Abstract
Using data from the Multi-Ethnic Study of Atherosclerosis (United States, 2000–2015), 6,527 racially/ethnically diverse adults (mean age, 62 (standard deviation, 10) years) free of known cardiovascular (CVD) had ankle brachial index (ABI) assessment of their bilateral dorsalis pedis/posterior tibial arteries (4 vessels total) and were followed for total mortality and incident CVD events/mortality. Individuals were classified into categories of 0-, 1-, 2-, 3- or 4-vessel peripheral artery disease (PAD) (ABI of ≤0.9). There were 1,202 deaths (18%), 656 incident CVD events (10%), and 282 CVD deaths (4.3%). Of the 6,527 individuals, 5,711 (87.5%) had 0-, 460 (7.0%) had 1-, 218 (3.3%) had 2-, 69 (1.1%) had 3-, and 69 (1.1%) had 4-vessel PAD, respectively. In multivariable Cox models, higher number of vessels with PAD was associated with higher risk of mortality (P for trend <0.001), CVD events (P for trend = 0.002), and CVD mortality (P for trend = 0.001). Compared with individuals who had 0-vessel disease, hazard ratios for mortality were 1.29 (95% confidence interval (CI): 1.06, 1.59) for 1-, 1.45 (95% CI: 1.14, 1.86) for 2-, 1.58 (95% CI: 1.13, 2.21) for 3-, and 2.15 (95% CI: 1.58, 2.94) for 4-vessel disease. A similar pattern was seen for CVD events/mortality. These results suggest the importance of accounting for ABI values of all 4 leg arteries in clinical practice and research.
Keywords: ankle brachial index, claudication, multivessel, primary prevention, screening
Abbreviations
- ABI
ankle brachial index
- CI
confidence interval
- CVD
cardiovascular disease
- DP
dorsalis pedis artery
- MESA
Multi-Ethnic Study of Atherosclerosis
- PAD
peripheral artery disease
- PT
posterior tibial artery
The ankle brachial index (ABI), a ratio of systolic blood pressure in the ankles to the arms, is a marker of lower extremity peripheral artery disease (PAD). Importantly, low ABI values are associated with increased risk for cardiovascular disease (CVD) events and total mortality in multiple populations (1, 2). Furthermore, ABI is predictive of mortality in individuals with asymptomatic PAD (3). However, the ABI calculation method has not been standardized across studies.
Current clinical guidelines recommend measuring bilateral brachial, dorsalis pedis (DP), and posterior tibial (PT) arteries and using the higher of the DP/PT in each leg for the numerator calculation of the ABI (4, 5). This yields 2 ABI values per individual, and most historical analyses used the lower of these 2 values to define each individual’s unique ABI value. While the use of this value is predictive of outcomes, it might not accurately characterize an individual’s burden of PAD. For example, an individual might have disease involvement ranging from 0 to 4 of the measured vessels or disease that is confined to one or both legs. Individuals with multivessel or bilateral lower extremity PAD might have additional risk of mortality and CVD events compared with those who have single vessel or unilateral disease.
Using a multiracial/ethnic cohort free of known CVD, this investigation sought to assess the disease burden of lower extremity PAD on total mortality, incident CVD events, and CVD mortality using the noninvasive ABI in a primary prevention population. We examined associations of different methods of categorizing lower extremity PAD burden with total mortality, hard CVD events, and CVD mortality. We hypothesized that higher number of vessels with low ABI would be associated with higher risk of total mortality, CVD events, and CVD mortality. Additionally, we hypothesized that bilateral PAD would be associated with higher risk of total mortality, CVD events, and CVD mortality compared with unilateral PAD and no disease.
METHODS
Participants
We used data from the Multi-Ethnic Study of Atherosclerosis (MESA). Details of its study design and protocol have been published (6). In brief, between July 2000 and August 2002, 6,814 non-Hispanic White, non-Hispanic Black, Hispanic, or Chinese men and women 45–84 years of age and free of clinically apparent CVD were recruited from 6 communities in the United States and participated in the baseline examination.
Data collection
At the baseline examination, standardized questionnaires were used to obtain demographic information, smoking history, alcohol use, cancer history, and medication use for high blood pressure, high cholesterol, and diabetes. Cancer history was obtained by asking participants to answer yes or no to the question, “Has a doctor ever told you that you had cancer?” Participants could further indicate whether they had prostate, breast, lung, colon, nonmelanoma skin, blood (leukemia, lymphoma, other), or other cancer. Cigarette smoking was calculated in pack-years and also defined as current, former, or never. Alcohol use was defined as current, former, or never. Resting systolic blood pressures were measured in seated position. Height was measured in centimeters. Physical activity was calculated (in metabolic equivalent–minutes/week) using a detailed questionnaire adapted from the Cross-Cultural Activity Participation Study (7). Leg pain with walking, a potential symptom of PAD, was assessed with the San Diego Claudication Questionnaire (8).
Laboratory
Total cholesterol, high-density lipoprotein cholesterol, glucose, and serum creatinine levels were measured from blood samples obtained after a 12-hour fast. Low-density lipoprotein cholesterol was calculated by the Friedewald equation (9). Diabetes categories were defined as treated diabetes (use of hypoglycemic medication), untreated diabetes (glucose of ≥126 mg/dL and no use of hypoglycemic medication), impaired fasting glucose (glucose values 100–125 mg/dL), and normal (10). Glomerular filtration rate was estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (11).
ABI protocol
Systolic blood pressure measurements in the bilateral brachial, posterior tibial (PT), and dorsalis pedis (DP) arteries were obtained in the supine position using a hand-held Doppler instrument with a 5-mHz probe. The leg cuff was inflated to a maximum of 300 mm Hg, and if a pulse was still detected at this level, the ABI was classified as “incompressible.” A subset of 384 MESA participants had replicate ABI measurements that showed excellent reproducibility (intraclass correlation coefficient = 0.93) (12). To avoid potential bias from subclavian stenosis, the higher of the brachial artery pressures was used as the denominator in these analyses (13). Vessel-specific ABIs were calculated for each of the 2 vessels (PT/DP) in each leg by taking the vessel pressure and dividing by the higher brachial artery pressure. Leg-specific ABIs were calculated by taking the higher of DP/PT systolic pressure in that leg and dividing by the higher brachial artery pressure, according to guidelines recommendations (see Figure 1 for all ABI calculations) (4, 5). The traditional ABI was calculated by taking the lower of the left or right leg ABI value. A vessel or leg was considered to have PAD if the vessel or leg-specific ABI was ≤0.9.
Figure 1.
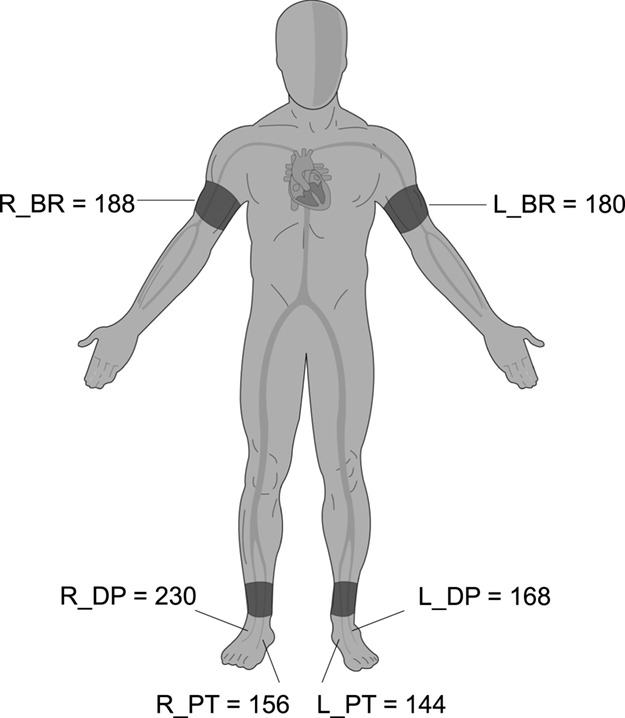
Vessel and leg-specific ankle brachial index (ABI) calculations, Multi-Ethnic Study of Atherosclerosis (MESA), United States, 2000–2015. This figure depicts the calculations for all the ABI values for a MESA participant. Each vessel-specific ABI value is calculated by taking the systolic pressure in that vessel and dividing by the higher of the 2 brachial systolic pressures. Leg-specific ABI values were calculated, according to clinical guideline recommendations (4, 5), by taking the higher of dorsalis pedis/posterior tibial systolic pressures in that leg and dividing by the higher of the 2 brachial systolic pressures. Traditional ABI is calculated by taking the lower of the 2 leg ABI values. For the scoring system in this analysis: right dorsalis pedis artery (R_DP) ABI: 230/188 = 1.22; right posterior tibial artery (R_PT) ABI: 156/188 = 0.83; left dorsalis pedis artery (L_DP) ABI: 168/188 = 0.89; left posterior tibial artery (L_PT) ABI: 144/188 = 0.79; right leg ABI: 230/188 = 1.22; left leg ABI: 168/188 = 0.89; and traditional ABI = 0.89. This participant has 3-vessel peripheral artery disease (PAD), defined by 3 vessels with an ABI of ≤0.9, and unilateral PAD, defined by 1 leg with an ABI of ≤0.9. R_BR, right brachial artery; L_BR, left brachial artery.
To score the burden of PAD, individuals were classified as having 0-, 1-, 2-, 3-, or 4-vessel PAD based on the number of ankle arteries with an ABI of ≤0.9. Furthermore, individuals were classified as having unilateral PAD, bilateral PAD, or none based on number of legs with an ABI of ≤0.9.
Mortality, CVD events, and CVD mortality
Follow-up began at the time of the baseline examination and continued until the first CVD event, death, loss to follow-up, or a follow-up call, ending in calendar year 2015. Details of CVD event ascertainment in MESA have been published (14). For this analysis, we considered hard CVD events, defined as myocardial infarction, resuscitated cardiac arrest, coronary death, stroke, and stroke death. CVD mortality consisted of death due to any atherosclerotic disease, stroke, and other CVD (such as arrhythmia, congestive heart failure, and periprocedural CVD complications).
Statistical analysis
Of the 6,814 individuals, we excluded 138 missing at least 1 ankle blood pressure (to avoid misclassification of complete vessel occlusion versus congenitally absent pulse), 36 missing both arm pressures, 92 with any ABI of ≥1.4 (to avoid PAD masked by stiff arteries), 5 who were found after the fact to have had a CVD event prior to baseline ABI assessment, and 16 missing mortality information, leaving a final analytical sample size of 6,527. Descriptive characteristics across number of vessels with PAD were computed by analysis of variance for continuous variables and χ2 for categorical variables.
We used multivariable Cox proportional hazards models to assess associations of burden of PAD with mortality and CVD event endpoints. We performed tests for the proportional hazards assumption across measures of PAD burden by evaluating interactions of PAD burden and time; none were significant. In order to examine the independence of PAD burden with increasing levels of adjustment, 3 separate Cox models were constructed for mortality, CVD events, and CVD mortality using complete-case analysis. To assess the magnitude of PAD disease burden for an individual presenting to a clinical visit for the first time, model 1 adjusted for age, sex, race/ethnicity, and height. To further assess the independence of PAD burden on mortality and CVD events, model 2 adjusted for traditional CVD and mortality risk factors: systolic blood pressure, antihypertensive medications, total cholesterol, high-density lipoprotein cholesterol, statin use, diabetes, smoking status, cigarette pack-years, estimated glomerular filtration rate, physical activity, alcohol use, and cancer history in addition to variables in model 1. Finally, model 3 added the traditional ABI value to model 2 to assess whether the PAD burden score offers incremental risk information independent of the traditionally used continuous ABI value. Modeling was performed separately for mortality, CVD events, and CVD mortality. The population attributable fraction for 4-vessel disease and mortality (model 2) was calculated using the formula by Miettinen (15). To assess the utility of the 4-vessel disease score across different ages, sexes, and races/ethnicities, we performed exploratory stratified analyses (model 2) to assess the hazard ratios associated with a 1-vessel increase in PAD on mortality by treating vessel score as a continuous variable.
Sensitivity analyses
We performed a series of sensitivity analyses. First, because most individuals with PAD are asymptomatic (16), they will not undergo routine evaluation with ABI in primary care settings (17). To mimic this situation, we refitted models 1–3 above after excluding individuals who reported leg pain with walking, a common symptom of PAD, to represent an asymptomatic population. Second, because having an ABI of >1.4 is associated with higher risk of CVD events and mortality (2), we repeated analyses including individuals with stiff vessels by scoring vessels with an ABI of >1.4 as having PAD. Third, because those with missing vessel pressure readings might have an occluded vessel (vs. congenitally absent or atrophic), we performed an analysis by scoring missing vessels as having PAD. Fourth, different cancers are likely differentially associated with PAD risk factors (i.e., smoking) and mortality. To evaluate for potential residual confounding of our cancer history (yes/no) variable, we refitted models by including the 7 possible cancer types as individual covariates. Finally, we tested the socioeconomic variables of education and income for the presence of additional statistical confounding. A 2-tailed P value of <0.05 was considered statistically significant for all analyses, including interaction terms. All data analysis was performed using SAS, version 9.4 (SAS Institute, Cary, North Carolina).
RESULTS
Over a mean follow-up time of 13.0 years, there were 1,202 (18.4%) deaths, of which 282 (4.3%) were CVD-related. With a mean follow-up time of 12.1 years, there were 656 (10.1%) incident CVD events. Of the participants, 5,711 (87.5%) had 0-vessel, 460 (7.0%) had 1-vessel, 218 (3.3%) had 2-vessel, 69 (1.1%) had 3-vessel, and 69 (1.1%) had 4-vessel PAD. Of those with 2-vessel disease, 114 (52.2%) had a traditional ABI of >0.9 in both legs. When examining laterality, 6,285 (96.3%) had no legs with PAD, 173 (2.7%) had unilateral PAD, and 69 (1.1%) had bilateral PAD.
Table 1 describes baseline characteristics across categories of number of vessels with PAD. When compared with those who did not have PAD, as number of vessels increased, individuals with PAD were older, were more likely to be Black, were less likely to be Hispanic, had higher blood pressure, had lower HDL cholesterol, had higher prevalence of diabetes, had lower estimated glomerular filtration rate, had higher cigarette smoking burden, had lower physical activity, and were more likely to have leg pain with walking.
Table 1.
Baseline Sociodemographic and Health-Related Characteristics According to Number of Vessels With Peripheral Artery Disease (n = 6,527), Multi-Ethnic Study of Atherosclerosis, United States, 2000–2002
No. of Vessels With ABI of  0.9
a 0.9
a
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||||||||||||
(n  5,711) 5,711)
|
(n  460) 460)
|
(n  218) 218)
|
(n  69) 69)
|
(n  69) 69)
|
||||||||||||
| Characteristic | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | P Value b |
| Age, years | 61.43 (10.09) | 63.94 (10.24) | 66.07 (10.18) | 70.61 (9.01) | 72.10 (8.18) | <0.001 | ||||||||||
| Sex | <0.001 | |||||||||||||||
| Female | 2,946 | 51.6 | 307 | 66.7 | 138 | 63.3 | 42 | 60.9 | 37 | 53.6 | ||||||
| Male | 2,765 | 48.4 | 153 | 33.3 | 80 | 36.7 | 27 | 39.1 | 32 | 46.4 | ||||||
| Race/ethnicity | <0.001 | |||||||||||||||
| Non-Hispanic White | 2,165 | 37.9 | 195 | 42.4 | 85 | 39.0 | 24 | 34.8 | 19 | 27.5 | ||||||
| Non-Hispanic Black | 1,459 | 25.5 | 187 | 40.7 | 96 | 44.0 | 29 | 42.0 | 35 | 50.7 | ||||||
| Hispanic | 1,347 | 23.6 | 44 | 9.6 | 27 | 12.4 | 9 | 13.0 | 10 | 14.5 | ||||||
| Chinese American | 740 | 13.0 | 34 | 7.4 | 10 | 4.6 | 7 | 10.1 | 5 | 7.2 | ||||||
| Health Conditions and Behaviors | ||||||||||||||||
| Systolic BP, mm Hg | 125.50 (20.76) | 130.91 (22.35) | 133.35 (25.28) | 138.95 (27.85) | 146.22 (29.84) | <0.001 | ||||||||||
| Antihypertensive use | 1,785 | 31.3 | 199 | 43.3 | 98 | 45.0 | 36 | 52.2 | 43 | 62.3 | <0.001 | |||||
| Total cholesterol, mg/dL | 194.17 (35.59) | 195.09 (38.27) | 196.36 (33.37) | 195.71 (35.12) | 194.12 (41.03) | 0.89 | ||||||||||
| HDL cholesterol, mg/dL | 50.76 (14.66) | 52.58 (15.09) | 52.18 (15.64) | 53.97 (16.27) | 48.12 (13.37) | 0.01 | ||||||||||
| Statin use | 780 | 13.7 | 103 | 22.5 | 39 | 17.9 | 20 | 29.0 | 23 | 33.3 | <0.001 | |||||
| Diabetes | <0.001 | |||||||||||||||
| Normal | 4,247 | 74.7 | 346 | 75.5 | 147 | 67.4 | 38 | 55.1 | 36 | 52.2 | ||||||
| Impaired fasting glucose | 780 | 13.7 | 65 | 14.2 | 27 | 12.4 | 10 | 14.5 | 9 | 13.0 | ||||||
| Untreated diabetes | 147 | 2.6 | 5 | 1.1 | 10 | 4.6 | 4 | 5.8 | 2 | 2.9 | ||||||
| Treated diabetes | 515 | 9.1 | 42 | 9.2 | 34 | 15.6 | 17 | 24.6 | 22 | 31.9 | ||||||
| eGFR, mL/minute/1.73 m2 | 78.46 (15.88) | 75.14 (16.63) | 76.00 (17.88) | 70.47 (16.32) | 67.07 (21.67) | <0.001 | ||||||||||
| Cancer history | 400 | 7.0 | 44 | 9.6 | 17 | 7.8 | 11 | 15.9 | 6 | 8.7 | 0.02 | |||||
| Leg pain walking | 1,344 | 23.5 | 121 | 26.4 | 70 | 32.1 | 27 | 39.1 | 28 | 40.6 | <0.001 | |||||
| Height, cm | 166.52 (10.04) | 165.13 (9.88) | 164.67 (9.47) | 163.81 (10.62) | 164.76 (9.96) | <0.001 | ||||||||||
| Physical activity, MET-minutes/week | 5,864.64 (5,983.20) | 5,490.36 (5,725.08) | 4,353.13 (4,298.42) | 4,791.07 (4,940.83) | 4,898.41 (6,113.92) | 0.001 | ||||||||||
| Cigarette use | <0.001 | |||||||||||||||
| Never | 2,933 | 51.4 | 213 | 46.8 | 83 | 38.4 | 26 | 38.2 | 28 | 41.2 | ||||||
| Former | 2,061 | 36.2 | 171 | 37.6 | 92 | 42.6 | 25 | 36.8 | 26 | 38.2 | ||||||
| Current | 707 | 12.4 | 71 | 15.6 | 41 | 19.0 | 17 | 25.0 | 14 | 20.6 | ||||||
| Smoking, pack-years | 10.52 (20.12) | 12.16 (18.91) | 19.84 (29.30) | 21.27 (28.21) | 25.50 (34.56) | <0.001 | ||||||||||
| Alcohol use | <0.001 | |||||||||||||||
| Never | 1,175 | 20.7 | 99 | 21.8 | 38 | 17.8 | 16 | 23.5 | 16 | 23.9 | ||||||
| Former | 1,309 | 23.1 | 120 | 26.4 | 70 | 32.7 | 22 | 32.4 | 28 | 41.8 | ||||||
| Current | 3,194 | 56.3 | 235 | 51.8 | 106 | 49.5 | 30 | 44.1 | 23 | 34.3 | ||||||
| ABI Measures | ||||||||||||||||
| Traditional ABIc | 1.12 (0.08) | 1.02 (0.07) | 0.91 (0.13) | 0.80 (0.10) | 0.71 (0.12) | |||||||||||
| Left DP ABI | 1.10 (0.09) | 0.95 (0.11) | 0.88 (0.13) | 0.81 (0.13) | 0.71 (0.12) | |||||||||||
| Right DP ABI | 1.10 (0.09) | 0.95 (0.11) | 0.87 (0.13) | 0.83 (0.14) | 0.71 (0.13) | |||||||||||
| Left PT ABI | 1.13 (0.09) | 1.01 (0.10) | 0.95 (0.13) | 0.84 (0.13) | 0.74 (0.11) | |||||||||||
| Right PT ABI | 1.13 (0.09) | 1.02 (0.10) | 0.93 (0.12) | 0.84 (0.13) | 0.72 (0.12) | |||||||||||
Abbreviations: ABI, ankle brachial index; BP, blood pressure; DP, dorsalis pedis artery; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; MET, metabolic equivalent; PT, posterior tibial artery; SD, standard deviation.
a Each ABI calculated by taking systolic pressure in the lower leg artery and dividing by highest of the 2 brachial systolic pressures.
b P value is from χ2 test for categorical variables and from analysis of variance for continuous variables.
c Calculated by taking the higher of the systolic pressure in the leg’s DP/PT and dividing by highest brachial systolic pressure. The lower of the 2 legs’ values is used for the traditional ABI.
The correlations among the 4 measured vessel-specific ABIs are displayed in Table 2. All the ABI values were moderately correlated. The smallest correlation (r = 0.60) was between the left PT ABI and the right DP ABI, and the highest correlation (r = 0.75) was between the left PT ABI and right PT ABI.
Table 2.
Correlations Among the 4 Vessel-Specific and Traditional Ankle Brachial Indexes (n = 6,527), Multi-Ethnic Study of Atherosclerosis, United States, 2000–2002
| ABI a | Left PT | Right PT | Left DP | Right DP | Traditional ABI b |
|---|---|---|---|---|---|
| Left PT | 1 | 0.75 | 0.71 | 0.60 | 0.85 |
| Right PT | 1 | 0.64 | 0.72 | 0.88 | |
| Left DP | 1 | 0.68 | 0.80 | ||
| Right DP | 1 | 0.79 | |||
| Traditional ABIb | 1 |
Abbreviations: ABI, ankle brachial index; DP, dorsalis pedis artery; PT, posterior tibial artery.
a Each ABI calculated by taking systolic pressure in the lower leg artery and diving by highest of the 2 brachial systolic pressures. Values are Pearson correlation coefficients (range, –1 to 1).
b Traditional ABI calculated by taking the systolic pressure in the higher of the DP/PT artery divided by highest of 2 brachial systolic pressures and choosing the lower value of the left or right leg.
Associations of the number of vessels with PAD with total mortality, CVD events, and CVD mortality are described in Table 3. In model 1, increasing number of vessels with PAD was associated with higher risk of mortality, CVD events, and CVD mortality (P for trend < 0.001). For mortality, compared with 0-vessel disease, risk was significant starting at 1-vessel PAD, with a hazard ratio of 1.30, increasing to 2.93 at 4 vessels. For CVD events, compared with 0-vessel disease, risk was significant starting with 2-vessel PAD, with a hazard ratio of 2.02 and a 4-vessel hazard ratio of 3.07. For CVD mortality, compared with 0-vessel disease, risk was significant starting with 2-vessel PAD, with a hazard ratio of 2.21 and a 4-vessel hazard ratio of 3.14. Adjustment for confounders and traditional CVD risk factors modestly attenuated the associations, but the overall pattern for mortality (P for trend < 0.001), CVD events (P for trend = 0.002), and CVD mortality (P for trend = 0.001) risk remained for increasing number of vessels with PAD. In model 3, adjusting for the traditional ABI value, the overall trending of higher number of diseased vessels with PAD persisted for mortality (P = 0.016) but not for CVD events (P = 0.42) or CVD mortality (P = 0.46).
Table 3.
Associations of Number of Vessels Having Peripheral Artery Disease With Total Mortality, Incident Cardiovascular Disease Events, and Cardiovascular Disease Mortality (n = 6,527), Multi-Ethnic Study of Atherosclerosis, United States, 2000–2015
| Model 1 c | Model 2 d | Model 3 e | |||||||
|---|---|---|---|---|---|---|---|---|---|
Outcome and No. ofVessels With ABI of  0.9a 0.9a
|
No. of Participants | No. of Events | Rate b | HR | 95% CI | HR | 95% CI | HR | 95% CI |
| Mortality | |||||||||
| 0 | 5,711 | 931 | 12.4 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 1 | 460 | 110 | 19.0 | 1.30 | 1.06, 1.59 | 1.29 | 1.06, 1.59 | 1.20 | 0.97, 1.49 |
| 2 | 218 | 75 | 28.7 | 1.71 | 1.34, 2.17 | 1.45 | 1.14, 1.86 | 1.23 | 0.92, 1.65 |
| 3 | 69 | 38 | 48.5 | 2.03 | 1.46, 2.82 | 1.58 | 1.13, 2.21 | 1.27 | 0.86, 1.88 |
| 4 | 69 | 48 | 74.2 | 2.93 | 2.18, 3.95 | 2.15 | 1.58, 2.94 | 1.64 | 1.09, 2.45 |
| P for trendf | <0.001 | <0.001 | 0.016 | ||||||
| Hard CVDg | |||||||||
| 0 | 5,704 | 531 | 7.6 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 1 | 460 | 48 | 9.0 | 1.12 | 0.83, 1.52 | 1.01 | 0.74, 1.36 | 0.93 | 0.68, 1.27 |
| 2 | 217 | 43 | 18.4 | 2.02 | 1.47, 2.78 | 1.61 | 1.16, 2.24 | 1.31 | 0.88, 1.97 |
| 3 | 69 | 12 | 17.2 | 1.43 | 0.80, 2.55 | 0.97 | 0.54, 1.73 | 0.74 | 0.39, 1.43 |
| 4 | 69 | 22 | 39.6 | 3.07 | 1.98, 4.74 | 2.08 | 1.33, 3.24 | 1.51 | 0.85, 2.67 |
| P for trendf | <0.001 | 0.002 | 0.422 | ||||||
| CVD death | |||||||||
| 0 | 5,711 | 212 | 2.8 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 1 | 460 | 25 | 4.3 | 1.27 | 0.84, 1.94 | 1.19 | 0.77, 1.83 | 1.04 | 0.66, 1.64 |
| 2 | 218 | 23 | 8.8 | 2.21 | 1.42, 3.43 | 1.98 | 1.27, 3.09 | 1.45 | 0.82, 2.54 |
| 3 | 69 | 9 | 11.5 | 1.97 | 1.00, 3.88 | 1.54 | 0.77, 3.05 | 1.03 | 0.46, 2.31 |
| 4 | 69 | 13 | 20.1 | 3.14 | 1.77, 5.60 | 2.20 | 1.21, 4.01 | 1.36 | 0.62, 2.98 |
| P for trendf | <0.001 | 0.001 | 0.462 | ||||||
Abbreviations: ABI, ankle brachial index; CI, confidence interval; CVD, cardiovascular disease; HDL, high-density lipoprotein; HR, hazard ratio; MI, myocardial infarction.
a Vessel-specific ABI was calculated by taking the systolic pressure in that vessel divided by highest of 2 arm systolic pressures.
b Crude incidence rate per 1,000 person-years.
c Model 1 adjusts for age, sex, race/ethnicity, and height.
d Model 2 adjusts for model 1 + systolic blood pressure, antihypertensive medications, total cholesterol, HDL cholesterol, statin use, diabetes, smoking status, cigarette pack-years, kidney function, physical activity, alcohol use, and cancer history.
e Model 3 adjusts for model 2 + ABI.
f P for trend calculated by treating number of vessels with ABI of ≤0.9 as continuous variable in regression models.
g Included MI, resuscitated cardiac arrest, MI death, stroke, and stroke death.
Table 4 shows the associations of the number of legs with PAD with total mortality, CVD events, and CVD mortality. In the minimally adjusting model 1, the risk of mortality, CVD events, and CVD mortality increased in a dose-dependent manner from none to unilateral to bilateral PAD (P for trend < 0.001 for all). In mortality analyses, unilateral PAD was associated with a hazard ratio = 1.83, whereas bilateral PAD was associated with a hazard ratio of 2.80, compared with no legs with PAD. A similar pattern was seen for CVD events and CVD mortality analyses, such that unilateral PAD hazard ratios of 1.50 and 2.16 and bilateral PAD hazard ratio of 2.93 and 2.99 carried incrementally higher risk than no legs with PAD for CVD events and CVD mortality, respectively. In model 2, confounders and traditional CVD risk factors attenuated the effect size, but the overall pattern persisted for mortality (P for trend < 0.001), CVD events (P for trend = 0.008), and CVD mortality (P = 0.001). In model 3, addition of traditional ABI significantly attenuated results for all outcomes such that number of legs of PAD did not significantly offer more information for event prediction than the traditional ABI.
Table 4.
Associations of Number of Legs Having Peripheral Artery Disease With Total Mortality, Incident Cardiovascular Disease Events, and Cardiovascular Disease Mortality (n = 6,527), Multi-Ethnic Study of Atherosclerosis, United States, 2000–2015
| Model 1 c | Model 2 d | Model 3 e | |||||||
|---|---|---|---|---|---|---|---|---|---|
Outcome and No. of Legs With ABI of  0.9a 0.9a
|
No. of Participants | No. of Events | Rate b | HR | 95% CI | HR | 95% CI | HR | 95% CI |
| Mortality | |||||||||
| None | 6,285 | 1,071 | 13.0 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Unilateral | 173 | 83 | 41.7 | 1.83 | 1.46, 2.3 | 1.45 | 1.15, 1.83 | 1.06 | 0.78, 1.45 |
| Bilateral | 69 | 48 | 74.2 | 2.80 | 2.08, 3.76 | 2.04 | 1.50, 2.79 | 1.40 | 0.94, 2.08 |
| P for trendf | <0.001 | <0.001 | 0.125 | ||||||
| Hard CVDg | |||||||||
| None | 6,278 | 602 | 7.9 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Unilateral | 172 | 32 | 17.7 | 1.50 | 1.04, 2.15 | 1.09 | 0.75, 1.58 | 0.73 | 0.45, 1.17 |
| Bilateral | 69 | 22 | 39.6 | 2.93 | 1.89, 4.52 | 1.99 | 1.28, 3.11 | 1.23 | 0.70, 2.15 |
| P for trendf | <0.001 | 0.008 | 0.80 | ||||||
| CVD death | |||||||||
| None | 6,285 | 245 | 3.0 | 1.00 | 1.00 | Referent | 1.00 | Referent | 1.00 |
| Unilateral | 173 | 24 | 12.1 | 2.16 | 1.40, 3.32 | 1.78 | 1.15, 2.76 | 1.12 | 0.61, 2.07 |
| Bilateral | 69 | 13 | 20.1 | 2.99 | 1.69, 5.31 | 2.09 | 1.16, 3.79 | 1.21 | 0.56, 2.63 |
| P for trendf | <0.001 | 0.001 | 0.62 | ||||||
Abbreviations: ABI, ankle brachial index; CI, confidence interval; CVD, cardiovascular disease; DP, dorsalis pedis artery; HDL, high-density lipoprotein; HR, hazard ratio; MI, myocardial infarction; PT, posterior tibial artery.
a Leg-specific ABI was calculated using the traditional method by taking the systolic pressure in the higher of the DP/PT artery divided by highest of 2 brachial systolic pressures.
b Crude incidence rate per 1,000 person-years.
c Model 1 adjusts for age, sex, race/ethnicity, and height.
d Model 2 adjusts for Model 1 + systolic blood pressure, antihypertensive medications, total cholesterol, HDL cholesterol, statin use, diabetes, smoking status, cigarette pack-years, kidney function, physical activity, alcohol use, and cancer history.
e Model 3 adjusts for Model 2 + ABI.
f P for trend calculated by treating number of vessels with ABI of ≤0.9 as continuous variable in regression models.
g Included MI, resuscitated cardiac arrest, MI death, stroke, and stroke death.
Table 5 presents associations of number of vessels with ABI of ≤0.9 among individuals who would not meet the threshold for PAD diagnosis based on a traditional ABI. In other words, individuals who have 2-vessel disease, but neither leg had a traditional ABI of ≤0.9, have 1.44, 2.15, and 1.75 times the risk of mortality, incident CVD events, and CVD mortality compared those with 0-vessel disease, respectively.
Table 5.
Associations of Number Vessels Having Peripheral Artery Disease With Total Mortality, Incident Cardiovascular Disease Events, and Cardiovascular Disease Mortality in Individuals Who Do Not Meet the Threshold of a Traditional Ankle Brachial Index of ≤0.9 in Either Leg, Multi-Ethnic Study of Atherosclerosis, United States, 2000–2015
| Model 2 c | |||||
|---|---|---|---|---|---|
Outcome and No. of Legs With ABI of 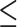 0.9a 0.9a
|
No. of Participants | No. of Events | Rate b | HR | 95% CI |
| Mortality | |||||
| 0 | 5,711 | 931 | 12.4 | 1.00 | Referent |
| 1 | 460 | 110 | 19.0 | 1.29 | 1.06, 1.59 |
| 2 | 114 | 30 | 21.3 | 1.44 | 0.99, 2.09 |
| Hard CVDd | |||||
| 0 | 5,704 | 531 | 7.6 | 1.00 | Referent |
| 1 | 460 | 48 | 9.0 | 1.01 | 0.74, 1.36 |
| 2 | 114 | 23 | 18.8 | 2.15 | 1.38, 3.34 |
| CVD death | |||||
| 0 | 5,711 | 212 | 2.8 | 1.00 | Referent |
| 1 | 460 | 25 | 4.3 | 1.19 | 0.77, 1.83 |
| 2 | 114 | 8 | 5.7 | 1.75 | 0.85, 3.58 |
Abbreviations: ABI, ankle brachial index; CI, confidence interval; CVD, cardiovascular disease; DP, dorsalis pedis artery; HDL, high-density lipoprotein; HR, hazard ratio; MI, myocardial infarction; PT, posterior tibial artery.
a Leg-specific ABI was calculated using the traditional method by taking the systolic pressure in the higher of the DP/PT artery divided by highest of 2 brachial systolic pressures.
b Crude incidence rate per 1,000 person-years.
c Model 2 adjusts for age, sex, race/ethnicity, height, systolic blood pressure, antihypertensive medications, total cholesterol, HDL cholesterol, statin use, diabetes, smoking status, cigarette pack-years, kidney function, physical activity, alcohol use, and cancer history.
d Included MI, resuscitated cardiac arrest, MI death, stroke, and stroke death.
The total population attributable fraction for mortality for at least 1 vessel with PAD was 7.3%. The population attributable fractions for 1-, 2-, 3- and 4-vessel disease were 2.1%, 1.9%, 1.2% and 2.1%, respectively.
In exploratory stratified analyses for mortality, the hazard ratios for each 1-vessel increase in PAD were 1.31 (95% confidence interval (CI): 1.08, 1.59) for individuals aged 62 years and younger, 1.21 (95% CI: 1.13, 1.29) in those older than 62 years, 1.23 (95% CI: 1.13, 1.34) in men, and 1.18 (95% CI: 1.08, 1.29) in women. For mortality according to race/ethnicity, the hazard ratios and 95% confidence intervals for each 1-vessel increase in PAD were 1.26 (95% CI: 1.13, 1.40) in non-Hispanic Whites, 1.36 (95% CI: 1.06, 1.75) in Chinese Americans, 1.12 (95% CI: 1.02, 1.23) in non-Hispanic Blacks, and 1.38 (95% CI: 1.18, 1.60) in Hispanics.
Table 6 displays results from our sensitivity analysis looking at individuals without potentially symptomatic PAD. In the subset without leg pain (n = 4,936, 75.6%), the overall pattern from the main analyses persisted, such that higher number of vessels with PAD was associated with significantly higher risk of mortality, CVD events, and CVD mortality. In other sensitivity analyses, the inclusion of individuals with stiff vessels (ABI of >1.4) or those with missing vessel ABIs as having PAD did not change the results. Additionally, inclusion of individual cancer subtypes or additional sociodemographic variables (education/income) did not significantly change the effect estimates.
Table 6.
Associations of Number Vessels Having Peripheral Artery Disease With Total Mortality, Incident Cardiovascular Disease Events, and Cardiovascular Disease Mortality in Individuals Who Did Not Have Leg Pain With Walking (n = 4,936), Multi-Ethnic Study of Atherosclerosis, United States, 2000–2015
| Model 1 c | Model 2 d | Model 3 e | |||||||
|---|---|---|---|---|---|---|---|---|---|
Outcome and No. of Vessels With ABI of 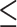 0.9a 0.9a
|
No. of Participants | No. of Events | Rate b | HR | 95% CI | HR | 95% CI | HR | 95% CI |
| Mortality | |||||||||
| 0 | 4,367 | 702 | 12.2 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 1 | 338 | 82 | 19.4 | 1.40 | 1.11, 1.76 | 1.42 | 1.12, 1.79 | 1.26 | 0.98, 1.62 |
| 2 | 148 | 51 | 28.4 | 1.76 | 1.32, 2.35 | 1.59 | 1.17, 2.14 | 1.22 | 0.85, 1.74 |
| 3 | 42 | 25 | 53.6 | 2.04 | 1.36, 3.07 | 1.73 | 1.15, 2.61 | 1.24 | 0.77, 2.00 |
| 4 | 41 | 26 | 67.2 | 3.04 | 2.04, 4.53 | 2.41 | 1.57, 3.70 | 1.56 | 0.92, 2.65 |
| P for trendf | <0.001 | <0.001 | 0.07 | ||||||
| Hard CVDg | |||||||||
| 0 | 4,360 | 406 | 7.6 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 1 | 338 | 34 | 8.8 | 1.09 | 0.77, 1.56 | 1.03 | 0.72, 1.47 | 0.88 | 0.60, 1.27 |
| 2 | 147 | 29 | 17.8 | 1.97 | 1.34, 2.88 | 1.72 | 1.15, 2.59 | 1.15 | 0.70, 1.88 |
| 3 | 42 | 10 | 24.5 | 1.92 | 1.01, 3.63 | 1.41 | 0.74, 2.69 | 0.87 | 0.42, 1.78 |
| 4 | 41 | 13 | 38.1 | 3.24 | 1.85, 6.57 | 2.14 | 1.21, 3.81 | 1.12 | 0.55, 2.30 |
| P for trendf | <0.001 | 0.001 | 0.95 | ||||||
| CVD death | |||||||||
| 0 | 4,367 | 163 | 2.8 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 1 | 338 | 17 | 4.0 | 1.24 | 0.75, 2.05 | 1.24 | 0.74, 2.06 | 0.96 | 0.56, 1.63 |
| 2 | 148 | 16 | 8.9 | 2.35 | 1.39, 3.98 | 2.40 | 1.40, 4.10 | 1.29 | 0.65, 2.56 |
| 3 | 42 | 7 | 15.0 | 2.44 | 1.13, 5.28 | 2.10 | 0.96, 4.58 | 0.97 | 0.38, 2.45 |
| 4 | 41 | 9 | 23.3 | 4.42 | 2.21, 8.81 | 3.39 | 1.64, 6.99 | 1.24 | 0.47, 3.27 |
| P for trendf | <0.001 | <0.001 | 0.74 | ||||||
Abbreviations: ABI, ankle brachial index; CI, confidence interval; CVD, cardiovascular disease; DP, dorsalis pedis artery; HDL, high-density lipoprotein; HR, hazard ratio; MI, myocardial infarction; PT, posterior tibial artery.
a Leg-specific ABI was calculated using the traditional method by taking the systolic pressure in the higher of the DP/PT artery divided by highest of 2 brachial systolic pressures.
b Crude incidence rate per 1,000 person-years.
c Model 1 adjusts for age, sex, race/ethnicity, and height.
d Model 2 adjusts for model 1 + systolic blood pressure, antihypertensive medications, total cholesterol, HDL cholesterol, statin use, diabetes, smoking status, cigarette pack-years, kidney function, physical activity, alcohol use, and cancer history.
e Model 3 adjusts for model 2 + ABI.
f P for trend calculated by treating number of vessels with ABI of ≤0.9 as continuous variable in regression models.
g Included MI, resuscitated cardiac arrest, MI death, stroke, and stroke death.
DISCUSSION
We found that multivessel PAD had a dose-dependent association with increased risk for mortality, incident CVD events, and CVD mortality in a diverse primary prevention population. This dose-dependent association persisted after adjustment for potential confounders. Furthermore, the dose-dependent association persisted even when conventional ABI values were included in the model (for mortality) and when the analysis was restricted to asymptomatic individuals. These data support the use of all 4 ABI values for each individual instead of a single representative value in clinical practice and research. Additionally, our results highlight the prognostic importance of the magnitude of lower extremity atherosclerosis on mortality, incident CVD, and CVD mortality.
The landmark ABI Collaboration, a meta-analysis of 16 population cohorts, found that ABI was inversely associated with mortality and CVD events in a dose-dependent pattern starting with ABI values of <1.1 (2). To highlight the powerful nature of the ABI, its measurement was not standardized across cohorts in this meta-analysis. For example, some cohorts measured only 1 ankle artery pressure in one leg while using different measuring devices (Doppler, oscillometry, or plethysmography) (5). As a result, the numerator and denominator for ABI calculation varied across cohorts. Even with this measurement variability, ABI showed a strong dose-dependent association with mortality and CVD event outcomes. Our results indicate that ABI should be measured according to current guidelines recommendations, (4, 5) but recommendations on calculating ABI and use of all the ankle pressure values might need modification.
Current guidelines recommend measurement of the bilateral brachial, DP, and PT artery pressures with Doppler probes (4). However, the current clinical recommendation is to use the higher of the DP/PT ankle pressure in each leg for the numerator in ABI calculation (4). If this ABI value is ≤0.9, the individual is considered to have PAD, and further workup should be initiated in the presence of symptoms. In relation to our study, this method of ABI calculation would require an individual to have 2-vessel disease to meet this threshold—both the DP and PT systolic pressures would have to be sufficiently low for the higher of those 2 values to lead to an ABI of ≤0.9. When considering a clinical workup and debating a possible medical or surgical intervention, this increased test specificity might be appropriate to minimize resource utilization and harm to patients. However, for risk characterization, our data shows that 1-vessel disease is associated with an increased risk of mortality. Furthermore, 2-vessel disease that was not detected with a traditional ABI of ≤0.9 was associated with a 2-fold increased risk of incident CVD events. This is consistent with 2 prior studies in clinical populations that showed increased risk of mortality (18) and CVD events (19) in individuals with a low ABI (lower of DP/PT used for numerator) of ≤0.9. Furthermore, our study takes this a step further by scoring the number of vessels that meet the threshold of ABI of ≤0.9 and demonstrates that it offers added information. In our mortality analysis, the trend of higher number of vessels with PAD’s association with mortality persisted after inclusion of the individual’s traditional ABI. A simple PAD vessel scoring of 0–4 after ABI calculation might further help classify individual risk for CVD events and mortality.
Our finding that a higher number of vessels with low ABI indicative of PAD was associated with higher risk of mortality and CVD events is not surprising. One prior study that examined the number of coronary vessels with coronary artery calcium, a measure of subclinical coronary artery disease, demonstrated a stronger mortality association in those with multivessel coronary artery calcium (20). Furthermore, atherosclerosis in multiple vascular beds is known to carry a higher risk of mortality. Multiple clinical trials that have examined subgroups of individuals with PAD have found that they are at higher risk of CVD or mortality compared with individuals with isolated coronary disease. For example, the FOURIER trial showed that participants with polyvascular disease had a poorer prognosis compared with participants who had a history of myocardial infarction or stroke alone (21). In the Coronary Artery Surgery Study, PAD was associated with higher mortality in individuals with stable coronary artery disease (22). In addition, another previous analysis from MESA showed that abdominal aortic calcium showed a stronger association than coronary artery calcium with total mortality (23). These findings consistently show that the systemic burden of extracoronary atherosclerosis appears important in predicting CVD and mortality risk. The number of vessels involved with PAD within the individual extends this finding and suggests that a noninvasive blood pressure test (the ABI) might allow assessment of the burden of PAD and refine risk prognostication.
While we found that multivessel subclinical PAD was associated with mortality, incident CVD events, and CVD mortality in asymptomatic individuals free of clinical CVD, the available data does not support routine screening with ABI. In 2018, the United States Preventive Services Task Force found limited data on the accuracy of ABI for identifying asymptomatic individuals who can benefit from treatment of PAD (24). This was based, in large part, on findings that asymptomatic individuals with PAD did not benefit from aspirin therapy (25, 26). A recent propensity-matched observational study found a reduction in cardiovascular events and mortality in statin-prescribed individuals with asymptomatic PAD (27). However, as McDermott and Criqui (28, 29) discuss in their 2 corresponding editorials to the statin study and United States Preventive Services Task Force recommendations, respectively, most, but not all, individuals with asymptomatic low ABI have another indication for cholesterol-lowering therapy. While our results and past studies demonstrate significantly higher mortality and CVD event rates in asymptomatic individuals with PAD, an effective intervention has yet to be identified in this population. A successful intervention would likely require a multidisciplinary and multipronged approach to aggressively modify CVD and mortality risk in those with PAD. An effective screening test requires an effective intervention after the test stratifies individuals into higher and lower risk of event. The current United States Preventive Services Task Force recommendation of not using ABI is not likely a reflection of ABI itself but the lack of a currently effective intervention in asymptomatic individuals. Future work needs to continue to evaluate potential strategies and interventions for reducing the burden of PAD in asymptomatic individuals, and if one develops, then ABI might be a useful screening tool in asymptomatic individuals.
Strengths and limitations
This study has notable strengths. The ABI and other measurements were performed and collected according to standardized protocols by trained study staff in a large multiracial/ethnic cohort. The use of the San Diego Claudication Questionnaire allowed us to perform a sensitivity analysis in asymptomatic individuals to mimic the screening setting in a primary care setting. This allowed us to demonstrate that multivessel PAD, even in asymptomatic individuals, is associated with increased risk for mortality and CVD events.
This study has important limitations. First, ABI is a noninvasive measure of peripheral artery disease, and we did not have a second (imaging) method such as arterial duplex or angiography to validate against the ABI. Second, ABI was measured only once per vessel in each individual and, thus, subject to measurement error and misclassification. However, a subset of 384 MESA participants had replicate ABI measurements at visit 3, with an overall intraclass correlation coefficient of 0.93 for these measures, demonstrating excellent reproducibility (12). Last, participants were notified of a low ABI (based on traditional ABI), and thus people with low ABI might have differentially received more aggressive cardiovascular preventive therapies. Here any such bias might still result in graded relationship.
Conclusion
Compared with those who had no PAD, participants with multivessel and bilateral lower extremity PAD had higher risks of mortality, CVD events, and CVD mortality. For accurate disease characterization, all values obtained during a recommended ABI measurement protocol should be considered in clinical practice and research.
ACKNOWLEDGMENTS
Author affiliations: Department of Family Medicine and Public Health, University of California San Diego, La Jolla, California (Jonathan T. Unkart, Matthew A. Allison, Maria Rosario G. Araneta, Joachim H. Ix, Michael H. Criqui); and Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland (Kunihiro Matsushita).
This research was supported by the National Heart, Lung, and Blood Institute (grants R01-HL-088451 and contracts N01-HC-95159 through N01-HC-95165 and N01-HC-95169, as well as 2T32HL079891 (University of California San Diego training grant support to J.T.U.)).
The authors thank the other investigators, the staff, and the participants of the Multi-Ethnic Study of Atherosclerosis study for their valuable contributions. A full list of participating Multi-Ethnic Study of Atherosclerosis investigators and institutions can be found at http://www.mesa-nhlbi.org. The authors also thank Matty James for assistance with Figure 1 creation.
Conflict of interest: none declared.
REFERENCES
- 1. Criqui MH, McClelland RL, McDermott MM, et al. The ankle-brachial index and incident cardiovascular events in the MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2010;56(18):1506–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ankle Brachial Index Collaboration, Fowkes FG, Murray GD, et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300(2):197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Diehm C, Allenberg JR, Pittrow D, et al. Mortality and vascular morbidity in older adults with asymptomatic versus symptomatic peripheral artery disease. Circulation. 2009;120(21):2053–2061. [DOI] [PubMed] [Google Scholar]
- 4. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135(12):e726–e779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aboyans V, Criqui MH, Abraham P, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126(24):2890–2909. [DOI] [PubMed] [Google Scholar]
- 6. Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. [DOI] [PubMed] [Google Scholar]
- 7. Ainsworth BE, Irwin ML, Addy CL, et al. Moderate physical activity patterns of minority women: the Cross-Cultural Activity Participation Study. J Womens Health Gend Based Med. 1999;8(6):805–813. [DOI] [PubMed] [Google Scholar]
- 8. Criqui MH, Denenberg JO, Bird CE, et al. The correlation between symptoms and non-invasive test results in patients referred for peripheral arterial disease testing. Vasc Med. 1996;1(1):65–71. [DOI] [PubMed] [Google Scholar]
- 9. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 10. American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(suppl 1):S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Allison MA, Aboyans V, Granston T, et al. The relevance of different methods of calculating the ankle-brachial index: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2010;171(3):368–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shadman R, Criqui MH, Bundens WP, et al. Subclavian artery stenosis: prevalence, risk factors, and association with cardiovascular diseases. J Am Coll Cardiol. 2004;44(3):618–623. [DOI] [PubMed] [Google Scholar]
- 14. Folsom AR, Kronmal RA, Detrano RC, et al. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA). Arch Intern Med. 2008;168(12):1333–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miettinen OS. Proportion of disease caused or prevented by a given exposure, trait or intervention. Am J Epidemiol. 1974;99(5):325–332. [DOI] [PubMed] [Google Scholar]
- 16. Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116(9):1509–1526. [DOI] [PubMed] [Google Scholar]
- 17. Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286(11):1317–1324. [DOI] [PubMed] [Google Scholar]
- 18. Nead KT, Cooke JP, Olin JW, et al. Alternative ankle-brachial index method identifies additional at-risk individuals. J Am Coll Cardiol. 2013;62(6):553–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Espinola-Klein C, Rupprecht HJ, Bickel C, et al. Different calculations of ankle-brachial index and their impact on cardiovascular risk prediction. Circulation. 2008;118(9):961–967. [DOI] [PubMed] [Google Scholar]
- 20. Tota-Maharaj R, Joshi PH, Budoff MJ, et al. Usefulness of regional distribution of coronary artery calcium to improve the prediction of all-cause mortality. Am J Cardiol. 2015;115(9):1229–1234. [DOI] [PubMed] [Google Scholar]
- 21. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713–1722. [DOI] [PubMed] [Google Scholar]
- 22. Eagle KA, Rihal CS, Foster ED, et al. Long-term survival in patients with coronary artery disease: importance of peripheral vascular disease. The Coronary Artery Surgery Study (CASS) Investigators. J Am Coll Cardiol. 1994;23(5):1091–1095. [DOI] [PubMed] [Google Scholar]
- 23. Criqui MH, Denenberg JO, McClelland RL, et al. Abdominal aortic calcium, coronary artery calcium, and cardiovascular morbidity and mortality in the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34(7):1574–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. US Preventive Services Task Force, Curry SJ, Krist AH, et al. Screening for peripheral artery disease and cardiovascular disease risk assessment with the ankle-brachial index: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320(2):177–183. [DOI] [PubMed] [Google Scholar]
- 25. Fowkes FG, Price JF, Stewart MC, et al. Aspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: a randomized controlled trial. JAMA. 2010;303(9):841–848. [DOI] [PubMed] [Google Scholar]
- 26. Belch J, MacCuish A, Campbell I, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ. 2008;337:a1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ramos R, Garcia-Gil M, Comas-Cufi M, et al. Statins for prevention of cardiovascular events in a low-risk population with low ankle brachial index. J Am Coll Cardiol. 2016;67(6):630–640. [DOI] [PubMed] [Google Scholar]
- 28. McDermott MM, Criqui MH. Reducing cardiovascular risk with ankle brachial index screening: new evidence for an old question? J Am Coll Cardiol. 2016;67(6):641–643. [DOI] [PubMed] [Google Scholar]
- 29. McDermott MM, Criqui MH. Ankle-brachial index screening and improving peripheral artery disease detection and outcomes. JAMA. 2018;320(2):143–145. [DOI] [PubMed] [Google Scholar]


