Abstract
Objectives
This study aims to explore the mechanism by which osteoblast autophagy participated in glucocorticoid-induced femoral head necrosis (FHN).
Materials and methods
Thirty male specific-pathogen-free C57 mice (age, one month; weighing 20-25 g) were randomly divided into blank control, dexamethasone and rapamycin-dexamethasone groups (n=10). After six weeks of intervention, right femoral head was obtained to observe morphology and to calculate percentage of empty lacunae. MC3T3-E1 cells were randomly divided into normal, dexamethasone, rapamycin and dexamethasone-rapamycin groups, and cultured for 24 h. Microtubule-associated protein 1 light chain 3 (LC3)-I, LC3-II, mammalian target of rapamycin (mTOR) and Beclin-1 protein expressions were detected by Western blot.
Results
In rapamycin-dexamethasone group, some bone trabeculae in medullary cavity ruptured and atrophied, and subchondral bone underwent local necrosis. The total apoptosis rates of dexamethasone and rapamycin-dexamethasone groups surpassed that of blank control group, and the former two groups had significantly different rates (p<0.001). LC3-II/LC3-I of dexamethasone group was lower than those of rapamycin and dexamethasone-rapamycin groups (p<0.001), and the ratio of rapamycin group surpassed that of dexamethasone-rapamycin group (p<0.001). Dexamethasone group had higher mTOR protein expression than those of rapamycin and dexamethasone- rapamycin groups (p<0.001), and the expression of rapamycin group was lower than that of dexamethasone-rapamycin group (p<0.001). The Beclin-1 protein expression of dexamethasone group was lower than those of rapamycin and dexamethasone- rapamycin groups (p<0.001), and the expression of rapamycin group exceeded that of dexamethasone-rapamycin group (p<0.05).
Conclusion
Osteoblast autophagy may play a crucial protective role in dexamethasone-induced FHN. The attenuation of autophagy may be related to the affected expressions of key autophagy regulators mTOR and Beclin-1.
Keywords: Autophagy, femoral head, glucocorticoid, necrosis, osteoblast
Introduction
Femoral head necrosis (FHN) is a pathological process typified by the death of bone active components.[1-3] With the aggravation of hormonal drug abuse, steroid-induced avascular necrosis of the femoral head (SANFH) has become a common bone and joint disease clinically, which usually endangers bilateral femoral heads with a wide range of osteonecrosis at the end stage. Because of the high disability rate after onset, most patients eventually choose hip replacement surgery.
In recent years, autophagy has been closely related to many aging and degenerative diseases, including aging-induced systemic bone loss. Autophagy is a self-eating behavior widely existing in cells, as an intracellular process transporting substances to lysosomes for degradation.[4] Like apoptosis and endoplasmic reticulum stress, autophagy is also a crucial physiological phenomenon in cells relying on a highly conserved autophagy process in each species to buffer the stress response of the external environment, to degrade harmful organelles, and to maintain normal metabolism and homeostasis of the internal environment.
Recently, the important roles of autophagy in human health and diseases have been highlighted, and the protective effects of autophagy under various stresses have been proven,[5] suggesting that the changes of autophagy activity dominate in the prevention and development of diseases. The influence of autophagy on bone metabolism is well documented. For instance, Liu et al.[6] found that autophagy was activated during the differentiation of osteoblasts. Besides, Zhang et al.[7] reported that the expressions of various autophagy-related genes were associated with bone density. However, they failed to determine whether autophagy exerted beneficial or adverse effects. Autophagy is of great significance to the maintenance of mature bone formation.[8] During aging, osteoblast function is weakened and bone loss is affected by the progressive damage to osteoblast autophagy.[9] In high glucose environments, autophagy is a crucial mechanism for osteoblast protection.[10] At present, autophagy is a well-established protective mechanism of cartilage itself. The autophagy level of chondrocytes gradually decreases along with aging, thus being one of the important reasons for articular cartilage degeneration.[11-14]
However, it remains controversial whether the autophagy level changes in the procession of hormone- induced FHN. Microtubule-associated protein 1 light chain 3 (LC3) is a key gene encoding autophagy- related proteins which are localized on the surfaces of pre-autophagic vesicles and autophagic vesicle membranes. It plays an essential role in the processing and modification of two ubiquitin-like proteins, and participates in autophagosome formation. Beclin-1 is a homologue of yeast autophagy-related 6 (Atg6) protein. Beclin-1 autophagy-related proteins are localized on the pre-autophagosome to bind phosphatidylinositol- 3-kinase (PI3K), forming a Beclin-1-Vps34-Vps15 core complex which plays a vital role in the early stage of autophagosome formation. Luo and Rubinsztein[15] reported that Beclin-1 overexpression in HeLa cells caused a continuous increase in the number of intracellular autophagosomes. Additionally, Qu et al.[16] found that Beclin-1-deficient mice underwent significantly reduced autophagic vacuole formation, suggesting that the regulation of Beclin-1 expression was consistent with autophagy. As a conserved serine/threonine protein kinase, mammalian target of rapamycin (mTOR) facilitates cell autophagy when its activity is inhibited, and suppresses autophagy when its activity is enhanced.[17] In this study, we aimed to explore the mechanism by which osteoblast autophagy participated in glucocorticoid-induced FHN.
Patients and Methods
This study was conducted at First Affiliated Hospital of Soochow University between November 2018 and November 2019. Thirty male, specific-pathogen-free and healthy C57 mice (age, one month; weighing 20-25 g) were purchased from Shanghai Jiao Tong University School of Medicine [animal license number: SCXK (Shanghai) 2018-0007]. MC3T3-E1 was obtained from American Type Culture Collection, USA.
Rapamycin was purchased from Sigma (Sigma Corp., St. Louis, MO, USA). Dexamethasone sodium phosphate injection was bought from Guangdong Sancai Shiqi Pharmaceutical Co., Ltd. (Guangzhou, China). Cell Counting Kit-8 (CCK8), Radio Immunoprecipitation Assay (RIPA) cell lysate, bicinchoninic acid (BCA) protein assay reagent and SDS-PAGE gel preparation kit were obtained from Beyotime Institute of Biotechnology Co., Ltd. (Shanghai, China). Minimum Essential Medium Eagle - alpha modification (α-MEM) was provided by Thermo Fisher Scientific (Suzhou) Instruments Co., Ltd. (Soochow, China). Hematoxylin and eosin (HE) staining kit was purchased from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China). mTOR antibody, LC3 B antibody, Beclin-1 antibody and β-actin antibody were bought from Cell Signal Technology (Danvers, MA, USA). Enhanced chemiluminescence (ECL) reagent was obtained from Invitrogen (Carlsbad, CA, USA). Polyvinylidene fluoride (PVDF) membrane and ultrapure water purification system were provided by Millipore (Burlington, MA, USA).
Thirty mice were randomly divided into blank control, dexamethasone and rapamycin- dexamethasone groups (n=10). The blank control group was intraperitoneally injected with 5 mg•kg-1 rapamycin-free solvent. The dexamethasone group was intraperitoneally injected with 2.5 mg•kg-1 dexamethasone sodium phosphate injection and 5 mg•kg-1 rapamycin-free solvent. The rapamycin- dexamethasone group was intraperitoneally injected with 2.5 mg•kg-1 dexamethasone sodium phosphate injection and 5 mg•kg-1 rapamycin. After six weeks of intervention, the mice were euthanized, from which the skin and subcutaneous tissue were cut from the right thigh, and muscles around the femur were disconnected. Finally, the complete right femoral head was obtained, fixed in 10% formaldehyde solution, decalcified in 10% ethylenediaminetetraacetic acid solution, paraffin-embedded and sectioned.
The right femoral head was scanned with micro-computed tomography (CT) to observe its morphology. The sections were placed in a 55°C oven for 30 min, infiltrated in xylene for 10 min and refreshed xylene for another 10 min, rinsed three times with phosphate-buffered saline (PBS) (five min each time), hydrated with absolute ethanol and gradient concentrations of ethanol solutions rinsed three times with PBS (five min each time) and stained with hematoxylin for 10 min. After excess staining solution was washed off with clean water for 10 min, the sections were washed with distilled water for 10 sec, discolored and differentiated with 1% hydrochloric acid-ethanol for 5 sec, stained with eosin for about one min, rinsed three times with PBS (five min each time) and then dehydrated with gradient concentrations of ethanol solutions. After dehydration was completed, the sections were transparentized with xylene for five min and mounted with neutral resin to observe the morphology of the femoral head under an optical microscope. Five fields of view were selected under high magnification, and 50 bone lacunae were counted in each field to calculate the percentage of empty lacunae, which was used to indicate the overall apoptosis rate. Percentage of empty lacunae = number of empty lacunae/number of bone lacunae × 100%.
The relative growth rate of MC3T3-E1 cells was detected by CCK8 assay. After passage in α-MEM to the third generation, the cells were counted, diluted and seeded into a 96-well plate at a density of 5×104/well. After cell adherence, the culture medium was discarded. Then the cells were added α-MEM containing dexamethasone at 0 mM (control), 10-8 M, 10-6 M or 10-4 M, and incubated for 24 h. Afterwards, 10 μL of CCK8 solution was added to each well, and the plate was placed in an incubator at 37°C for another one h. The 96-well plate was taken out from the incubator, and the optical density (OD) was measured by a microplate reader at 450 nm to calculate the relative growth rate. Relative cell growth rate= (ODexperimental group - ODmedium)/(ODcontrol group - ODmedium).
According to the optimal pre-experimental results (dexamethasone concentration: 10-6 M; incubation time: 24 h), the third-generation MC3T3-E1 cells were randomly divided into four groups, and cultured with α-MEM (normal group), α-MEM containing 10-6 M dexamethasone (dexamethasone group), α-MEM containing 100 nM rapamycin (rapamycin group), and α-MEM containing 10-6 M dexamethasone and 100 nM rapamycin (rapamycin-dexamethasone group) for 24 h.
MC3T3-E1 cells were lysed with 200 μL of pre- cooled RIPA lysis buffer, and centrifuged at 4°C and 12,000 rpm for five min to collect the supernatant. Total protein concentration was measured by the BCA method. Subsequently, proteins were separated by 5% SDS-PAGE, and the products were electronically transferred onto a PVDF membrane. The membrane was thereafter washed three times with Tris buffered saline with Tween 20 (TBST) (10 min each time), blocked in the blocking solution at room temperature for one h, incubated with primary antibodies against LC3B, mTOR and Beclin-1 (1:300 diluted) and β-actin (1:1,000 diluted) overnight at 4°C, washed, incubated with secondary antibodies against at room temperature in dark for 60 min, and washed by TBST. Afterwards, the membrane was developed by using ECL reagent, and the gray values of protein bands were analyzed by Image Lab (Image Lab LLC, Sarasota, FL, USA) software.
Statistical analysis
All data were statistically analyzed by IBM SPSS version 20.0 software (IBM Corp., Armonk, NY, USA). Comparisons among different groups were performed by one-way analysis of variance, and pairwise intergroup comparisons were conducted with the least significant difference (LSD)-t test. P<0.05 was considered statistically significant.
Results
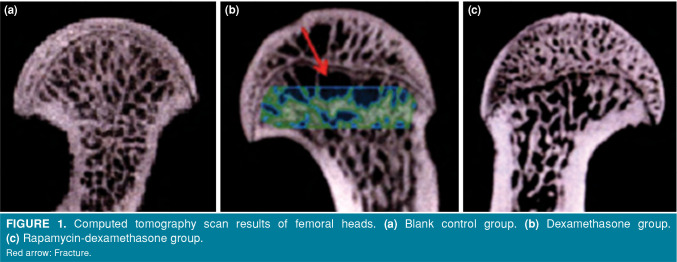
The CT scan showed that in the blank control group, the trabeculae of the femoral head were in good shape and evenly distributed, without the formation of subchondral necrosis zone (Figure 1a). In the dexamethasone group, the subchondral necrosis zone of the femoral head was formed, the trabeculae were occluded, and that in the medullary cavity was thin, sparse, fractured, and the cortical bone was thickened (Figure 1b). In the rapamycin-dexamethasone group, the CT imaging findings of the femoral head were between the dexamethasone group and the blank control group. The fracture and atrophy of the trabeculae in the medullary cavity and the partial necrosis of the subchondral bone were observed (Figure 1c).
Figure 1. Computed tomography scan results of femoral heads. (a) Blank control group. (b) Dexamethasone group. (c) Rapamycin-dexamethasone group. Red arrow: Fracture.
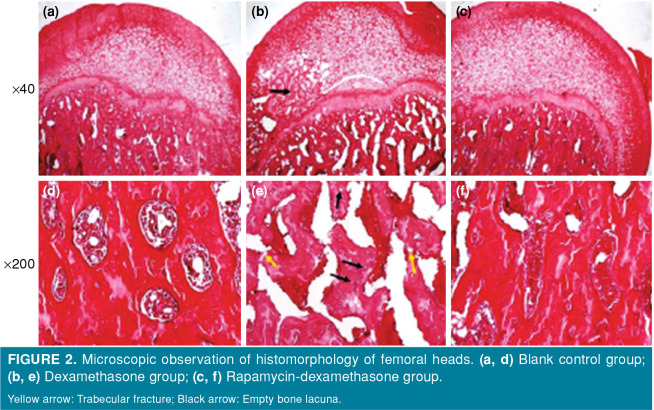
Microscopy showed that the trabeculae of the femoral head in the blank control group were in good shape, without empty bone lacunae, adipocyte and lipid deposition, or tissue necrosis and fibrosis (Figure 2a and d). The trabeculae of the femoral head in the dexamethasone group were sparse and disordered, the number of adipocytes increased, the lipid deposited in the bone cells, and a large number of empty bone lacunae were formed, with local tissue necrosis, fibrosis and decreased cell number (Figure 2b and e). In the rapamycin dexamethasone group, some of the trabeculae of the femoral head were fractured and atrophied, and a small amount of empty bone lacunae were observed (Figure 2c and f).
Figure 2. Microscopic observation of histomorphology of femoral heads. (a, d) Blank control group; (b, e) Dexamethasone group; (c, f) Rapamycin-dexamethasone group. Yellow arrow: Trabecular fracture; Black arrow: Empty bone lacuna.
The total apoptosis rates of the dexamethasone group (64.0±4.4%) and the rapamycin-dexamethasone group (33.3±3.3%) were significantly higher than that of the blank control group (6.3±0.8%) (p<0.001), and the former two groups also had significantly different rates (p<0.001) (Table I).
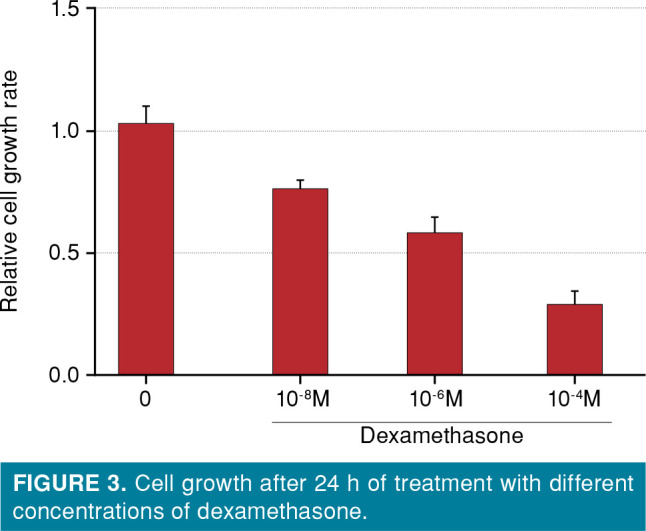
The growth rate of MC3T3-E1 cells untreated with dexamethasone exceeded those of the cells treated with different concentrations of dexamethasone for 24 h (p<0.001) (Figure 3).
Figure 3. Cell growth after 24 h of treatment with different concentrations of dexamethasone.
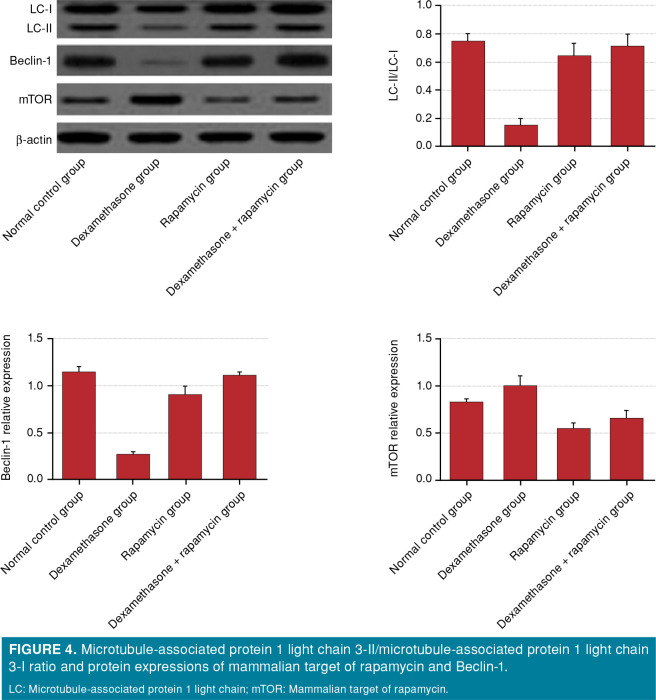
The LC3-II/LC3-I ratio of the normal group was higher than that of the dexamethasone group (p<0.001), lower than that of the rapamycin group (p<0.001), and similar to that of the dexamethasone- rapamycin group (p>0.05). LC3-II/LC3-I of the dexamethasone group was lower than those of rapamycin and dexamethasone-rapamycin groups (p<0.001). The rapamycin group had higher LC3-II/ LC3-I than that of the dexamethasone-rapamycin group (p<0.001). The mTOR protein expression of the normal group was lower than that of the dexamethasone group (p<0.05), and higher than those of rapamycin and dexamethasone-rapamycin groups (p<0.001). The dexamethasone group had higher mTOR protein expression than those of rapamycin and dexamethasone-rapamycin groups (p<0.001). The mTOR protein expression of the rapamycin group was lower than that of the dexamethasone- rapamycin group (p<0.001). The Beclin-1 protein expression of the normal group was higher than that of the dexamethasone group, but similar to those of rapamycin and dexamethasone-rapamycin groups (p>0.05). The Beclin-1 protein expression of the dexamethasone group was lower than those of rapamycin and dexamethasone-rapamycin groups (p<0.001). The rapamycin group had higher Beclin-1 protein expression than that of the dexamethasone- rapamycin group (p<0.05) (Figure 4).
Figure 4. Microtubule-associated protein 1 light chain 3-II/microtubule-associated protein 1 light chain 3-I ratio and protein expressions of mammalian target of rapamycin and Beclin-1. LC: Microtubule-associated protein 1 light chain; mTOR: Mammalian target of rapamycin.
Discussion
The pathogenesis of FHN is the interruption or damage of blood supply to the femoral head, leading to apoptosis of bone cells.[18] At present, there are many theories on the pathogenesis of hormonal FHN, such as osteoporosis, intraosseous hypertension, intravascular coagulation, osteotoxin effect, osteocyte apoptosis, and fat metabolism disorder,[1-3] but its exact pathogenesis remains unclear.
Autophagy is an important protective mechanism of cartilage itself, and the reduction of autophagy is considered to be one of the important causes of articular cartilage degeneration. Zhang et al.[19] found that mesenchymal stem cells can promote their survival by increasing the level of autophagy in hypoxia and serum-free conditions. Besides, Gao et al.[20] reported that autophagy also played a key protective role in the process of hormone- induced degeneration and apoptosis of bone cells by in vitro bone cell culture, and the number of bone cell death after autophagy significantly increased. In this study, a steroid-induced FHN model was established by intraperitoneal injection of 2.5 mg•kg-1 dexamethasone sodium phosphate. After the model was established, the femoral head tissue obtained was stained with HE, and the morphology of the femoral head was observed under a microscope. The results showed that under the intervention of dexamethasone, the adipocytes in femoral head of the mice increased, the lipid deposited in the bone cells, the trabeculae of the femoral head were sparse and disordered, and the number of empty bone lacunae increased in the dexamethasone group, which was more than that of the blank control group and the dexamethasone and rapamycin group. According to the results of micro- CT, in the mouse hormone FHN model constructed by dexamethasone alone, subchondral necrosis zone of femoral head was formed, trabeculae were occluded, trabeculae in medullary cavity became thin, sparse and fractured in the medullary cavity, and thickened bone cortex appeared. However, after intraperitoneal injection of rapamycin, the femoral head was in good shape, some trabeculae were fractured and atrophied, and the subchondral necrosis zone was significantly less than that of the dexamethasone-constructed hormonal FHN mice.
In vitro, Western blot was used to detect the expression of autophagy-related proteins mTOR, Beclin-1 and LC3 in MC3T3-E1 cells. The results showed that the ratio of LC3-II/LC3-I and the content of Beclin-1 protein in MC3T3-E1 cells of the dexamethasone group were significantly lower than those of other groups, while the expression of mTOR protein was significantly higher than that of other groups. The ratio of LC3-II/LC3-I in MC3T3-E1 cells in the dexamethasone-rapamycin group was significantly higher than that of the dexamethasone group, and the expression of mTOR protein was lower than that of the dexamethasone group. Therefore, dexamethasone stimulation alone can reduce the level of autophagy, while rapamycin can antagonize the inhibition of autophagy. Li et al.[21] explored the mechanism underlying dexamethasone-induced osteoblast injury, and found that 24 h of treatment with 1 and 10 μmol/L dexamethasone significantly up-regulated Beclin-1 and LC3 expressions (p<0.05). The study of Li et al.[21] showed that dexamethasone significantly promoted autophagy, and that overactivated autophagy may be a crucial factor contributing to SANFH. In contrast, we herein found that dexamethasone suppressed autophagy, which may be one of the causes of FHN. The inconsistent results may be attributed to different dexamethasone doses. Meanwhile, cell autophagy may be a double- edged sword. In other words, either overactivated or decreased autophagy has a negative effect, finally inducing hormonal FHN.
Moreover, Liao et al.[22] reported that compared with the control group, the LC3-II/LC3-I ratio of dexamethasone group gradually decreased from 12 h (p<0.01) to 24 h (p<0.05), reaching the minimum at 48 h. They verified that autophagy was inhibited by dexamethasone treatment time-dependently. Furthermore, Luo et al.[23] reviewed the role of autophagy in steroid-induced FHN. They concluded that autophagy was a self-protection mechanism for FHN, and autophagy and apoptosis participated in the pathogenesis of steroid-induced FHN, with the level of autophagy associated with the dose of hormone.
The LC3 is a recognized autophagy marker. When autophagy is formed, cytosolic LC3 (i.e. LC3-I) can hydrolyze a small segment of polypeptide and transform into an autophagosome model (i.e. LC3-II). The LC3-II/LC3-I ratio can be used to estimate the level of autophagy.[24] The mTOR complex and Beclin-1 complex are key molecules regulating autophagy in cells, which play a crucial role in the development of autophagy.[25,26] It is generally accepted that mTOR plays a regulatory role in autophagy through two mechanisms: (i) mTOR-mediated signal transduction acts on downstream effectors, such as transcription initiation factor 4E binding protein 1 and ribosomal protein S6 kinase, and initiates related gene transcription and translation to control autophagy; (ii) mTOR kinase acts directly on Atg protein to regulate autophagosome formation.[27,28] Autophagy is promoted when mTOR activity is inhibited, and suppressed when mTOR activity is increased. Beclin-1, also known as BECN1, is a homolog of the yeast autophagy protein 6 (ATG6) gene and a specific gene of mammals involved in autophagy. Beclin-1 gene regulates the localization of other Atg proteins in the structure of autophagy precursor and regulates autophagy activity by forming complex with type III PI3K.[29,30] It is well established that up-regulation of Beclin-1 expression in mammalian cells can stimulate the occurrence of autophagy, but it cannot happen after knockings out this gene.[25,28]
Rapamycin used in this study is a classical autophagy enhancer, and the regulation pathway of autophagy mainly involves the classical PI3K/protein kinase B/rapamycin target protein signaling pathway. However, rapamycin can induce and promote autophagy by inhibiting mTOR, and the influence of many factors on autophagy is related to the change of mTOR activity.[31,32] Herein, after rapamycin enhanced autophagy level, the apoptosis level and cell growth inhibition rate decreased significantly. It indicates that autophagy can inhibit the death of MC3T3-E1 cells stimulated by dexamethasone to a certain extent, and autophagy plays a protective role on osteoblasts (MC3T3-E1), which will provide a new idea for the treatment of hormonal FHN.
The number of used animals is limited. Besides, the findings must be further verified by clinical studies.
In conclusion, autophagy plays a crucial protective role in the pathogenesis of hormonal FHN. The decrease of autophagy level induced by dexamethasone may be one of the causes of FHN, and its mechanism may be related to the expression of mTOR and Beclin-1, which are the key regulators of autophagy stimulated by dexamethasone. In the future, the improvement of autophagy level can be an important target for the prevention or treatment of hormonal FHN.
Footnotes
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Financial Disclosure: The authors received no financial support for the research and/or authorship of this article.
References
- 1.Haberal B, Şahin O, Şimşek EK, Mahmuti A, Tuncay İC. Outcomes for core decompression with multiple drilling of the osteonecrosis of the femoral head in patients with solid organ transplantation. Eklem Hastalik Cerrahisi. 2018;29:159–164. doi: 10.5606/ehc.2018.61348. [DOI] [PubMed] [Google Scholar]
- 2.Maurel DB, Boisseau N, Benhamou CL, Jaffre C. Alcohol and bone: review of dose effects and mechanisms. Osteoporos Int. 2012;23:1–16. doi: 10.1007/s00198-011-1787-7. [DOI] [PubMed] [Google Scholar]
- 3.Kang JS, Park S, Song JH, Jung YY, Cho MR, Rhyu KH. Prevalence of osteonecrosis of the femoral head: a nationwide epidemiologic analysis in Korea. J Arthroplasty. 2009;24:1178–1183. doi: 10.1016/j.arth.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 4.Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat Cell Biol. 2010;12:814–822. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kroemer G, Mariño G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu F, Fang F, Yuan H, Yang D, Chen Y, Williams L, et al. Suppression of autophagy by FIP200 deletion leads to osteopenia in mice through the inhibition of osteoblast terminal differentiation. J Bone Miner Res. 2013;28:2414–2430. doi: 10.1002/jbmr.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L, Guo YF, Liu YZ, Liu YJ, Xiong DH, Liu XG, et al. Pathway-based genome-wide association analysis identified the importance of regulation-of-autophagy pathway for ultradistal radius BMD. J Bone Miner Res. 2010;25:1572–1580. doi: 10.1002/jbmr.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manolagas SC, Parfitt AM. What old means to bone. Trends Endocrinol Metab. 2010;21:369–374. doi: 10.1016/j.tem.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinstein RS, Wan C, Liu Q, Wang Y, Almeida M, O'Brien CA, et al. Endogenous glucocorticoids decrease skeletal angiogenesis, vascularity, hydration, and strength in aged mice. Aging Cell. 2010;9:147–161. doi: 10.1111/j.1474-9726.2009.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartolomé A, López-Herradón A, Portal-Núñez S, García-Aguilar A, Esbrit P, Benito M, Guillén C. Autophagy impairment aggravates the inhibitory effects of high glucose on osteoblast viability and function. Biochem J. 2013;455:329–337. doi: 10.1042/BJ20130562. [DOI] [PubMed] [Google Scholar]
- 11.Caramés B, Hasegawa A, Taniguchi N, Miyaki S, Blanco FJ, Lotz M. Autophagy activation by rapamycin reduces severity of experimental osteoarthritis. Ann Rheum Dis. 2012;71:575–581. doi: 10.1136/annrheumdis-2011-200557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lotz MK, Caramés B. Autophagy and cartilage homeostasis mechanisms in joint health, aging and OA. Nat Rev Rheumatol. 2011;7:579–587. doi: 10.1038/nrrheum.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasaki H, Takayama K, Matsushita T, Ishida K, Kubo S, Matsumoto T, et al. Autophagy modulates osteoarthritis- related gene expression in human chondrocytes. Arthritis Rheum. 2012;64:1920–1928. doi: 10.1002/art.34323. [DOI] [PubMed] [Google Scholar]
- 14.Yang RT, Zhang C, Liu Y, Zhou HH, Li ZB. Autophagy prior to chondrocyte cell death during the degeneration of Meckel's cartilage. Anat Rec (Hoboken) 2012;295:734–741. doi: 10.1002/ar.22433. [DOI] [PubMed] [Google Scholar]
- 15.Luo S, Rubinsztein DC. Apoptosis blocks Beclin 1-dependent autophagosome synthesis: an effect rescued by Bcl-xL. Cell Death Differ. 2010;17:268–277. doi: 10.1038/cdd.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 2003;112:1809–1820. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang F, Shan S, Huo Y, Xie Z, Fang Y, Qi Z, et al. MiR- 155-5p inhibits PDK1 and promotes autophagy via the mTOR pathway in cervical cancer. Int J Biochem Cell Biol. 2018;99:91–99. doi: 10.1016/j.biocel.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Pouya F, Kerachian MA. Avascular Necrosis of the Femoral Head: Are Any Genes Involved. Arch Bone Jt Surg. 2015;3:149–155. [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Q, Yang YJ, Wang H, Dong QT, Wang TJ, Qian HY, et al. Autophagy activation: a novel mechanism of atorvastatin to protect mesenchymal stem cells from hypoxia and serum deprivation via AMP-activated protein kinase/mammalian target of rapamycin pathway. Stem Cells Dev. 2012;21:1321–1332. doi: 10.1089/scd.2011.0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao J, Cheng TS, Qin A, Pavlos NJ, Wang T, Song K, et al. Glucocorticoid impairs cell-cell communication by autophagy-mediated degradation of connexin 43 in osteocytes. Oncotarget. 2016;7:26966–26978. doi: 10.18632/oncotarget.9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Li YS, Li LJ, Xie X, Yang Y, Deng ZH, et al. Overactivated autophagy contributes to steroid-induced avascular necrosis of the femoral head. Exp Ther Med. 2017;14:367–372. doi: 10.3892/etm.2017.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao Y, Zhang P, Yuan B, Li L, Bao S. Pravastatin Protects Against Avascular Necrosis of Femoral Head via Autophagy. Front Physiol. 2018;9:307–307. doi: 10.3389/fphys.2018.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo P, Gao F, Han J, Sun W, Li Z. The role of autophagy in steroid necrosis of the femoral head: a comprehensive research review. Int Orthop. 2018;42:1747–1753. doi: 10.1007/s00264-018-3994-8. [DOI] [PubMed] [Google Scholar]
- 24.Fritzen AM, Frøsig C, Jeppesen J, Jensen TE, Lundsgaard AM, Serup AK, et al. Role of AMPK in regulation of LC3 lipidation as a marker of autophagy in skeletal muscle. Cell Signal. 2016;28:663–674. doi: 10.1016/j.cellsig.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Pattingre S, Espert L, Biard-Piechaczyk M, Codogno P. Regulation of macroautophagy by mTOR and Beclin 1 complexes. Biochimie. 2008;90:313–323. doi: 10.1016/j.biochi.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 26.Eskelinen EL. New insights into the mechanisms of macroautophagy in mammalian cells. Int Rev Cell Mol Biol. 2008;266:207–247. doi: 10.1016/S1937-6448(07)66005-5. [DOI] [PubMed] [Google Scholar]
- 27.Scott RC, Schuldiner O, Neufeld TP. Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev Cell. 2004;7:167–178. doi: 10.1016/j.devcel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Rosenfeldt MT, Ryan KM. The role of autophagy in tumour development and cancer therapy. e36Expert Rev Mol Med. 2009;11 doi: 10.1017/S1462399409001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scarlatti F, Maffei R, Beau I, Codogno P, Ghidoni R. Role of non-canonical Beclin 1-independent autophagy in cell death induced by resveratrol in human breast cancer cells. Cell Death Differ. 2008;15:1318–1329. doi: 10.1038/cdd.2008.51. [DOI] [PubMed] [Google Scholar]
- 30.Gaytán M, Morales C, Sánchez-Criado JE, Gaytán F. Immunolocalization of beclin 1, a bcl-2-binding, autophagy-related protein, in the human ovary: possible relation to life span of corpus luteum. Cell Tissue Res. 2008;331:509–517. doi: 10.1007/s00441-007-0531-2. [DOI] [PubMed] [Google Scholar]
- 31.Korolchuk VI, Saiki S, Lichtenberg M, Siddiqi FH, Roberts EA, Imarisio S, et al. Lysosomal positioning coordinates cellular nutrient responses. Nat Cell Biol. 2011;13:453–460. doi: 10.1038/ncb2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poüs C, Codogno P. Lysosome positioning coordinates mTORC1 activity and autophagy. Nat Cell Biol. 2011;13:342–344. doi: 10.1038/ncb0411-342. [DOI] [PubMed] [Google Scholar]