Abstract
Despite widespread information about the Human Papillomavirus (HPV) vaccine, uptake continues to be low (CDC, 2010). HPV vaccine uptake may be maximized by better understanding factors likely to influence parents' decisions to vaccinate their age-recommended children. Previous reviews have summarized barriers and facilitators likely to influence parents' decision to vaccinate their adolescents (mostly daughters) against HPV; however, less attention has been given to summarize and evaluate the methodology. The aim of this study is to systematically review the methodology used in observational studies of HPV vaccine uptake from a parental perspective. A systematic search of Academic Search Premier, CINAHL, ERIC, Medline and PsycInfo to obtain relevant articles after FDA vaccine approval (2006-to present) yielded 446 studies, of which 17 studies were eligible. Results showed the majority of studies were cross-sectional, with random sampling from list-assisted sources begin the most common method for data collection. Most studies used convenience samples and relied on parental self-report. Also, the majority of studies explored vaccine initiation, but only a few explored regimen completion and timely completion of vaccine regimen. Given that the effectiveness of the HPV vaccine is based on established recommendations for a three dose regimen within a timely interval, studies on factors likely to influence regimen completion and timely completion of regimen are essential to maximize the effectiveness and public health benefits of the vaccine. Research with more diverse samples, better and increased use of random sampling techniques, and the use of precise and objective measures of vaccine uptake to supplement parental self-report, is necessary to reduce selection and information biases in future studies. Studies to inform on factors likely to influence parents' decisions to vaccinate their sons against HPV are also needed.
Human Papillomavirus (HPV) is the most common sexually transmitted infection (STI) in the US with at least half of the sexually active population likely to become infected in their lifetime [1]. There is no cure for HPV and persistent infection has been associated with various types of cancer (cervical, vulvar, vaginal, penile, anal,oropharyngeal) and genital warts [2]. In 2006, the US Food and Drug Administration (FDA) approved a quadrivalent vaccine (Gardasil®), which protects against the HPV types that cause 70% of cervical cancer cases (16 & 18) and 90% of genital warts cases (6 & 11). Gardasil® is available for females and males aged 9–26 (approved for boys in 2009), and specifically recommended for females and males aged 11–12 [3]. In 2009, the FDA approved a bivalent vaccine (Ceravix®), which protects against types 16 and 18 and is available for girls aged 9–26. The recommended vaccine regimen is to receive three doses of the vaccine (time 0, one-two months after time one, and the final within six months of first) [3]. Both vaccines have the potential to significantly reduce the HPV public health burden with widespread uptake administered prior to sexual debut, ideally during early adolescence [2].
According to estimates from the Centers for Disease Control and Prevention (CDC), 2010 HPV vaccination rates in the US were low (48.7% of girls aged 13–17 had at least one dose, and 32% completed the series) [4]. An objective for Healthy People 2020 is to increase HPV vaccination coverage among adolescents to at least 80% [5]. HPV vaccine uptake could be maximized by developing a better understanding of factors influencing vaccination decisions, which largely refers to parents of adolescents [6]. Although previous literature reviews have summarized facilitators and barriers to parents’ decisions to vaccinate their adolescents, [7, 8] less attention has been given to the methodology of these studies. Biases in study design, differing sample characteristics, and statistical measurement error may lead to inaccurate findings and increased risk for misguided conclusions [9]. Thus, the overarching goal of this study is to systematically review the methodology used in observational studies of HPV vaccine uptake from a parental perspective. The specific aims are to: (a) describe population characteristics and methodology used in studies of HPV vaccine uptake among daughters/sons from a parent’s perspective, (b) systematically review how vaccine uptake has been defined and measured across studies, (c) summarize themes of variables hypothesized to be associated with HPV vaccine, and (d) provide recommendations to improve study design and assessment of vaccine uptake in observational studies.
Methods
The methodology was based on guidelines from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [10], and included peer-reviewed studies reporting quantitative data from parents or caretakers about their child’s HPV vaccine uptake. A “child” was defined from a parental perspective without imposing age restrictions. Inclusion criteria for studies were that the study 1) was published in English, 2) sampled US parents/caretakers (given the potential influence of culture, policy, and healthcare systems in HPV vaccine uptake) and 3) included HPV vaccine uptake as an outcome of interest. Studies that included combined samples of parents/caretakers and non-parent/non-caretakers were excluded. Also excluded were intervention and qualitative studies, case reports, reviews, commentaries, virology studies, and presentations of clinical guidelines. Intervention studies were excluded because the primary focus of this review was on prevalence of vaccine uptake, not behavior change.
For the initial selection of eligible studies, two authors searched the literature using multiple databases (Academic Search Premier, the Cumulative Index to Nursing and Allied Health Literature Plus, Educational Resource Information Center, Medline, and PsycInfo). To obtain articles published after FDA vaccine approval, searches were limited to studies published between 2006 and February 2012. Terms selected for the keyword search anywhere in text, abstract and title were HPV OR human papillomavirus AND vaccine AND parent, parental, mother, maternal, father, paternal, and caregiver.
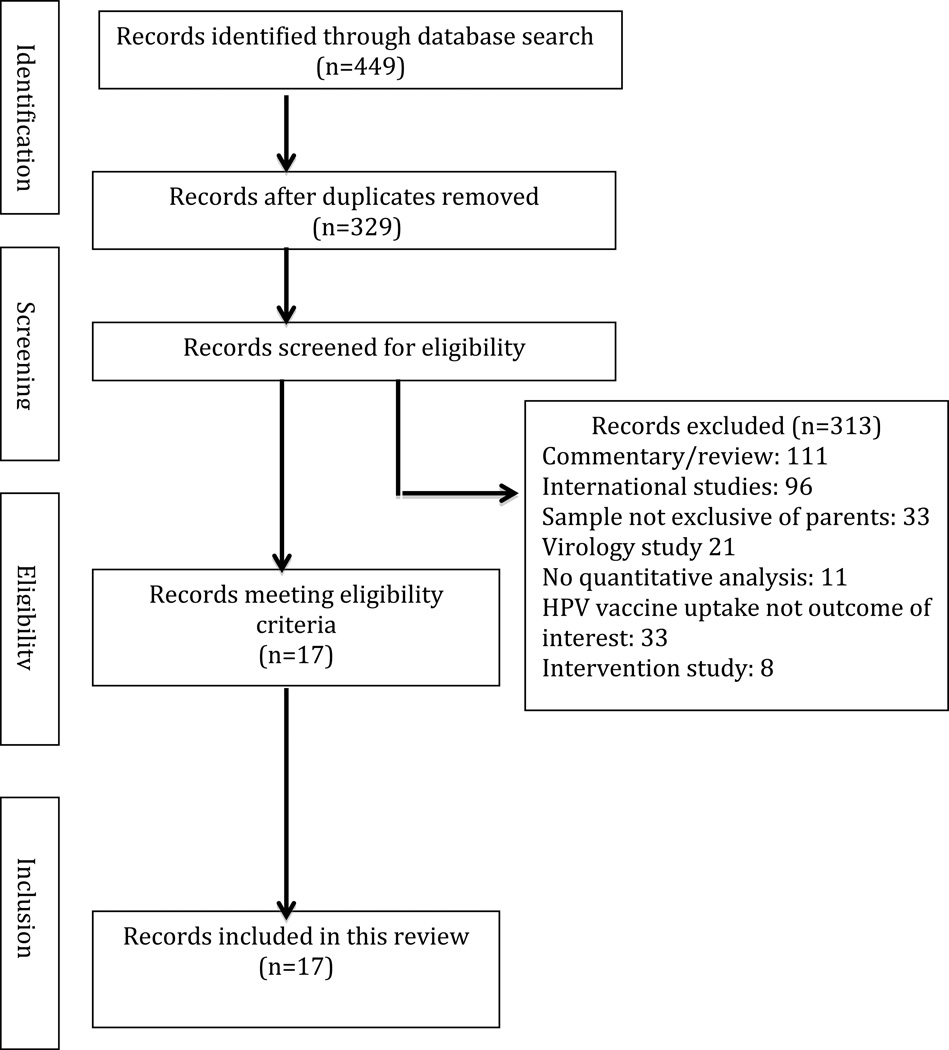
A total of 449 articles were identified. Following the removal of duplicates (n = 120), 329 articles were screened for inclusion, 313 were excluded based on eligibility criteria, and 17 were eligible for review. See Figure 1 for study selection process and exclusion details.
Figure 1.
Flow diagram demonstrating article screening and eligibility
A standardized data abstraction form was created based on results from previous reviews [7, 9, 11, 12]. Information on study design, participant characteristics, application of a theoretical framework, constructs assessed, and measures used to assess HPV vaccine uptake were abstracted from eligible studies. HPV vaccine uptake is defined based on definitions from previous reviews [7, 9]; and construct definitions and examples of measures from the National Cancer Institute [13].
A standardized codebook was created, and all authors reviewed two studies to identify questions and/or ambivalent items. Modifications were made to the coding sheet until 93% inter-rater joint probability agreement was obtained. Each eligible study was coded independently by one of two coders. Data were entered and analyzed using SPSS v.19.
Results
Study Design
The majority of studies were cross-sectional (82%). Table 1 summarizes features of study design and Table 2 assesses study characteristics. All data were collected after HPV vaccine approval for women (June, 2006), 6% used data collected a year after FDA approval [14], and 88% used data collected July 2007-September 2010 [15–29]. One study did not provide information about when the data were collected [30]. One study reported collecting data for sons after vaccine approval for males (October 2009) [26].
Table 1.
Study design characteristics
| Study | Study Design | Source of Data | Mode of Data Collection |
Response Rate |
|---|---|---|---|---|
| Brewer, et al., 2011 [16] |
Prospective | CHIME Projecta | Computer administered telephone interview |
74% |
| Cates, et al., 2010 [17] |
Cross-sectional | BRFSS/CHAMPb (2008) |
Computer administered telephone interview |
77% |
| Chao, et al., 2009 [14] |
Retrospective | KPSC Medical Recordsc |
Electronic Medical Records |
NA |
| Dorell, et al., 2011 [18] |
Cross-sectional | NIS-Teend (2009) |
Telephone survey w/caregivers followed by Mailed survey to Providers |
59% |
| Gerend, et al., 2009 [19] |
Cross-sectional | Medical setting survey |
Paper pencil survey | NR |
| Gottlieb, et al., 2009 [20] |
Cross-sectional | CHIME Projecta | Computer administered telephone interview |
74% |
| Guerry, et al., 2011 [21] |
Cross-sectional | List-Assisted Public School Roster |
Telephone survey | 48% |
| Kennan, et | Cross-sectional | Pittsburgh Girls | In-home interview | 85% |
| al., 2012 [22] | Study | |||
| Krieger, et al., 2010 [29] |
Cross-sectional | Academic Setting survey |
Paper pencil survey | 72% |
| Reiter, et al., 2009a [23] |
Cross-sectional | CHIME Projecta | Computer administered telephone interview |
74% |
| Reiter, et al., 2009b [24] |
Prospective | CHIME Projecta (Follow up data) |
Computer administered telephone interview |
74% |
| Reiter, et al., 2010 [25] |
Cross-sectional | BRFSS/CHAMPb (2008) |
Computer administered telephone interview |
77% |
| Reiter, et al., 2011 [26] |
Cross-sectional | List-Assisted HIS Surveye |
Online survey | 63% |
| Rosenthal, et al., 2008 [15] |
Cross-sectional | Medical setting survey |
Paper pencil survey | 83% |
| Wong, et al., 2011 [27] |
Cross-sectional | NHISf (2008) |
Paper pencil survey | 72% |
| Yeganeh, et al. (2010) [28] |
Cross-sectional | Medical setting survey |
In person interview | NR |
| Ziarnowski, et al., 2009 [29] |
Cross-sectional | CHIME Projecta | Computer administered telephone interview |
74% |
NA=Not applicable
NR= Not reported
Carolina HPV Immunization Measurement and Evaluation
Behavioral Risk Factor Surveillance System Survey/Child Health Assessment and Monitoring Program Survey
Kaiser Permanente Southern Electronic Medical Records
National Immunization Survey-Teen
HPV Immunization in Sons Study
National Health Interview Survey
Table 2.
Summary assessment of study features
| Study | Use of Random sampling |
Ethnic & racial diversity of sample* |
Explicit Criteria provided for identification of index child |
Assessment of multiple aspects of vaccine uptake |
Use of objective data to complement parental self report |
Theoretical framework used to explore correlates of interest |
Adequate description of measures is provided |
|---|---|---|---|---|---|---|---|
| Brewer, et al., 2011 [16] |
Yes | Yes | Yes | Yes | |||
| Cates, et al., 2010 [17] |
Yes | Yes | Yes | Yes | |||
| Chao, et al., 2009 [14] |
Yes | Yes | Yes | Yes | Yes | ||
| Dorell, et al., 2011 [18] |
Yes | Yes | Yes | Yes | Yes | ||
| Gerend, et al., 2009 [19] |
Yes | ||||||
| Gottlieb, et al., 2009 [20] |
Yes | Yes | Yes | Yes | |||
| Guerry, et al., 2011 [21] |
Yes | Yes | Yes | ||||
| Kennan, et al., 2012 [22] |
Yes | Yes | Yes | ||||
| Krieger, et al., 2010 [30] |
Yes | Yes | |||||
| Reiter, et al., 2009a [23] |
Yes | Yes | Yes | ||||
| Reiter, et al., 2009b [24] |
Yes | Yes | |||||
| Reiter, et al., 2010 [25] |
Yes | Yes | |||||
| Reiter, et al., 2011 [26] |
Yes | Yes | Yes | ||||
| Rosenthal, et al., 2008 [15] |
Yes | Yes | |||||
| Wong, et al., 2011 [27] |
Yes | Yes | Yes | Yes | |||
| Yeganeh, et al. (2010) [28] |
Yes | Yes | |||||
| Ziarnowski, et al., 2009 [29] |
Yes | Yes | Yes |
Ethnic and racial diversity of samples was determined based on the inclusion of no more than 67% of non-Hispanic White, which reflects recent national population estimates reported by the U.S. Census Bureau [36]
Sources of data
Twelve different sources of data were used in the 17 studies. Primary source data were used in 41% [15, 19, 21, 22, 26, 28, 30]; 29% used data from the Carolina HPV Immunization Measurement and Evaluation (CHIME) project [16, 20, 23, 24, 29], 24% used data from state or national databases [17, 18, 25, 27], and 6% used data from existing medical records [14].
Six of the data sources were collected using random digit-dial or random sampling from list-assisted sources, including a study that selected random household numbers from public school rosters [21]. Other sources of data were collected from medical (33%) and academic settings (8%). Approximately 33% of data sources were collected using telephone surveys, 33% paper/pencil questionnaires, 17% face-to-face interviews, 8% online surveys, and 8% existing medical records. Most studies (82%) reported a response rate ranging from 48%–85%. Lowest participation was reported in a study using random household numbers from public school rosters [21]; highest participation occurred when recruitment was performed in medical settings [15, 19, 28] or in-home interviews in the community [22].
Participant Characteristics
Most studies used convenience samples. See Table 3 for a description of participant characteristics. Most (47%)data were collected in the South [15–17, 20, 23–25, 29], 18% in the West [14, 21, 28], 6% in the Midwest [30], 6% in the East[22], and 6% in the Southeast [19]. Three studies used nationwide samples [18, 26, 27]. Of the 7 studies providing information about residential setting (urban vs. rural), 47–83% of participants resided in urban settings. Regarding sample size, 12% had fewer than 100 participants, 18% had101–500 participants, 47% had 501–1000 participants, and 4 had more than a 1000 (23%). The largest sample was collected from existing medical records [14].
Table 3.
Participant Characteristics, year of data collection and percentage of vaccine initiation
| Study | Geographic Location |
Sample size & Participant Characteristics |
Year of Data Collection |
% Vaccine Initiation |
|---|---|---|---|---|
| N= 650 | ||||
| Brewer, et al., 2011 [16] |
South (NC) | Daughter’s age range=10–18 yrs Ethnicities included: White (56%), African-American (35%), Latinos (9%) |
Baseline 2007 Follow up 2008 |
12% at baseline 27% at FU* |
| N= 696 | ||||
| Cates, et al., 2010 [17] |
South (NC) | Daughter’s age range=10–17 yrs Ethnicities included: White (63%), African-American (23%), Other (14%) |
2008 | 31% |
| N= 148,350 dyads | ||||
| Chao, et al., 2009 [14] |
West (CA) | Daughter’s age range=9–17 yrs Ethnicities included: White (22%), African-American (10%), Asian-American (7%), Latino (37%), Other (24%) |
2006–2007 | 27% (41% Completed regimen) |
| N = 18,228 | ||||
| Dorell, et al., 2011 [18] |
Nationwide | Daughter’s age range=13–17 yrs Ethnicities included: White (60%), African-American (15%), Asian-American (3%), Latino (18%), Other (4%) |
2008–2010 | 40% (53% Completed regimen) |
| N= 82 | ||||
| Gerend, et al., 2009 [19] |
Southeastern (FL) |
Daughter’s age range=9–17 yrs Ethnicities included: White (71%), African-American (20%), Other (4%), Not reported (5%) |
2008 | 47% |
| N= 889 | ||||
| Gottlieb, et al., 2009 [20] |
South (NC) | Daughter’s age range=10–18 yrs Ethnicities included: White (52%), African-American (38%), Latinos (5%), Other (5%) |
2007 | 10% |
| N= 509 | ||||
| Guerry, et al., 2011 [21] |
West (CA) |
Daughter’s age range=11–18 yrs Ethnicities included: African- American (16%), Latino (81%), Other (3%) |
2007–2008 | 23% |
| N= 2,098 | ||||
| Kennan, et al., 2012 [22] |
East (PA) | Daughter’s age range=12–15 yrs Ethnicities included: White (41%), African-American (58%), Asian-American (.9%, Excluded) |
2008 | 63% |
| N= 182 dyads | ||||
| Krieger, et al., 2010 [30] |
Midwest (OH) | Daughter’s age range=18–31 yrs Ethnicities included: White (87%), African-American (7%), Asian-American (3%), Other (1%), Unreported (2%) |
NR | NR |
| N= 889 | ||||
| Reiter, et al., 2009a [23] |
South (NC) | Daughter’s age range=10–18 yrs Ethnicities included: White (70%), African-American(23%), Other (7%) |
2007 | 12% |
| N= 229 | ||||
| Reiter, et al., 2009b [24] |
South (NC) | Daughter’s age range=11–20 yrs Ethnicities included: White (77%), African-American (17%), Other (6%) |
Baseline 2007 Follow up 2008 |
100% at baseline 86% at FU were on timely completion |
| N= 617 | ||||
| Reiter, et al., 2010 [25] |
South (NC) | Daughter’s age range=10–17 yrs Ethnicities included: White (68%), African-American (20%), Other (12%) |
2008 | 31% |
| N=547 | ||||
| Reiter, et al., 2011 [26] |
Nationwide | Son’s age range=11–17 yrs Ethnicities included: White (68%), African-American (12%), Latino (15%), Other (5%) |
2010 | 2% (<1% completed regimen) (males) |
| N= 153 | ||||
| Rosenthal, et al., 2008 [15] |
South (TX) | Daughter’s age range=11–17 yrs Ethnicities included: White (34%), African-American (39%), Latino (20%), Other (7%) |
2007–2008 | 26% |
| N= 2,205 | ||||
| Wong, et al., 2011 [27] |
Nationwide | Daughter’s age range=9–17 yrs Ethnicities included: White (25%), African-American (21%), Latinos (21%), Asian-American (7%), Other (30%) |
2008 | 23% (9% Completed regimen) |
| N= 95 | ||||
| Yeganeh, et al. (2010) [28] |
West (CA) | Daughter’s age range=9–19 yrs Ethnicities included: Latino (91%), Other (9%) |
2008 | 37% (26% Completed |
| N= 889 | ||||
| Ziarnowski, et al., 2009 [29] |
South (NC) | Daughter’s age range=10–18 yrs Ethnicities included: White (70%), African-American (23%), Other (7%) |
2007 | 12% |
NR= Not reported
Of those not vaccinated at baseline
Study assessed timely completion of dosage regardless of vaccine doses.
Of studies reporting parental gender, the majority included female and male caregivers (71%); however, sample sizes for male caregivers/fathers were generally small (5–17% of sample), except for a study in which male caregivers made up 46% of the sample [26]. Parental age ranged from 20–77 years (M=42, SD=3.5). Most parents had some college education (56–87%), and an annual household income greater than $50,000 (60–70%), except for a single study of low-income minority mothers (58% of sample had an annual household income <$25,000) [28]. Most parents were married and spoke English. Of studies reporting the number of children in the household, these ranged from 1–7 (M=2.4, SD=1.2). All but one study assessed vaccine uptake among daughters only, and of these the most included daughters aged 9–18 (81%). Two studies extended the age range to 19 and 20 year olds [24, 28], and one study assessed vaccine uptake among adult daughters aged 18–31 [30]. Only one study assessed vaccine uptake among sons, and their age ranged from 11–17 [26].
The majority of studies included White and African-American parents, but only about half of studies reported inclusion of Latinos. Four studies reported sample sizes for Asian-Americans [14 18, 27, 30]. Two studies included Latino sample sizes greater than 80% [21, 28], and 1 study provided information on ethnic subgroups by assessing country of birth [28]. Three studies provided participants with the choice of answering questions in a language other than English (Spanish) [21, 27, 28]. Most parents were insured (range 89–96%). Other parental characteristics assessed were religion/religiosity [16, 26, 28, 30], political affiliation [16, 26, 28], and maternal health and sexual history [14, 16, 21, 23, 25, 28].
Framework for Analyses
Four studies (24%) reported using a theoretical framework to identify correlates of HPV vaccine uptake, which included the Health Belief Model [16, 19, 23], Theory of Planned Behavior [19], the Extended Parallel Process Model (EPPM) [30], and the Risk Reception Attitude (RRA) framework [30]. Three general aspects of vaccine uptake were explored: (a) vaccine initiation (at least 1 shot) [14–23, 25–29], (b) regimen completion (all 3 shots) [14, 16, 18, 23, 27], and (c) timely completion of regimen based on the Advisory Committee of Immunization Practices (ACIP) recommendations [14, 16, 23]. All but 1 of the studies explored correlates of vaccine uptake. Of these, 1 study reported descriptive data on regimen completion and timely completion [16] and another used a predictor model to explore factors related to regimen completion [14]. Only one study explored regimen completion among daughters and that focus was specifically on whether reported pain from the first shot (from a parental perspective) predicted series completion [23].
Assessment of HPV Vaccine Uptake
Two of the 17 studies assessed vaccine uptake objectively through existing medical records [14, 18]. All others relied on parental self-report. Of studies using parental self-report, 41% described the specific questions used to assess vaccine uptake [17, 20, 21, 23, 25, 29, 30]. The most frequently used question to assess vaccine initiation was “has [daughter] had any shots of the HPV vaccine?” [17, 20, 21, 23, 29]. In at least 2 of these studies, this question was not followed by clarification regarding the number of doses, limiting assessment of regimen completion [17, 25]. In contrast, one study used the question “how many doses of the HPV vaccine has your daughter received?” [30]. Among those studies not describing the specific questions used to assess vaccine uptake, 2 reported asking the number of doses received along with a time frame for when doses were acquired to assess timely completion [16, 23] defined by either having received all three doses of the vaccine or having received 1 or 2 doses no more than 2 months past the time recommended [16, 23]. The remaining studies assessed vaccine initiation based on whether or not a daughter has been “vaccinated” [15, 19, 28].
Of the 15 studies using parental self-report, 53% indicated that among parents with more than one daughter, interviewers randomly selected an index daughter from household rosters to be used as the referent [16, 17, 20, 21, 23, 25, 27, 29]. One study asked parents to identify the son with the most recent birthday as referent [26], and another study used parent-child dyads to facilitate identification of the index child [30]. Five studies did not specify how parents with multiple daughters answered questions on vaccine uptake even when provided an age-range referent [15, 18, 19, 22, 28].
Prevalence of HPV Vaccine Uptake
Except for a study that conditioned participation to 100% vaccination status [24], rates of HPV vaccine initiation ranged from 10–63%. Among these studies, lowest vaccine initiation rates (10–12%) were reported in studies using data from the CHIME project, which included a large sample size of parents randomly selected from counties with high rates of cervical cancer in the south [20, 23, 29]. Studies reporting the highest rates of vaccine initiation among daughters (47–63%) were a study that included small sample sizes of parents attending community health clinics [19] and a study using a larger sample of community households in Pennsylvania [22].
Changes in vaccine initiation rates over time were also observed. For example, vaccine initiation rates during from 2006–2007 were considerably lower (10–27%) than rates reported in studies conducted from 2008–2010 (23–63%). Additionally, initiation of the HPV vaccine varied by daughter’s age group in some studies, with older daughters (ages 16–18) being 3 times more likely than younger daughters (ages 10–12) to initiate vaccination [20, 25]. Although not consistent across studies, some studies showed HPV vaccine initiation to vary by parental age group (parents >=40 more likely to initiate vaccination for their daughters) [20]. The only study that assessed vaccine uptake among males indicated a low uptake rate (2%) [26].
In terms of ethnic/racial differences in HPV vaccine uptake, inconsistencies were observed across studies. In a study using a large number of medical records, Chao, et al. [14] found African-Americans (25%) and women who identified themselves as “Other” (24%) reported lower vaccine initiation for their daughters compared to Whites (28%), Latinas (29%), and Asian-Americans (29%). Similar results for African American and Latinas were reported in Dorell et al [18]. In contrast, Gottlieb, et al. [20] found African-Americans reporting similar HPV vaccine initiation rates to Whites, with Latinos having the lowest reported rates.
Five studies reported vaccination completion rates [14, 26, 27, 28], which ranged from <1% [26] to 53% [18], with the lowest rate reported among sons. One study reported on timely completion (41% completed within ACIP recommendations) [14].
Assessment of measures used to assess correlates of HPV vaccine uptake
Most studies (71%) provided a detailed description or reference of the measures used to assess correlates of HPV vaccine uptake [14, 15, 17, 18, 20–23, 25, 26, 29, 30]. The majority of studies used single item questions to assess correlates [14, 17–20, 25, 28, 29], while the remaining used a combination of single and multiple-item measures [15, 16, 21–23, 26, 30]. Of studies using multiple item measures, most reported the reliability of the measures (Cronbach’s α = 0.5–0.9). Three studies using surveys reported piloting questions before data collection [15, 21, 26]. Six studies provided a reference to additional information on the development of survey items [18, 19, 23, 26, 27, 30]. Two studies used translated versions of the measures (Spanish); however, no comment or reference was provided for the translation methodology [21, 28].
Constructs assessed for association to HPV vaccine uptake
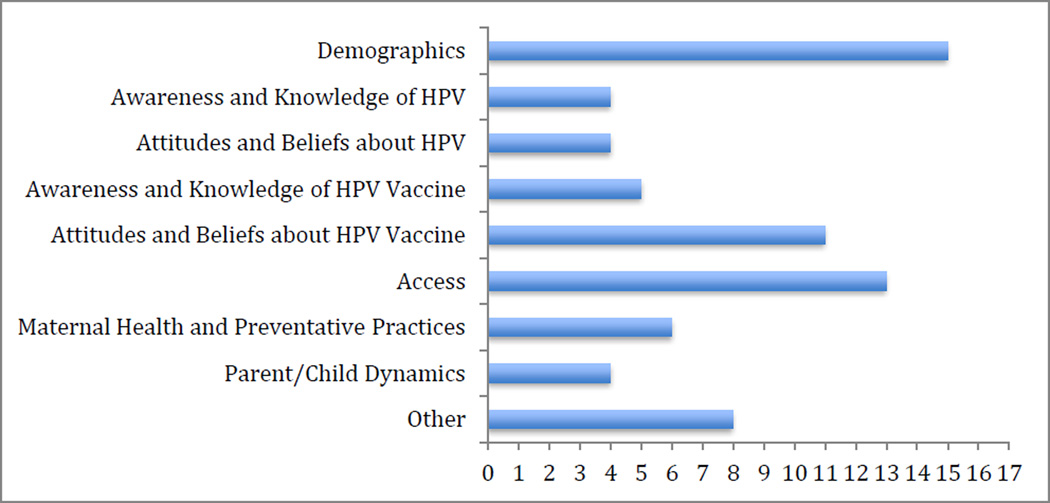
Figure 2 summarizes general constructs hypothesized to be associated with HPV vaccine uptake. The most prevalent constructs (assessed in 88% of studies) were demographics and access-related factors (e.g., provider’s recommendation, insurance coverage) (76%), followed by parental attitudes and beliefs about HPV and the vaccine. Specific attitudes and beliefs about HPV assessed in the included studies were perceived HPV susceptibility of self and daughter. Examples of attitudes and beliefs about HPV vaccination assessed included perceived effectiveness, safety/harm and benefits, and perceived barriers (e.g., cost, limited information). Other constructs explored included maternal health history (e.g., history of cervical cancer, HPV and STIs), maternal preventive practices (e.g., history of pap smear and STI testing) (38% of studies), HPV awareness and knowledge (25%), vaccine awareness and knowledge (29%) and parent-child dynamics (e.g., parental monitoring style, parent-child communication style, shared decision making about vaccine) (24%). To a lesser extent the association of other constructs such as daughter’s sexual intimate behavior, pubertal development, age of mother’s sexual initiation and daughter’s dating status, and their association to HPV vaccine uptake have also been explored.
Figure 2. Constructs assessed in the included studies for association with HPV vaccine initiation.
*Constructs assessed under the “Other” category include daughter’s sexual intimate behavior, pubertal development, age of mother’s sexual initiation and daughter’s dating status.
Having received a provider’s recommendations for the HPV vaccine was found to be among the strongest predictors of vaccine initiation in 6 of 8 studies assessing this factor [16–18, 23, 26].Parents reporting having a previous provider’s recommendation for the vaccine were more likely to have daughters who have initiated vaccination. Percentage of daughters who have received a provider’s recommendation for the vaccine ranged from 21–53.1%.
Discussion
The present review evaluated study designs, participant characteristics, construct themes of factors associated with HPV vaccine uptake and measures used to assess HPV vaccine initiation and completion across 17 studies of HPV vaccine uptake from a US parental perspective. Most studies explored the prevalence and factors associated with HPV vaccine initiation, with a limited number focusing on completion and timely completion. Given that the HPV vaccine’s effectiveness is based on a three dose regimen within a timely interval, the need for studies on factors influencing timely completion are essential to maximizing the effectiveness and public health benefits of the vaccine.
Only one study explored HPV vaccine initiation and completion among sons (revealing 2% initiation and <1% completion rates), and few included small samples of male caregivers/fathers. . Given that the vaccine is now available for males, additional studies are needed to understand parents’ decisions about vaccinating their sons. As the structure of the American family is changing, with an increase in single parent-households and more males full-time caregivers [31], studies assessing male caregivers/fathers’ HPV vaccine decision making are needed. Equally needed are studies exploring differences in the parental perspectives of male and female caregivers who care for the same child in order to develop campaigns and interventions aimed at increasing HPV vaccine acceptance within the household.
An assessment of study designs, participant characteristics, and measures used to assess HPV vaccine uptake and correlates showed there are possible selection and information biases across studies. Only about a third of the studies reported use of random sampling techniques, inclusion of diverse samples, providing explicit criteria for the selection of respondents (e.g., index child), assessment of vaccine regimen completion and timely completion, using of objective measures to assess uptake, using theoretical frameworks to identify correlates, and providing detailed descriptions of measures used. A noticeable weakness among several of the studies was the use of convenience samples and response rate disparities across recruitment settings. Such biases could misrepresent parental vaccination intentions, which can influence the target populations of public health interventions. To reduce the risk of selection biases, future studies should focus on increasing random sampling techniques, and including more diverse samples. Studies focusing on assessing parental perspectives on HPV vaccine uptake for children among Asian-American, biracial and/or parents/caregivers who identify themselves as “other” (e.g., Middle Eastern populations) are needed. Previous research has shown marked health disparities within some ethnic subgroups, such as Latinos and Asian-Americans, in terms of socioeconomic characteristics, access to health-care factors, and health behaviors including preventive practices [32, 33],which may influence HPV vaccine uptake. Studies using statistical analyses that can control for within ethnic subgroup differences are also recommended. -
Several challenges were identified regarding the measurement of HPV vaccine uptake, which may increase risk for information biases. A common limitation noted in all but two studies was the lack of objective data to verify uptake/completion. Although research has shown that parental self-report may be somewhat accurate [18], verifiable objective data may be preferable, especially for regimen completion and timely completion.
Another identified challenge to the measurement of HPV vaccine uptake involves the proper identification of a referent child. This is particularly relevant for caregivers/parents to more than one child who could vary in age, but still be included within the age range for vaccination. Given that daughter’s age has been found to be significantly associated with HPV vaccine uptake [20, 23], parents with an older and a younger daughter may experience confusion when answering questions. The use of household rosters to provide caregivers with an opportunity to answer HPV vaccine uptake questions for each child in the household may provide for more accurate assessment of and added incremental validity to the assessment of HPV vaccine uptake.
Another important consideration includes specifying the number of vaccine doses received and the dates each dose was received, in order to allow for the assessment of timely completion. Previous research shows that rates of timely completion of the HPV vaccine regimen are low (42%–60%), and timely completion of one or two doses does not guarantee timely completion of the full series [34, 35]. Studies assessing factors associated with timely completion of the HPV vaccine are important to develop interventions aimed at increasing adherence to recommended guidelines.
In the studies reviewed, HPV vaccine initiation rates (10–63% for daughters, 2% for sons) were somewhat comparable with those reported by the 2010 National Immunization Survey-Teen (49% for daughters, <1% for sons) [4]. Regimen completion rates for daughters varied greatly (9–53% for daughters), and were low for sons (<1% for sons). Given that data collection for most of the studies was conducted between 2006 and 2008, it is possible that HPV vaccine uptake rates may have increased since then, providing a plausible explanation for the lower vaccination rates reported in this review. More recent studies of HPV vaccine uptake among daughters and sons from a parental perspective are needed to determine how well localized studies (e.g., those conducted at single sites or states) represent national estimates.
The included studies utilized a variety of research questions and themes to explore correlates of HPV vaccine uptake from a parental perspective, but few reported using a theoretical framework to identify correlates. Future studies should consider the use of theoretical frameworks to identify and explore relevant factors likely to influence vaccine uptake. Also, although the included studies explored a wide range of factors associated with HPV vaccine uptake, the themes explored did not include the study of how specific cultural factors from a parental perspective (e.g., level of acculturation, cultural beliefs and values, mistrust) may be associated to HPV vaccine uptake. This knowledge is necessary for the development of culture sensitive interventions to reduce disparities in HPV vaccination.
Limitations of this review
This study complements previous reviews on HPV vaccine uptake by providing a detailed analysis of the assessment and methodology used in the study of HPV vaccine uptake from a parental perspective as explored in observational studies. Nevertheless, this review is not without limitations, most of which stem from the exclusion criteria used to select eligible studies. It is possible that studies using other methodologies (e.g., qualitative analyses) and samples (e.g., combined samples of parents and non-parent adults) may facilitate clarification to some of the questions raised in the present review. Also important to emphasize is that findings from this review refer only to observational studies conducted in the US, and that international studies may vary greatly from the included studies. Finally, comparison of findings across studies was difficult given differences in measures used and reported measures of association.
Conclusion
Numerous studies have explored caregivers’ or parents’ intentions to vaccinate their children against HPV, but fewer studies have focused on exploring factors likely to influence whether or not parents have their children initiate and complete the HPV vaccine series. Research has shown that despite motivation to vaccinate their children against HPV, many parents are unable to fulfill their intentions. Developing a better understanding of barriers and resources influencing parental action to vaccinate their children is necessary for the development of prevention interventions aimed to better achieve the public health benefits of HPV vaccination. This review has identified areas that need further research and methodological issues that should be taken into consideration.
Highlights.
Studies on HPV vaccine regimen completion/timely completion of regimen are scant
Studies on HPV vaccine uptake among males from a parental perspective are needed.
Multiple selection and information biases were identified in the included studies.
Recommendations to reduce the aforementioned biases in future studies are provided
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dunne EF, Unger ER, Sternberg M, McQuillan G, Swan DC, Patel SS, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297(8):813–819. doi: 10.1001/jama.297.8.813. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F. Chapter 2:The burden of HPV-related cancers. Vaccine. 2006;24(S3):S11–S25. doi: 10.1016/j.vaccine.2006.05.111. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. FDA Licensure of Bivalent Human Papillomavirus Vaccine (HPV2, Cervarix) for Use in Females and Updated HPV Vaccination Recommendations from the Advisory Committee on Immunization Practices (ACIP) MMWR Rep. 2010;59(20):613–648. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. National, state, and local area vaccination coverage among adolescents aged 13–17 years-United States, 2010. Morbidity & Mortality Weekly Report. 2011;60:1117–1123. [PubMed] [Google Scholar]
- 5.U.S. Department of Health and Human Services. Healthy People. Washington, D.C: 2020. [Accessed January 11, 2011]. Office of Disease Prevention and Health Promotion. Available at http://www.healthypeople.gov/2020/default.aspx. [Google Scholar]
- 6.Gonik B. Strategies for fostering HPV vaccine acceptance. Infect Dis Obstet Gynecol. 2006:369–379. doi: 10.1155/IDOG/2006/36797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartlett JA, Peterson JA. The uptake of Human Papillomavirus (HPV) vaccine among adolescent females in the United States: A review of the literature. J Sch Nurse. 2011;27:434–446. doi: 10.1177/1059840511415861. [DOI] [PubMed] [Google Scholar]
- 8.Trim K, Nagji N, Elit L, Roy K. Parental knowledge, attitudes, and behaviours towards Human Papillomavirus vaccination for their children: A systematic review from 2001 to 2011. Obstet Gynecol Intl. 2011:1–12. doi: 10.1155/2012/921236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen JD, Coronado GD, Williams RS, Glenn B, Escoffery C, Fremandez M, et al. A systematic review of measures used in studies of human papillomavirus (HPV) vaccine acceptability. Vaccine. 2010;2:4027–4037. doi: 10.1016/j.vaccine.2010.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Loannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009;6:7. doi: 10.1371/journal.pmed.1000100. e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liddon N, Hood J, Wynn BA, Markowitz LE. Acceptability of Human Papillomavirus Vaccine for males: A review of the literature. J Adolesc Health. 2010;46:113–123. doi: 10.1016/j.jadohealth.2009.11.199. [DOI] [PubMed] [Google Scholar]
- 12.Mullen PD, Allen JD, Glanz K, Fernandez ME, Bowen DJ, Pruitt SL, et al. Measures used in studies of informed decision making about cancer screening: A systematic review. Ann Behav Med. 2006;32:188–201. doi: 10.1207/s15324796abm3203_4. [DOI] [PubMed] [Google Scholar]
- 13.National Cancer Institute. Health behavior and constructs: Theory, measurement & Research. 2011a Available at http://cancercontrol.cancer.gov/brp/constructs/
- 14.Chao C, Slezak J, Coleman K, Jacobsen S. Papanicolaou screening behavior in mothers and human papillomavirus vaccine uptake in adolescent girls. Am J Public Health. 2009;99:1137–1142. doi: 10.2105/AJPH.2008.147876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenthal SL, Rupp R, Zimet GD, Meza HM, Loza ML, Short MB, et al. Uptake of HPV vaccine: Demographics, sexual history and values, parenting style, and vaccine attitudes. J Adolesc Health. 2008;43:239–245. doi: 10.1016/j.jadohealth.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Brewer NT, Gottlieb SL, Reiter PL, McRee A, Liddon N, Markowitz L, et al. Longitudinal predictors of human papillomavirus vaccine initiation among adolescent girls in a high-risk geographic area. Sex Transm Dis. 2011;38:197–204. doi: 10.1097/OLQ.0b013e3181f12dbf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cates JR, Shafer A, Carpentier FD, Reiter PL, Brewer NT, McRee AL, et al. How parents hear about Human Papillomavirus vaccine: Implications for uptake. Journal of Adolescent Health. 2010;47:305–308. doi: 10.1016/j.jadohealth.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dorell CG, Yankey D, Santibanez TA, Markowitz LE. Human Papillomavirus vaccination series initiation and completion, 2008–2009. Pediatrics. 2011;128:830–839. doi: 10.1542/peds.2011-0950. [DOI] [PubMed] [Google Scholar]
- 19.Gerend MA, Wibley E, Bland H. Parental response to human papillomavirus vaccine availability: Uptake and intentions. J Adolesc Health. 2009;45:528–531. doi: 10.1016/j.jadohealth.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Gottlieb SL, Brewer NT, Sternberg MR, Smith JS, Ziarnowski K, Liddon N, et al. Human papillomavirus vaccine initiation in an area with elevated rates of cervical cancer. J Adolesc Health. 2009;45:430–437. doi: 10.1016/j.jadohealth.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 21.Guerry SL, De Rosa CJ, Markowitz SE, Walker S, Liddon N, Kerndt PR, et al. Human papillomavirus vaccination initiation among adolescent girls in high risk communities. Vaccine. 2011;29:2235–2241. doi: 10.1016/j.vaccine.2011.01.052. [DOI] [PubMed] [Google Scholar]
- 22.Keenan K, Hipwell A, Stepp S. Race and sexual behavior predict uptake of the Human Papillomavirus vaccine. Health Psychology. 2012;31:31–34. doi: 10.1037/a0026812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reiter PL, Brewer NT, Gottlieb SL, McRee A, Smith JS. Parents’ health beliefs and HPV vaccination of their adolescent daughters. Soc Sci Med. 2009;69:475–480. doi: 10.1016/j.socscimed.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 24.Reiter PL, Brewer NT, Gottlieb SL, McRee AL, Smith JS. How much will it hurt? HPV vaccine side effects and influence on completion of three three-dose regimen. Vaccine. 2009;27:6840–6844. doi: 10.1016/j.vaccine.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reiter PL, Cates JR, McRee A, Gottlieb SL, Shafer A, Smith JS, et al. Statewide HPV vaccine initiation among adolescent females in North Carolina. Sex Trans Dis. 2010;37:549–556. doi: 10.1097/OLQ.0b013e3181d73bf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reiter PL, McRee AL, Kadis JA, Brewer NT. HPV vaccine and adolescent males. Vaccine. 2011;29:5595–5602. doi: 10.1016/j.vaccine.2011.06.020. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong CA, Berkowitz Z, Dorell CG, Anhang Price R, Lee J, Saralya M. Human Papillomavirus vaccine uptake among 9- to 17-year-old girls: National Health Interview Survey, 2008. Cancer. 2011;117:5612–5620. doi: 10.1002/cncr.26246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeganeh N, Curtis D, Kuo A. Factors influencing HPV vaccination status in a Latino population; and parental attitudes towards vaccine mandates. Vaccine. 2010;28:4186–4191. doi: 10.1016/j.vaccine.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Ziarnowski KL, Brewer NT, Weber B. Present choices, future outcomes: Anticipated regret and HPV vaccination. Prev Med. 2009;48:411–414. doi: 10.1016/j.ypmed.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 30.Krieger JL, Kam JA, Katz ML, Roberto AJ. Does mother know best? An actor-partner model of college-age women’s human papillomavirus vaccination behavior. Hum Commun Res. 2011;37:107–124. [Google Scholar]
- 31.American Psychological Association (APA) The changing role of the modern day father. 2012 Retrieved January 2012 from http://www.apa.org/pi/families/resources/changing-father.aspx.
- 32.Lee HY, Ju E, Vang PD, Lundquist M. Breast and cervical cancer screening disparity among Asian American women: Does race/ethnicity matter? J Womens Health. 2010;19:1877–1884. doi: 10.1089/jwh.2009.1783. [DOI] [PubMed] [Google Scholar]
- 33.Ramirez RR, De la Cruz GP. The Hispanic population in the United States: March 2002: Population characteristics. Current Population Reports. 2003:20–545. [Google Scholar]
- 34.Chou B, Krill LS, Horton BB, Barat CE, Trimble CL. Disparities in Human Papillomavirus vaccine completion among vaccine initiators. Obstet Gynecol. 2011;118:14–20. doi: 10.1097/AOG.0b013e318220ebf3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Widdice MD, Bernstein DI, Leonard AC, Marsolo KA, Kahn JA. Adherence to the HPV vaccine dosing intervals and factors associated with completion of 3 doses. Pediatrics. 2011;127:77–84. doi: 10.1542/peds.2010-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.U.S. Census Bureau. DetailedFinder Tables-American Fact. [Accessed March 29, 2011];Population Estimates. 2010 Available at http://factfinder2.census.gov.