34% of variance in maternal CRP. Step 2 entry of mothers’ retrospective ratings of autonomy-supportive early caregiving were also significantly associated with current maternal CRP levels, F(5,39) = 5.37, (p < 0.01), R2 = 0.41, β = −0.27, t = −2.15 (p < 0.05), significantly improving model prediction (R2change = 0.07, F = 4.62, p < 0.05). That is, above and beyond the influence of a mother's current BMI, daily cigarette smoking, depression symptoms, and SES, her recollections of more autonomy-supportive caregiving in early childhood were associated with lower levels of chronic inflammation in the present (See Table 3).
TABLE 3.
Summary of hierarchical regression analysis for positive early caregiving predicting maternal CRP
| Variable | Model 1 |
Model 2 |
||||
|---|---|---|---|---|---|---|
| B | SE B | β | B | SE B | β | |
| Autonomy support predicting Maternal CRP | ||||||
| Cigarette use | 0.03 | 0.04 | 0.07 | 0.03 | 0.06 | 0.07 |
| BMI | 0.18 | 0.04 | 0.56*** | 0.16 | 0.04 | 0.51*** |
| Depression score | −0.03 | 0.04 | −0.11 | −0.04 | 0.04 | −0.14 |
| SES | −0.13 | 0.19 | −0.10 | −0.12 | 0.18 | −0.09 |
| Recollected autonomy support | −0.02 | 0.01 | −0.27* | |||
| R2 | 0.34 | 0.41 | ||||
| F for change in R2 | 5.10** | 4.62* | ||||
| Warm guidance predicting maternal CRP | ||||||
| Cigarette use | 0.03 | 0.06 | 0.07 | 0.01 | 0.06 | 0.01 |
| BMI | 0.18 | 0.04 | 0.56*** | 0.17 | 0.04 | 0.54*** |
| Depression score | −0.03 | 0.04 | −0.11 | −0.04 | 0.04 | −0.14 |
| SES | −0.13 | 0.19 | −0.10 | −0.05 | 0.19 | −0.04 |
| Recollected warm guidance | −0.02 | 0.01 | −0.27* | |||
| R2 | 0.39 | 0.40 | ||||
| F for change in R2 | 5.10** | 3.95* | ||||
Note. n = 44.
p ≤ .05
p ≤ .01
p ≤ .001.
Next, mothers’ SASB ratings of warm and protective early caregiving, entered at step 2, were also found to predict current maternal CRP levels, F(5,39) = 5.17, (p < 0.01), β = −0.27, t = −1.99 (p = 0.05), R2 = 0.40 (R2change = 0.06, F = 3.95, p = 0.05) after controlling for all covariates described above (see Table 3). Mothers who recalled experiencing more warm guidance/protection from their early caregivers displayed lower levels of chronic inflammation. Conversely, higher chronic, low-grade inflammation in mothers was significantly associated with less support for autonomy and less warm guidance/provided by caregiver in early childhood, even after controlling for the effects of SES and maternal BMI, depression, and cigarette use on inflammation scores.
3.2.2 ∣. PEP
Next, we conducted an HLR examining SASB ratings of autonomy-supportive caregiving as a predictor of maternal resting PEP, after controlling for mother's current BMI, daily cigarette smoking, depression symptoms, and rating of SES. At step 1, the control variables were not statistically significantly associated with mother resting PEP scores, F(4,33) = 0.86, (p > 0.05), R2 = 0.18, At step 2, we observed a trend (i.e., p = 0.05). However, autonomy-supportive caregiving was associated with longer maternal resting PEP, F(5,32) = 2.45 (p = 0.055), R2 = 0.28, β = 0.31, t = 2.02, p = 0.05). The addition of reported autonomy-supportive caregiving increased model prediction (R2change = 0.09, F = 4.08 p = 0.05; see Table 4). In sum, after controlling for maternal health factors, we observed trend-level associations between more caregiver autonomy-granting caregiving in mother's childhood and longer maternal resting PEP (i.e., lower SNS arousal), explaining approximately 9% of variance in mothers’ resting PEP scores.
TABLE 4.
Summary of hierarchical regression analysis for autonomy support predicting maternal resting PEP
| Variable | Model 1 |
Model 2 |
||||
|---|---|---|---|---|---|---|
| B | SE B | β | B | SE B | β | |
| Cigarette use | 0.24 | 0.33 | 0.19 | 0.22 | 0.32 | 0.11 |
| BMI | −0.43 | 0.24 | −0.29** | −0.35 | 0.24 | −0.23 |
| Depression score | −0.37 | 0.21 | −0.32** | −0.33 | 0.20 | −0.28 |
| SES | −1.27 | 1.09 | −0.21 | −1.31 | 1.04 | −0.22 |
| Recollected autonomy support | 0.10 | 0.05 | 2.02* | |||
| R2 | 0.18 | 0.28 | ||||
| F for change in R2 | 1.86 | 4.09* | ||||
Notes. n = 37.
p≤ 0.05.
p<0.10.
Given the lack of statistically significant bivariate correlation observed between maternal PEP and SASB Intrex ratings of warm guidance/protective caregiving, we did not conduct HLR to examine associations between these variables.
3.3 ∣. Associations between biomarkers of mother and child stress reactivity
Finally, we explored two-generational associations between measures of mother and child peripheral physiology. Using a family-wise alpha = 0.025, two hierarchical linear regressions were conducted with mother CRP levels and their children's PEP scores (a) at rest and (b) during social interactions. In each HLR model, child age and gender were included as covariates. Step 1 entry of child age and gender were not statistically significant in either of the models. At step 2, statistically significant associations were observed between mother CRP and children's PEP scores at rest, F(3,38) = 6.15 (p < 0.01), R2 = 0.33, β = −0.58, t = −4.24 (p < 0.001) and during the social interactions, F(3,38) = 7.10 (p < 0.01), R2 = 0.38, β = −0.60, t = −4.40 (p < 0.001; see Table 5). Over and above the contributions of child age and gender, maternal CRP levels significantly predicted remaining variance in (a) children's resting PEP, (R2change = 0.32, F = 18.01, p < 0.001) and (b) children's PEP during social interactions, (R2change = 0.38, F = 19.19, p < 0.001 see Table 5). In other words, after controlling for children's age and gender, chronic, low-grade maternal CRP levels accounted for 32%–38% of the variance in children's sympathetic activation, with greater low-grade maternal inflammation predicting shorter child PEP (i.e., heightened SNS arousal) across resting and social interaction contexts.
TABLE 5.
Summary of hierarchical regression analysis for maternal CRP predicting child PEP
| Variable | Model 1 |
Model 2 |
||||
|---|---|---|---|---|---|---|
| B | SE B | β | B | SE B | β | |
| Maternal CRP predicting child PEP at rest | ||||||
| Child age | 0.06 | 0.14 | 0.06 | 0.13 | 0.12 | 0.15 |
| Child gender | −0.20 | 0.43 | −0.08 | −0.51 | 0.36 | −0.20 |
| Maternal CRP | −2.28 | 0.54 | −0.58*** | |||
| R2 | 0.01 | 0.33 | ||||
| F for change in R2 | 0.15 | 18.02*** | ||||
| Maternal CRP Predicting Child PEP during Social Engagement | ||||||
| Child age | 0.10 | 0.15 | 0.11 | 0.17 | 0.12 | 0.20 |
| Child gender | −0.48 | 0.43 | −0.19 | −0.80 | 0.36 | −0.31* |
| Maternal CRP | −2.33 | 0.53 | −0.60*** | |||
| R2 | 0.04 | 0.38 | ||||
| F for change in R2 | 0.70 | 19.19*** | ||||
Note. n = 37. Higher values on gender indicate female.
p ≤ .05
p ≤ .01
p ≤ .001.
4 ∣. DISCUSSION
In this study, we examined associations between quality of caregiving experienced by mothers in their own childhoods and physiological functioning in adulthood. We found robust associations between mothers’ retrospective reports of the quality of their own early caregiving and their current levels of chronic inflammation. Above and beyond the effects of several known correlates of chronic inflammation—including maternal smoking (Taheri et al., 2007; Yanbaeva et al., 2007), SES (Alley et al., 2006), depressive symptoms (Pikhart et al., 2009; Valkanova & Ebmeier, 2013), and BMI (Ghanim et al., 2004; Rawson et al., 2003; Taheri et al., 2007)—mothers in this sample who recalled greater warm guidance and autonomy support from their own caregivers in early childhood displayed lower current CRP levels. In other words, more supportive and autonomy-promotive caregiving experienced in early childhood was associated with lower adulthood chronic, low-grade inflammation. These findings further support the notion that autonomy-supportive parenting is highly protective for behavioral and physiological function (e.g., Belsky et al., 2008; Bindman, et al., 2015; Noll et al., 2015), and may direct future research in clarifying if the presence of autonomy support and warm guidance experienced during childhood extends beyond known outcomes (i.e., behavioral and physiological self-regulation) to potentially impact the long-term functioning of physiological stress response systems.
We also observed an association, though weaker, between autonomy-supportive parenting and mothers’ resting SNS activation. Mothers who recalled receiving more support for autonomy from caregivers during early childhood tended to display lower SNS arousal (i.e., longer PEP) at rest. Research generally supports a link between SNS activity and early adversity (Ali & Pruessner, 2012; Esposito et al., 2016; Gunnar et al., 2009; Hengesch et al., 2018), however, current understanding of how more normative variations in early experience may shape in-the-moment SNS activation is relatively limited. Our findings here are consistent with evidence of SNS hyperactivity among those exposed to poor early caregiving (McLaughlin et al., 2015; Oosterman, Schipper, Fisher, Dozier, & Schuengel, 2010), and they extend the literature by identifying specific positive parenting behaviors that are associated with calmer SNS functioning in adulthood. Taken together, these findings linking markers of maternal SNS activity with chronic low-grade inflammation are consistent with a growing body of literature indicating that positive, supportive, autonomy-affirming relationships experienced during childhood are likely to predict physiological functioning both in childhood and into adulthood (Ali & Pruessner, 2012; Anda et al., 2009; Dube et al., 2009; Miller et al., 2011). This also supports Nusslock & Miller's neuroimmune network hypothesis (Nusslock & Miller, 2016), which posits that repeated exposure to early life stress, even in the form of suboptimal parent-child relationships, may result in sensitization of stress response systems including the SNS, leading to heightened neural-immune signaling and, eventually, chronic low-grade inflammation. Thus, whereas many studies have linked exposure to early life stress and harsh parenting to concurrent physiological activity in single systems or single diseases, our findings underscore the notion set forth by Nusslock and Miller (2016) that SNS and immune function are linked and may both relate to the quality of positive early relationships.
Contrary to our hypothesis, we found no linkages between quality of early caregiving and maternal SNS activation during social interactions, but rather, only with maternal SNS activation at rest. Consistent with Obradovic (2012), these results could align with the theory that early life experiences give rise to a particular physiological phenotype rather than to patterns of hyper- or hyporeactivity in response to task activation. The association found here between autonomy-supportive parenting experienced in childhood and resting SNS activation in adulthood could offer avenues for further research regarding the protective nature of parenting on later physiological functioning. However, it is also possible that the lack of association between positive early caregiving and mothers’ PEP activity while interacting with her child may be related to low statistical power, particularly given the percentage of missing PEP data for mothers during the social interaction task, as compared to assessment of PEP at rest. Further research with larger samples is needed to clarify the nature PEP activity in social interactions between mothers and children.
We also explored associations between mother and child markers of peripheral physiology to capture SAM system activation during a prosocial exchange. We found significant links between higher maternal chronic inflammation (i.e., higher CRP) and greater child sympathetic activation (i.e., shorter PEP) both at rest and during social interactions (i.e., with one's mother and with a female stranger). Children of mothers showing more chronic low-grade inflammation displayed greater sympathetic activation at rest and during physically close, social interactions with their mother and a female stranger. We consider several plausible explanations for these associations. First, it is possible that elevated maternal chronic inflammation is related to heightened child sympathetic activation at rest and during social interactions due to a common, hereditary trait underlying exaggerated stress responding (Li-Tempel et al., 2016). Evidence drawn from twin studies indeed suggests that individual differences in cardiovascular reactivity to stress are moderately heritable (Rose & Chesney, 1986; Turner & Hewitt, 1992), and many diseases associated with chronic inflammation are also known to be heritable (Heap & Van Heel, 2009). However, our pattern of findings does not seem wholly consistent with a hereditary explanation, given that only mothers’ inflammation, and not their SNS activity, was related to their child's SNS activity. Alternatively, there is strong evidence for the epigenetic transmission of stress responding from mother to child, particularly in rodent model research (e.g., Franklin et al., 2010; Meaney, 2001). Although much of this work has identified maternal behavior as the mechanism (Champagne & Meaney, 2001; Weaver et al., 2004), physiological effects of stress may be transmitted to offspring independent of variations in levels of environmental stress and caregiving (Harper, 2005). For example, Franklin et al. (2010) found that mouse pups exposed to early life stress displayed behavioral disturbances and altered DNA methylation. Similar patterns of DNA methylation and gene expression were present in the subsequent two generations of rodents, despite these mice being raised under normal conditions (e.g., no experience of stress). It is possible that the link we observed between mother's chronic inflammation and their child's SNS response is related to a similar epigenetic transmission process, whereby mothers’ early caregiving experiences influenced their own physiological responsivity, thus influencing the genetic traits passed on to their offspring. Further, animal and human studies have shown that prenatal stressors are linked with fetal changes in HPA functioning (Koehl et al., 1999; Seckl, 2008) and sustained HPA dysregulation at 6 months (Lyons-Ruth, Wolfe, & Lyubchik, 2000), 5 years (Gutteling, de Weerth, & Buitelaar, 2005) and 10 years of age (O'Connor, 2005), demonstrating a significant relationship between prenatal stress and children's physiological responding. Given strong previous evidence that chronic inflammation is stress-related, it may be that the link observed here between maternal low-grade inflammation and heightened child sympathetic activation is precipitated by exposure to elevated maternal stress in utero. Alternately, it is possible that the association between maternal chronic inflammation and child SNS activity may be partially mediated by quality of parent-child interactions, further investigations that undertake assessment of maternal stress exposure during pregnancy and observational coding of parenting practices will be essential next step lines of inquiry.
We found no significant associations between mother's chronic inflammation and child inflammation. As our sample included 3–5-year-old preschool children, it is plausible that chronic inflammation related to environmental stress is difficult to detect in early childhood. Elevated CRP levels have been observed in children as young as 8 years (Byrne et al., 2013; Slopen et al., 2013), though there is limited research on profiles of chronic inflammation in children exposed to environmental stress. The results of our exploratory analysis between child SNS activity and mother chronic inflammation show a promising avenue for further investigation. As the current understanding of how stress exposure relates to SNS activity in children is somewhat limited, our results provide an initial description of physiological linkages in at-risk mothers and their children. Future studies should employ longitudinal design to assess physiological responding across systems and quality of parenting across multiple generations, in order to confirm and explicate the current findings. If future studies are able to clarify the biological or behavioral mechanisms, underlying two-generational associations observed here, this may provide an avenue to explore potentially malleable targets for intervention.
4.1 ∣. Limitations and future directions
Several limitations should be considered when interpreting these results. First, the sample size of the current study is relatively small (n = 52), which imposes limits on the statistical power available to detect associations between study variables. This issue was particularly salient for examining mother's PEP scores during social interaction, as there were also considerable missing data in the task. Maternal PEP scores were obtained during a social engagement taskwhich was not designed to activate PEP activity in caregivers per se, but rather for their preschool aged children (Beauchaine, 2012; Brenner, Beauchaine, & Sylvers, 2005). As such, the experience of a prosocial connection during the social engagement task might be inherently rewarding for mothers. Regardless, future research would do well to incorporate incentivized tasks to detect meaningful PEP activation in parents.
As there is a robust link between early experiences of maltreatment on later physiological functioning (Anda et al., 2009; Dube et al., 2009), future work is needed to characterize the constellation of early adversity exposure in order to more fully understand the role of childhood trauma in the caregiving—stress activity linkages observed here. Because we did not assess for childhood maltreatment in the current study, we cannot ascertain whether the associations we observed between early positive caregiving and adult stress activity may in fact be driven by experiences of child maltreatment among those reporting the lowest levels of positive caregiving.
The cross-sectional design of this study limits interpretation of findings to non-causal inferences only. Further exploration into the ways in which early experiences of positive caregiving map onto variation in SNS activation and other biomarkers of stress activity in adults is needed using prospective, longitudinal designs and larger samples of adults. Longitudinal designs would also be beneficial for modeling the developmental trajectories of CRP elevations and SNS activation over time in early childhood through adolescence, as relatively little is known about the physiological profiles of children's stress activity, particularly in the context of environmental stress. Finally, our sample was comprised of mostly Caucasian mothers (69.2%) and was therefore limited in racial/ethnic diversity. Recent evidence has emerged suggesting possible differences across racial/ethnic groups in patterns of chronic inflammation indexed via CRP, especially in social contexts (i.e., perceived social support predicting lower levels of CRP in African Americans; Uchino et al, 2016). Future research should consider ethnicity/race when examining CRP in social contexts as there are often significant sociocultural differences across groups, particularly in the family environment.
Despite these limitations, the results provide initial evidence that maternal recollections of a caregiving environment characterized by warm guidance and autonomy support may relate to levels of chronic inflammation in adulthood. This underscores the developmental importance of these particular aspects of positive parenting, as has been set forth in previous literature (Belsky et al., 2008; Bindman et al., 2015; Noll et al., 2015) and suggests they may be associated with biological functioning as well. However, further research is needed to confirm this directly. Positive parenting has been shown to be a significant protective factor for psychosocial and physiological development in children (Bindman et al., 2015); if confirmed in future studies using prospective longitudinal designs, warm guidance and autonomy-supportive caregiving may serve a protective function for adult health over time, including against chronic diseases such as CVD, diabetes, and some forms of cancer (Lehman, Taylor, Kiefe, & Seeman, 2005, 2009; Taylor et al., 2006). The current study also provides support for the coactivation of the SNS and immune systems related to environmental experience, as proposed by Nusslock and Miller (2016). Future research should continue to examine measures of multiple physiological systems and should implement longitudinal study designs across stages of development (e.g., prenatal, infancy, early childhood, adolescence) in order to disentangle the biobehavioral transactions that shape stress activity over time.
Acknowledgments
Funding information
National Institute of Mental Health, Grant/Award Number: R01 MH079328
REFERENCES
- Abou-Raya A, & Abou-Raya S (2006). Inflammation: A pivotal link between autoimmune diseases and atherosclerosis. Autoimmunity Reviews, 5(5), 331–337. 10.1016/j.autrev.2005.12.006 [DOI] [PubMed] [Google Scholar]
- Ali N, & Pruessner JC (2012). The salivary alpha amylase over cortisol ratio as a marker to assess dysregulations of the stress systems. Physiology & Behavior, 106(1), 65–72. 10.1016/j.physbeh.2011.10.003 [DOI] [PubMed] [Google Scholar]
- Alley DE, Seeman TE, Kim JK, Karlamangla A, Hu P, & Crimmins EM (2006). Socioeconomic status and C‐reactive protein levels in the US population: NHANES IV. Brain, Behavior, and Immunity, 20(5), 498–504. 10.1016/j.bbi.2005.10.003 [DOI] [PubMed] [Google Scholar]
- Anda RF, Dong M, Brown DW, Felitti VJ, Giles WH, Perry GS, … Dube SR (2009). The relationship of adverse childhood experiences to a history of premature death of family members. Biomedical Central Public Health, 9(1), 106 10.1186/1471-2458-9-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, McDonald PG, … Sood AK (2006). The influence of bio-be-havioural factors on tumour biology: Pathways and mechanisms. Nature Reviews Cancer, 6(3), 240 10.1038/nrc1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP (2012). Physiological markers of emotion and behavior dysregulation in externalizing psychopathology. Monographs of the Society for Research in Child Development, 77, 79–86. 10.1111/j.1540-5834.2011.00665.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Gatzke-Kopp L, & Mead HK (2007). Polyvagal theory and developmental psychopathology: Emotion dysregulation and conduct problems from preschool to adolescence. Biological Psychology, 74(2), 174–184. 10.1016/j.biopsycho.2005.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Booth la Force C, Bradley RH, Brownell CA, Burchinal M, Campbell SB, … Kelly JF (2008). Mothers' and fathers' support for child autonomy and early school achievement. Developmental Psychology, 44(4), 895–907. 10.1037/0012-1649.44.4.895 [DOI] [PubMed] [Google Scholar]
- Benjamin LS (1974). Structural analysis of social behavior. Psychological Review, 81, 392–425. 10.1037/h0037024 [DOI] [Google Scholar]
- Benjamin LS (1996). A clinician-friendly version of the Interpersonal Circumplex: Structural Analysis of Social Behavior (SASB). Journal of Personality Assessment, 66(2), 248–266. 10.1207/s15327752jpa6602_5 [DOI] [PubMed] [Google Scholar]
- Bernier A, Carlson SM, & Whipple N (2010). From external regulation to self-regulation: Early parenting precursors of young children’s executive functioning. Child Development, 81(1), 326–339. 10.1111/j.1467-8624.2009.01397.x [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Binkley PF, Uchino BN, Quigley KS, & Fieldstone A (1994). Autonomic cardiac control. III. Psychological stress and cardiac response in autonomic space as revealed by pharmacological blockades. Psychophysiology, 31(6), 599–608. 10.1111/j.1469-8986.1994.tb02352.x [DOI] [PubMed] [Google Scholar]
- Bertoni AG, Burke GL, Owusu JA, Carnethon MR, Vaidya D, Barr RG, … Rotter JI (2010). Inflammation and the incidence of Type 2 diabetes: The Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care, 33, 804–810. 10.2337/dc09-1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindman SW, Pomerantz EM, & Roisman GI (2015). Do children’s executive functions account for associations between early autonomy-supportive parenting and achievement through high school? Journal of Educational Psychology, 107(3), 756 10.1037/edu0000017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell A, Snodgrass J, Madimenos F, & Sugiyama L (2010). Life history, immune function, and intestinal helminths: Trade-offs among immunoglobulin E, C-reactive protein, and growth in an Amazonian population. American Journal of Human Biology, 10.1002/ajhb.21092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Granger DA, Willoughby M, Mills-Koonce R, Cox M, Greenberg MT, … FLP Investigators. (2011). Salivary cortisol mediates effects of poverty and parenting on executive functions in early childhood. Child Development, 82(6), 1970–1984. 10.1111/j.1467-8624.2011.01643.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner SL, Beauchaine TP, & Sylvers PD (2005). A comparison of psychophysiological and self-report measures of BAS and BIS activation. Psychophysiology, 47, 108–115. 10.1111/j.1469.8986.2005.00261.x [DOI] [PubMed] [Google Scholar]
- Byrne ML, O’Brien-Simpson NM, Reynolds EC, Walsh KA, Laughton K, Waloszek JM, … Allen NB (2013). Acute phase protein and cytokine levels in serum and saliva: A comparison of detectable levels and correlations in a depressed and healthy adolescent sample. Brain, Behavior, and Immunity, 34, 164–175. 10.1016/j.bbi.2013.08.010 [DOI] [PubMed] [Google Scholar]
- Cannon WB (1929). Organization for physiological homeostasis. Physiological Reviews, 9(3), 399–431. 10.1152/physrev.1929.9.3.399 [DOI] [Google Scholar]
- Champagne F, & Meaney MJ (2001). Like mother, like daughter: Evidence for non-genomic transmission of parental behavior and stress responsivity. Progress in Brain Research, 133, 287–302. 10.1016/S0079-6123(01)33022-4 [DOI] [PubMed] [Google Scholar]
- Dandona P, Aljada A, Chaudhuri A, Mohanty P, & Garg R (2005). Metabolic syndrome: A comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation, 111, 1448–1454. 10.1161/01.CIR.0000158483.13093.9D [DOI] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, & Poulton R (2007). Childhood maltreatment predicts adult inflammation in a life-course study. Proceedings of the National Academy of Sciences, 104(4), 1319–1324. 10.1073/pnas.0610362104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Caspi A, Williams B, Ambler A, Sugden K, Mika J, … Arseneault L (2011). Biological embedding of stress through inflammation processes in childhood. Molecular Psychiatry, 16, 244–246. 10.1038/mp.2010.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, … Caspi A (2009). Adverse childhood experiences and adult risk factors for age-related disease: Depression, inflammation, and clustering of metabolic risk markers. Archives of Pediatrics & Adolescent Medicine, 163(12), 1135–1143. 10.1001/archpediatrics.2009.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deci EL, & Ryan RM (1985). Intrinsic motivation and self-determination in human behavior. New York, NY: Plenum Press. [Google Scholar]
- Deci EL, & Ryan RM (2012). Motivation, personality, and development within embedded social contexts: An overview of self-determination theory In Ryan RM (Ed.), Oxford library of psychology. The Oxford handbook of human motivation (pp. 85–107). New York, NY: Oxford University Press. [Google Scholar]
- Derogatis LR, & Melisaratos N (1983). The brief symptom inventory: An introductory report. Psychological Medicine, 13(3), 595–605. 10.1017/S0033291700048017 [DOI] [PubMed] [Google Scholar]
- Dozier M, Peloso E, Lewis E, Laurenceau JP, & Levine S (2008). Effects of an attachment-based intervention on the cortisol production of infants and toddlers in foster care. Development and Psychopathology, 20(3), 845–859. 10.1017/S0954579408000400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube SR, Fairweather D, Pearson WS, Felitti VJ, Anda RF, & Croft JB (2009). Cumulative childhood stress and autoimmune diseases in adults. Psychosomatic Medicine, 71(2), 243–250. 10.1097/PSY.0b013e3181907888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, & Anda RF (2003). Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: The adverse childhood experiences study. Pediatrics, 111(3), 564–572. 10.1542/peds.111.3.564 [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Kouros CD, Erath S, Cummings EM, Keller P, & Staton L (2009). Marital conflict and children’s externalizing behavior: Pathways involving interactions between parasympathetic and sympathetic nervous system activity. Monographs of the Society for Research in Child Development, 74(1), vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito EA, Koss KJ, Donzella B, & Gunnar MR (2016). Early deprivation and autonomic nervous system functioning in post-institutionalized children. Developmental Psychobiology, 58(3), 328–340. 10.1002/dev.21373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, & Kim P (2007). Childhood poverty and health: Cumulative risk exposure and stress dysregulation. Psychological Science, 18(11), 953–957. 10.1111/j.1467-9280.2007.02008.x [DOI] [PubMed] [Google Scholar]
- Fisher PA, & Stoolmiller M (2008). Intervention effects on foster parent stress: Associations with child cortisol levels. Development and Psychopathology, 20(3), 1003–1021. 10.1017/S0954579408000473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford ES, Galuska DA, Gillespie C, Will JC, Giles WH, & Dietz WH (2001). C-reactive protein and body mass index in children: findings from the Third National Health and Nutrition Examination Survey, 1988-1994. The Journal of Pediatrics, 138(4), 486–492. [DOI] [PubMed] [Google Scholar]
- Franklin TB, Russig H, Weiss IC, Gräff J, Linder N, Michalon A, … Mansuy IM (2010). Epigenetic transmission of the impact of early stress across generations. Biological Psychiatry, 68(5), 408–415. 10.1016/j.biopsych.2010.05.036 [DOI] [PubMed] [Google Scholar]
- Gatzke-Kopp L, & Ram N (2018). Developmental dynamics of autonomic function in childhood. Psychophysiology, , 55(11), e13218. 10.1111/psyp.13218 [DOI] [PubMed] [Google Scholar]
- Ghanim H, Aljada A, Hofmeyer D, Syed T, Mohanty P, & Dandona P (2004). Circulating mononuclear cells in the obese are in a proinflammatory state. Circulation, 110(12), 1564–1571. 10.1161/01.CIR.0000142055.53122.FA [DOI] [PubMed] [Google Scholar]
- Grolnick WS, Deci EL, & Ryan RM (1997). Internalization within the family: The self-determination theory perspective In Grusec JE, & Kuczynski L (Eds.), Parenting and children's internalization of values: A handbook of contemporary theory (pp. 135–161). Hoboken, NJ: John Wiley & Sons Inc. [Google Scholar]
- Grolnick WS, & Pomerantz EM (2009). Issues and challenges in studying parental control: Toward a new conceptualization. Child Development Perspectives, 3(3), 165–170. 10.1111/j.1750-8606.2009.00099.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grolnick WS, & Ryan RM (1989). Parent styles associated with children's self-regulation and competence in school. Journal of Educational Psychology, 81(2), 143–154. 10.1037/0022-0663.81.2.143 [DOI] [Google Scholar]
- Gunnar MR, & Donzella B (2002). Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology, 27(1–2), 199–220. 10.1016/S0306-4530(01)00045-2 [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Frenn K, Wewerka SS, & Van Ryzin MJ (2009). Moderate versus severe early life stress: Associations with stress reactivity and regulation in 10–12-year-old children. Psychoneuroendocrinology, 34(1), 62–75. 10.1016/j.psyneuen.2008.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M, & Quevedo K (2007). The neurobiology of stress and development. Annual Review of Psychology, 58, 145–173. 10.1146/annurev.psych.58.110405.085605 [DOI] [PubMed] [Google Scholar]
- Gutteling BM, de Weerth C, & Buitelaar JK (2005). Prenatal stress and children’s cortisol reaction to the first day of school. Psychoneuroendocrinology, 30, 541–549. 10.1016/j.psyneuen.2005.01.002 [DOI] [PubMed] [Google Scholar]
- Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, & Kivimäki M (2015). Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain, Behavior, and Immunity, 49, 206–215. 10.1016/j.bbi.2015.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper L (2005). Epigenetic inheritance and the intergenerational transfer of experience. Psychological Bulletin, 131(3), 340 10.1037/0033-2909.131.3.340 [DOI] [PubMed] [Google Scholar]
- Heap GA, & van Heel DA (2009). The genetics of chronic inflammatory diseases. Human Molecular Genetics, 18(1), 101–106. 10.1093/hmg/ddp001 [DOI] [PubMed] [Google Scholar]
- Hengesch X, Elwenspoek MM, Schaan VK, Larra MF, Finke JB, Zhang X, … Schächinger H (2018). Blunted endocrine response to a combined physical-cognitive stressor in adults with early life adversity. Child Abuse & Neglect, 10.1016/j.chiabu.2018.04.002 [DOI] [PubMed] [Google Scholar]
- Irwin MR, & Cole SW (2011). Reciprocal regulation of the neural and innate immune systems. Nature Reviews Immunology, 11(9), 625 10.1038/nri3042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TV, Abbasi A, & Master VA (2013). Systematic review of the evidence of a relationship between chronic psychosocial stress and C-reactive protein. Molecular Diagnosis & Therapy, 17(3), 147–164. 10.1007/s40291-013-0026-7 [DOI] [PubMed] [Google Scholar]
- Kalvin CB, Bierman KL, & Gatzke-Kopp LM (2016). Emotional reactivity, behavior problems, and social adjustment at school entry in a high-risk sample. Journal of Abnormal Child Psychology, 44(8), 1527–1541. 10.1007/s10802-016-0139-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehl M, Darnaudry M, Dulluc J, Van Reeth O, Moal ML, & Maccari S (1999). Prenatal stress alters circadian activity of hypothalamo-pituitary-adrenal axis and hippocampal corticosteroid receptors in adult rats of both gender. Journal of Neurobiology, 40, 302–315. [DOI] [PubMed] [Google Scholar]
- Kuhlman KR, Chiang JJ, Horn S, & Bower JE (2017). Developmental psychoneuroendocrine and psychoneuroimmune pathways from childhood adversity to disease. Neuroscience & Biobehavioral Reviews, 80, 166–184. 10.1016/j.neubiorev.2017.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DA, Smith GD, Rumley A, Lowe GD, & Ebrahim S (2005). Associations of fibrinogen and C-reactive protein with prevalent and incident coronary heart disease are attenuated by adjustment for confounding factors: British Women’s Heart and Health Study. Thrombosis and Haemostasis, 93, 955–963. 10.1160/TH04-12-0805 [DOI] [PubMed] [Google Scholar]
- Lehman BJ, Taylor SE, Kiefe CI, & Seeman TE (2005). Relation of childhood socioeconomic status and family environment to adult metabolic functioning in the CARDIA study. Psychosomatic Medicine, 67(6), 846–854. 10.1097/01.psy.0000188443.48405.eb [DOI] [PubMed] [Google Scholar]
- Lehman BJ, Taylor SE, Kiefe CI, & Seeman TE (2009). Relationship of early life stress and psychological functioning to blood pressure in the CARDIA Study. Health Psychology, 28(3), 338–346. 10.1037/a0013785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li-Tempel T, Larra MF, Sandt E, Mériaux SB, Schote AB, Schächinger H, … Turner JD (2016). The cardiovascular and hypothalamus-pituitary-adrenal axis response to stress is controlled by glucocorticoid receptor sequence variants and promoter methylation. Clinical Epigenetics, 8(1), 12 10.1186/s13148-016-0180-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano DL, Norman G, Knox D, Wood BL, Miller BD, Emery CF, & Bernston GG (2007). Where to B in dZ/dt. Psychophysiology, 44, 113–119. [DOI] [PubMed] [Google Scholar]
- Luecken LJ, & Lemery KS (2004). Early caregiving and physiological stress responses. Clinical Psychology Review, 24(2), 171–191. 10.1016/j.cpr.2004.01.003 [DOI] [PubMed] [Google Scholar]
- Lyons-Ruth K, Wolfe R, & Lyubchik A (2000). Depression and the parenting of young children: Making the case for early preventive mental health services. Harvard Review Psychiatry, 8(148–153). 10.1080/hrp_8.3.148 [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, & Balkwill F (2008). Cancer-related inflammation. Nature, 454(7203), 436 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- McDade T, Burhop J, & Dohnal J (2004). High-sensitivity enzyme immunoassay for C-reactive protein in dried blood spots. Clinical Chemistry, 50(3), 652–654. 10.1373/clinchem.2003.029488 [DOI] [PubMed] [Google Scholar]
- McDade TW, Hawkley LC, & Cacioppo JT (2006). Psychosocial and behavioral predictors of inflammation in middle-aged and older adults: The Chicago health, aging, and social relations study. Psychosomatic Medicine, 68(3), 376–381. 10.1097/01.psy.0000221371.43607.64 [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Tibu F, Fox NA, Zeanah CH, & Nelson CA (2015). Causal effects of the early caregiving environment on development of stress response systems in children. Proceedings of the National Academy of Sciences, 112(18), 5637–5642. 10.1073/pnas.1423363112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ (2001). Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience, 24(1), 1161–1192. 10.1146/annurev.neuro.24.1.1161 [DOI] [PubMed] [Google Scholar]
- Miller GE, & Chen E (2010). Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychological Science, 21(6), 848–856. 10.1177/0956797610370161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, & Parker KJ (2011). Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychological Bulletin, 137(6), 959–997. 10.1037/a0024768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moilanen KL, Shaw DS, Dishion TJ, Gardner F, & Wilson M (2010). Predictors of longitudinal growth in inhibitory control in early childhood. Social Development, 19(2), 326–347. 10.1111/j.1467-9507.2009.00536.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll LK, Clark CA, & Skowron EA (2015). Multigenerational links between mothers' experiences of autonomy in childhood and preschoolers' respiratory sinus arrhythmia: Variations by maltreatment status. Development and Psychopathology, 27(4pt2), 1443–1460. 10.1017/S0954579415000863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, & Miller GE (2016). Early-life adversity and physical and emotional health across the lifespan: A neuro-immune network hypothesis. Biological Psychiatry, 80(1), 23–32. 10.1016/j.biopsych.2015.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor TG, Ben-Shlomo Y, Heron J, Golding J, Adams D, & Glover V (2005). Prenatal anxiety predicts individual differences in cortisol in pre-adolescent children. Biological Psychiatry, 58, 211–217. 10.1016/j.biopsych.2005.03.032 [DOI] [PubMed] [Google Scholar]
- Obradović J (2012). How can the study of physiological reactivity contribute to our understanding of adversity and resilience processes in development? Development and Psychopathology, 24(2), 371–387. 10.1017/S0954579412000053 [DOI] [PubMed] [Google Scholar]
- Oosterman M, De Schipper JC, Fisher P, Dozier M, & Schuengel C (2010). Autonomic reactivity in relation to attachment and early adversity among foster children. Development and Psychopathology, 22(1), 109–118. 10.1017/S0954579409990290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, … Rifai N (2003). Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation, 107(3), 499–511. 10.1161/01.CIR.0000052939.59093.45 [DOI] [PubMed] [Google Scholar]
- Pikhart H, Hubacek JA, Kubinova R, Nicholson A, Peasey A, Capkova N, … Bobak M (2009). Depressive symptoms and levels of C-reactive protein. Social Psychiatry and Psychiatric Epidemiology, 44(3), 217–222. 10.1007/s00127-008-0422-1 [DOI] [PubMed] [Google Scholar]
- Pomerantz EM, Grolnick WS, & Price CE (2005). The role of parents in how children approach achievement In Elliot AJ, & Dweck CS (Eds.), Handbook of competence and motivation (pp. 259–278). New York, NY: The Guilford Press. [Google Scholar]
- Rawson ES, Freedson PS, Osganian SK, Matthews CE, Reed G, & Ockene IS (2003). Body mass index, but not physical activity, is associated with C-reactive protein. Medicine and Science in Sports and Exercise, 35(7), 1160–1166. 10.1249/01.MSS.0000074565.79230.AB [DOI] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, & Seeman TE (2002). Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin, 128(2), 330 10.1037//0033-2909.128.2.330 [DOI] [PubMed] [Google Scholar]
- Ridker PM (2003). Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation, 107(3), 363–369. 10.1161/01.CIR.0000053730.47739.3C [DOI] [PubMed] [Google Scholar]
- Riis JL, Granger DA, Minkovitz CS, Bandeen-Roche K, DiPietro JA, & Johnson SB (2016). Maternal distress and child neuroendocrine and immune regulation. Social Science & Medicine, 151, 206–214. 10.1016/j.socscimed.2015.12.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose RJ, & Chesney MA (1986). Cardiovascular stress reactivity: A behavior-genetic perspective. Behavior Therapy, 17(4), 314–323. 10.1016/S0005-7894(86)80064-8 [DOI] [Google Scholar]
- Seckl JR (2008). Glucocorticoids, developmental ‘programming’ and the risk of affective dysfunction. Progress in Brain Research, 167, 17–34. 10.1016/s0079-6123(07)67002-2 [DOI] [PubMed] [Google Scholar]
- Sesso HD, Buring JE, Rifai N, Blake GJ, Gaziano JM, & Ridker PM (2003). C-reactive protein and the risk of developing hypertension. Journal of the American Medical Association, 290, 2945–2951. 10.1001/jama.290.22.2945 [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, & McEwen BS (2009). Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. Journal of the American Medical Association, 301(21), 2252–2259. 10.1001/jama.2009.754 [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Garner AS, Siegel BS, Dobbins MI, Earls MF, McGuinn L, … Committee on Early Childhood, Adoption, and Dependent Care. (2012). The lifelong effects of early childhood adversity and toxic stress. Pediatrics, 129(1), 232–246. 10.1542/peds.2011-2663 [DOI] [PubMed] [Google Scholar]
- Slopen N, Kubzansky LD, McLaughlin KA, & Koenen KC (2013). Childhood adversity and inflammatory processes in youth: A prospective study. Psychoneuroendocrinology, 38(2), 188–200. 10.1016/j.psyneuen.2012.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass J, Leonard W, Tarskaia L, McDade T, Sorensen M, Alekseev V, & Krivoshapkin V (2007). Anthropometric correlates of C-reactive protein among indigenous Siberians. Journal of Physiological Anthropology, 26(2), 241–246. 10.2114/jpa2.26.241 [DOI] [PubMed] [Google Scholar]
- Stenhammar C, Olsson GM, Bahmanyar S, Hulting AL, Wettergren B, Edlund B, & Montgomery SM (2010). Family stress and BMI in young children. Acta Paediatrica, 99(8), 1205–1212 [DOI] [PubMed] [Google Scholar]
- Taheri S, Austin D, Lin L, Nieto FJ, Young T, & Mignot E (2007). Correlates of serum C-reactive protein (CRP)—no association with sleep duration or sleep disordered breathing. Sleep, 30(8), 991–996. 10.1093/sleep/30.8.991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Lehman BJ, Kiefe CI, & Seeman TE (2006). Relationship of early life stress and psychological functioning to adult C-reactive protein in the Coronary Artery Risk Development in Young Adults Study. Biological Psychiatry, 60, 819–824. 10.1016/j.biopsych.2006.03.016 [DOI] [PubMed] [Google Scholar]
- Turner JR, & Hewitt JK (1992). Twin studies of cardiovascular response to psychological challenge: A review and suggested future directions. Annals of Behavioral Medicine [Google Scholar]
- Uchino BN, Cacioppo JT, & Kiecolt-Glaser JK (1996). The relationship between social support and physiological processes: A review with emphasis on underlying mechanisms and implications for health. Psychological Bulletin, 119(3), 488 10.1037/0033-2909.119.3.488 [DOI] [PubMed] [Google Scholar]
- Uchino BN, Ruiz JM, Smith TW, Smyth JM, Taylor DJ, Allison M, & Ahn C (2016). Ethnic/racial differences in the association between social support and levels of C-reactive proteins in the North Texas Heart Study. Psychophysiology, 53(1), 64–70. 10.1111/psyp.12499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkanova V, Ebmeier KP, & Allan CL (2013). CRP, IL-6 and depression: A systematic review and meta-analysis of longitudinal studies. Journal of Affective Disorders, 150(3), 736–744. 10.1016/j.jad.2013.06.004 [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, … Meaney MJ (2004). Epigenetic programming by maternal behavior. Nature Neuroscience, 7(8), 847 10.1038/nn1276 [DOI] [PubMed] [Google Scholar]
- Willemen AM, Schuengel C, & Koot HM (2009). Physiological regulation of stress in referred adolescents: The role of the parent–adolescent relationship. Journal of Child Psychology and Psychiatry, 50(4), 482–490. 10.1111/j.1469-7610.2008.01982.x [DOI] [PubMed] [Google Scholar]
- Wismer Fries AB, Zigler T, Kurian J, Jacoris S, & Pollak SD (2005). Early experience in humans is associated with changes in neuropeptides critical for regulating social behaviour. Proceedings of the National Academy of Sciences, USA, 102, 17237–17240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanbaeva DG, Dentener MA, Creutzberg EC, Wesseling G, & Wouters EF (2007). Systemic effects of smoking. Chest, 131(5), 1557–1566. 0.1378/chest.06-2179 [DOI] [PubMed] [Google Scholar]


