To the Editor:
We would like to thank our colleagues for their letter in response to our manuscript. The letter raises important theoretical concerns about the impact of procoagulant factors in plasma on the coagulation cascade in the context of patients with severe COVID-19.1 Generally, we agree with the word of caution regarding the use of convalescent plasma in the context of patients with severe COVID-19 and evidence of dysregulated hemostasis, as observed among patients who required extracorporeal membrane oxygenation (ECMO) support.2 Indeed, fresh frozen plasma contains physiologic ratios of both procoagulant and anticoagulant proteins.3 Theoretically, if transfused to a patient that is prothrombotic, plasma can contribute to ongoing dysregulated hemostasis. Despite the theoretical concerns enumerated by our colleagues, some evidence suggests that transfusion of fresh frozen plasma in nonbleeding critically ill patients does not aggravate their inflammatory response, and it might stabilize endothelial condition.4
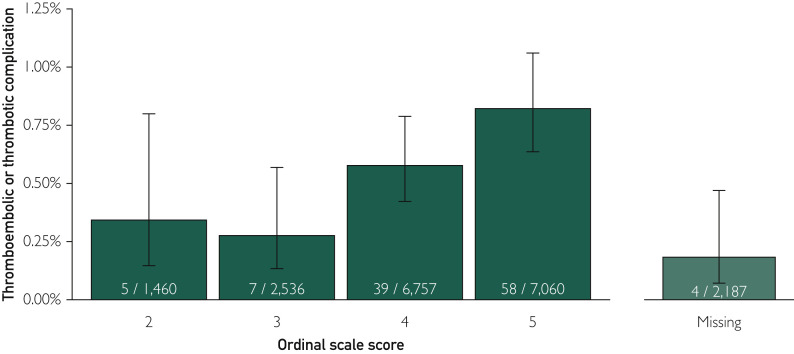
As noted in our original article,5 the low rate (∼0.5%) of thrombotic and thromboembolic events—113 events in 20,000 patients with COVID-19—is encouraging, particularly given the high prevalence of both COVID-19 associated–respiratory failure and hypoxemia in the observed patients. Herein, the rate of thrombotic and thromboembolic events was stratified using a 6-level ordinal scale to assess the clinical course of COVID-19,6 with higher scores indicating worse condition at time of enrollment (Figure ). The rate of thrombotic and thromboembolic events appears to increase with more advanced clinical course of COVID-19; however, the rate of events is objectively low among patients in the most severe category of COVID-19 (∼0.8%).
Figure.
The rate of thrombotic and thromboembolic events stratified using a 6-level ordinal scale to assess the clinical course of COVID-19. Scores on the ordinal scale were defined as follows6: a score of 1 indicated not hospitalized; 2, hospitalized and not receiving supplemental oxygen; 3, hospitalized and receiving supplemental oxygen; 4, hospitalized and receiving oxygen supplementation administered by a high-flow nasal cannula or noninvasive ventilation; 5, hospitalized and receiving mechanical ventilation; and 6, death. Wilson confidence interval calculation for binomial proportions was used to estimate the 95% confidence intervals for each point estimate.
In summary, we agree with the word of caution provided by our colleagues, particularly among patients with COVID-19 who have a dysregulated coagulation system promoting hypercoagulation. The coagulation profile of plasma and its likely effect on hemostatic balance should be a factor in clinical decisions about the therapeutic use of convalescent plasma. However, the low rate of thrombotic and thromboembolic events provides strong support of the safety profile of convalescent plasma, even among hospitalized patients with severe COVID-19.
Footnotes
Potential Competing Interests: The authors report no competing interests.
References
- 1.Giannis D., Ziogas I.A., Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020;127:104362. doi: 10.1016/j.jcv.2020.104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel B.V., Arachchillage D.J., Ridge C.A. Pulmonary angiopathy in severe COVID-19: Physiologic, imaging, and hematologic observations. Am J Respir Crit Care Med. 2020;202(5):690–699. doi: 10.1164/rccm.202004-1412OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gratz J., Ponschab M., Iapichino G.E. Comparison of fresh frozen plasma vs. coagulation factor concentrates for reconstitution of blood: An in vitro study. Eur J Anaesthesiol. 2020;37(10):879–888. doi: 10.1097/EJA.0000000000001202. [DOI] [PubMed] [Google Scholar]
- 4.Straat M., Muller M.C., Meijers J.C. Effect of transfusion of fresh frozen plasma on parameters of endothelial condition and inflammatory status in non-bleeding critically ill patients: a prospective substudy of a randomized trial. Crit Care. 2015;19(1):163. doi: 10.1186/s13054-015-0828-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joyner M.J., Bruno K.A., Klassen S.A. Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients. Mayo Clin Proc. 2020;95(9):1888–1897. doi: 10.1016/j.mayocp.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavalcanti A.B., Zampieri F.G., Rosa R.G. Hydroxychloroquine with or without azithromycin in mild-to-moderate covid-19. N Engl J Med. 2020;383(21):2041–2052. doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]