Abstract
Purpose
Reported rates of acute kidney injury (AKI) have varied significantly among studies of coronavirus disease 2019 (COVID-19) published to date. The present meta-analysis was conducted to gain clarity regarding AKI incidence and renal replacement therapy (RRT) use in COVID-19 patients.
Methods
The PubMed, Embase, Web of Science, medRxiv, and bioRxiv databases were systematically searched for COVID-19-related case reports published through 25 July 2020. Pooled analyses were conducted using R.
Results
The pooled incidence of AKI in 51 studies including 21,531 patients was 12.3% (95% CI 9.5–15.6%), with higher rates of 38.9% in 290 transplant patients (95% CI 27.3–51.9%), 39.0% in 565 ICU patients (95% CI 23.2–57.6%) and 42.0% among 1745 deceased patients (95% CI 30.3–54.7%). RRT usage was reported in 39 studies of 17,664 patients, with an overall pooled use of 5.4% (95% CI 4.0–7.1%), with higher rates of 15.6% in 117 transplant patients (95%CI 9.9–23.8%) and 16.3% in 776 ICU patients (95% CI 11.1–23.3%).
Conclusion
AKI and RRT use among COVID-19 patients represent a major public health concern, and early and appropriate intervention should be called upon to improve the prognosis of patients suffering from AKI.
Keywords: COVID-19, Acute kidney injury, Renal replacement therapy
1. Introduction
Within the past two decades, two previously unknown coronaviruses known as severe acute respiratory syndrome coronavirus (SARS-COV) and Middle East respiratory syndrome coronavirus (MERS-COV) have caused serious epidemic outbreaks associated with high mortality rates [1], [2]. A new form of pneumonia associated with a novel coronavirus emerged in late 2019 in Wuhan, China [3], and rapidly began spreading between humans after initial local spread in a seafood market. The disease caused by this novel SARS-related coronavirus (SARS-CoV-2), which was designated coronavirus disease 2019 (COVID-19), was declared a global pandemic by the World Health Organization in March 2020. As of July 31, 2020, over 17 million COVID-19 cases and 670,000 deaths associated with this virus had been reported [4]. Early symptoms of COVID-19 include fever, a dry cough, and dyspnea [5]. The disease can progress rapidly and can cause high rates of mortality or severe life-threatening organ damage in some patients.
One retrospective analysis of 536 SARS patients found that while acute kidney injury (AKI) was uncommon among these patients (36 cases), its incidence was associated with a 91.7% mortality rate [6]. A male patient that died of acute pneumonia and renal failure in Saudi Arabia in 2012 was the first patient from whom MERS-CoV was isolated [2], underscoring the potential for severe renal damage associated with these viruses. Xu et al. [7] determined that SARS-CoV and SARS-CoV-2 are highly homologous, and computational models revealed that SARS-CoV-2 exhibits a strong affinity for human angiotensin-converting enzyme 2 (ACE2). There is evidence to suggest that COVID-19 infection has the potential to induce kidney damage in infected patients. For example, Li et al. [8] found that blood urea nitrogen was elevated in 30.1% (59/193) of analyzed COVID-19 patients, while creatinine levels were elevated in 22.3% (43/193) of these patients. These same patients also frequently exhibited proteinuria (59.9%; 88/147) and hematuria (48.3%; 71/147), and most exhibited abnormal renal radiographic findings consistent with edema and inflammation. AKI incidence in COVID-19 patients has also been found to be an independent predictor of poor prognosis and elevated mortality rates [9]. Chan et al. reported a high pooled mortality rate of 93.27% among 65 patients with AKI [10]. In the present study, we thus undertook a comprehensive approach to reviewing relevant literature to conduct a systematic review and meta-analysis regarding rates of AKI and renal replacement therapy (RRT) use among COVID-19 patients.
2. Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement and Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines were used to guide the conceptualization and execution of this meta-analysis [11], [12].
2.1. Search strategy
The PubMed, Embase, Web of Science, medRxiv, and bioRxiv databases were systematically searched for relevant studies published as of 25 July 2020, without any language restrictions, using the following search terms: “COVID-19”, “2019-nCoV”, “SARA-CoV-2”, “novel coronavirus” “Acute Kidney Injury”, and “Acute Renal Failure”. The search strategy was presented in Online Appendix 1. COVID-19-related reports in relevant literature reviews and the references of included studies were also searched to identify other relevant analyses.
2.2. Study selection
Studies eligible for inclusion in this meta-analysis were: (1) observational studies reporting rates of AKI and RRT use in COVID-19 patients; (2) published studies that met with relevant requirements, regardless of whether or not AKI definition was mentioned; and (3) pre-print articles that included an explicit definition of AKI.
Studies were excluded from this analysis if they were abstracts, editorials, reviews, letters, conference abstracts, or commentaries. For any studies reporting on patient populations within the same hospital during overlapping time periods, only the study with the most comprehensive dataset was included in this analysis.
2.3. Data extraction
Two investigators (XPY and SST) independently reviewed studies, extracted data, and assessed study quality. Any discrepancies were resolved by a third investigator (HG).
Data extracted from each study included study design, study title, first author, year of publication, country, patient information, AKI incidence, and rates of RRT use.
Observational study quality was evaluated using the Newcastle-Ottawa Scale.
Primary study outcomes included AKI incidence among total COVID-19 patients, among COVID-19 patients admitted to the intensive care unit (ICU), and among deceased COVID-19 patients. Secondary outcomes included RRT use in total COVID-19 patients and among COVID-19 patients admitted to the ICU.
2.4. Statistical analysis
The “meta” package for R (v.4.0.2) was used to conduct the present meta-analysis when there are five or more studies reporting the same outcome with the same definition. The normality of data distributions was assessed, and data were used in their raw form when normally distributed, whereas they were otherwise subjected to Logit transformation. Freeman-Tukey Double arcsine transformation was conducted for variables for which many values were either 0 or 1 in order to facilitate variance stabilization. A continuous correction of 0.5 was used for studies with rates of 0. Corresponding 95% confidence intervals (CIs) were computed for all pooled result analyses. The I2 statistic was used to assess heterogeneity among studies. When I2 >50%, a random-effects model was used for analyses, whereas a fixed-effects model was otherwise used. Potential sources of heterogeneity were identified through sensitivity and subgroup analyses.
3. Results
3.1. Study characteristics
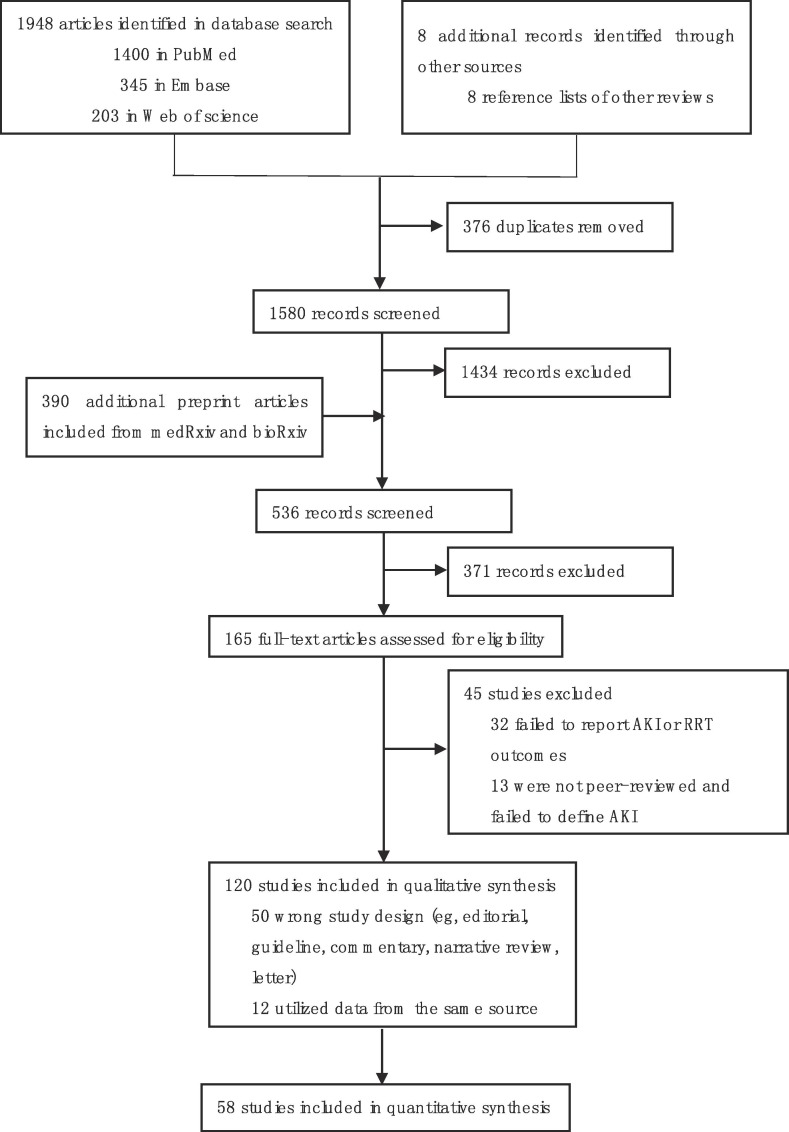
The PRISMA flow diagram was shown in Fig. 1 . Our search strategy identified 165 potentially studies, of which 13 were not peer-reviewed and failed to define AKI, 32 failed to report AKI or RRT outcomes in patients, 50 with wrong design and 12 utilized data from the same source. The remaining 58 studies of 22,671 patients were included in the present meta-analysis [8], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69]. Individual studies incorporated 7 – 5700 patients, with average patient ages ranging from 37 to 79.7 years (Table 1 ). The Newcastle-Ottawa scale was used to assess the quality of these included studies (Table 2 ). Studies came from China (n = 29), the USA (n = 12), Italy (n = 3), the UK (n = 2), Spain (n = 2), Kuwait (n = 2), France (n = 1), Germany (n = 1), Canada (n = 1), Thailand (n = 1), India (n = 1), Poland (n = 1) and Turkey (n = 1). One study included data from 12 countries. All studies were observational in nature, including 54 retrospective studies and 4 prospective studies. Of these studies, 15 were multi-center analyses.
Fig. 1.
PRISMA flow diagram.
Table 1.
Characteristics of included studies.
| Study | Location | Study design | N | Average age | Male | AKI(total) | AKI(ICU) | AKI(death) | RRT(total) | RRT(ICU) |
|---|---|---|---|---|---|---|---|---|---|---|
| Wang2020[13] | China | ROS | 138 | 56 | 75 (54.3%) | 5 (3.6%) | 3 (8.3%) | 2 (1.5%) | 2 (5.6%) | |
| Wang 2020 [14] | China | POS | 116 | 54 | 67 (57.8%) | 0 | 0 | |||
| Richardson 2020 [15] | USA | ROS | 5700 | 63 | 3437 (60.1%) | 1370 (24.0%) | 347 (62.7%) | 225 (3.9%) | ||
| Huang 2020 [16] | China | ROS | 41 | 49 | 30 (73.2%) | 3 (7.3%) | 3 (23.1%) | 3 (7.3%) | 3 (23.1%) | |
| Cheng 2020 [17] | China | POS | 701 | 63 | 367 (52.4%) | 36 (5.1%) | ||||
| Chen 2020 [18] | China | ROS | 274 | 62 | 171 (62.4%) | 29 (10.6%) | 28 (24.8%) | 3 (1.1%) | ||
| Guan 2020 [19] | China | ROS | 1099 | 42 | 640 (58.2%) | 6 (0.5%) | 9 (0.8%) | |||
| Alberici 2020 [20] | Italy | ROS | 20 | 59 | 16 (80.0%) | 6 (30.0%) | 1 (5.0%) | |||
| Yang 2020 [21] | China | ROS | 52 | 59.7 | 35 (67.3%) | 15 (28.8%) | 15 (28.8%) | 12 (37.5%) | 9 (17.3%) | 9 (17.3%) |
| Zhou 2020 [22] | China | ROS | 191 | 56 | 119 (62.3%) | 28 (14.7%) | 27 (50.0%) | 10 (5.2%) | ||
| Chen 2020 [23] | China | ROS | 99 | 55.5 | 67 (67.7%) | 3 (3.0%) | 9 (9.1%) | |||
| Arentz 2020 [24] | USA | ROS | 21 | 70 | 11 (52.4%) | 4 (19.1%) | 4 (19.1%) | |||
| Banerjee 2020 [25] | UK | ROS | 7 | 47.6 | 4 (57.1%) | 4 (57.1%) | ||||
| Deng 2020 [26] | China | ROS | 225 | NR | 124 (55.1%) | 20 (8.9%) | 20 (18.3%) | |||
| Guo 2020 [27] | China | ROS | 187 | 58.5 | 91 (48.7%) | 18 (9.6%) | ||||
| Lei 2020 [28] | China | ROS | 34 | 55 | 14 (41.2%) | 2 (5.9%) | 2 (13.3%) | 1 (2.9%) | 1 (6.7%) | |
| Shi 2020 [29] | China | ROS | 416 | 64 | 205 (49.3%) | 8 (1.9%) | 2 (0.5%) | |||
| Wang 2020 [30] | China | ROS | 107 | 51 | 57 (53.3%) | 14 (13.1%) | 14 (73.7%) | 4 (3.7%) | ||
| Zhang 2020 [31] | China | ROS | 221 | 55 | 108 (48.9%) | 10 (4.5%) | 5 (2.3%) | |||
| Zhang 2020 [32] | China | ROS | 645 | NR | 328 (50.9%) | 2 (0.3%) | 0 | |||
| Hu 2020 [33] | China | ROS | 323 | 61 | 166 (51.4%) | 17 (5.3%) | 10 (38.5%) | 72 (22.3%) | 4 (15.4%) | |
| Brill 2020 [34] | UK | ROS | 450 | 72 | 272 (60.4%) | 85 (18.9%) | 54 (31.2%) | |||
| Cheung 2020 [35] | USA | ROS | 10 | 79.7 | 2 (20.0%) | 1 (10.0%) | ||||
| Salacup 2020 [36] | USA | ROS | 242 | 66 | 123 (50.8%) | 24 (9.9%) | ||||
| Piva 2020 [37] | Italy | POS | 33 | 64 | 30 (90.9%) | 1 (3.4%) | 1 (3.4%) | |||
| Argenziano 2020 [38] | USA | ROS | 850 | NR | 511 (60.1%) | 288 (33.9%) | 184 (78.0%) | 117 (13.8%) | 83 (35.2%) | |
| Anish 2020 [39] | Canada | ROS | 117 | 69 | 79 (67.5%) | 16 (13.7%) | 16 (13.7%) | |||
| Aggarwal 2020 [40] | India | ROS | 32 | 54.5 | 19 (59.4%) | 13 (40.6%) | ||||
| Haydar 2020 [41] | USA | ROS | 242 | 66 | 123 (50.8%) | 26 (10.7%) | ||||
| Yi 2020 [42] | China | ROS | 54 | 63 | 33 (61.1%) | 2 (3.7%) | ||||
| Wan 2020 [43] | China | ROS | 135 | 47 | 72 (53.3%) | 5 (3.7%) | 5 (3.7%) | |||
| Nowak 2020 [44] | Poland | ROS | 169 | 63.7 | 87 (51.5%) | 17 (10.1%) | 10 (21.7%) | 1 (0.6%) | ||
| Goyal 2020 [45] | USA | ROS | 375 | 62.2 | 238 (63.5%) | 18 (4.8%) | ||||
| Hardy 2020 [46] | USA | ROS | 15 | 51 | 10 (66.7%) | 6 (40.0%) | 2 (13.3%) | |||
| Crespo 2020 [47] | Spain | ROS | 15 | 73.6 | 11 (73.3%) | 5 (33.3%) | 5 (71.4%) | 3 (20.0%) | ||
| Borobia 2020 [48] | Spain | ROS | 2226 | 61 | 1074 (48.2%) | 173 (7.8%) | 120 (26.1%) | |||
| Cravedi 2020 [49] | 12 centers | ROS | 144 | 62 | 94 (65.3%) | 74 (52.1%) | 26 (59.1%) | |||
| Husain-Syed 2020 [50] | Germany | POS | 23 | 60 | 19 (82.6%) | 12 (52.2%) | 10 (83.3%) | 3 (13.0%) | 3 (25.0%) | |
| Nair 2020 [51] | USA | ROS | 10 | 57 | 6 (60.0%) | 5 (50.0%) | ||||
| Akalin 2020 [52] | USA | ROS | 28 | 60 | 22 (78.6%) | 6 (21.4%) | ||||
| Wang 2020 [53] | China | ROS | 344 | 64 | 179 (52.0%) | 86 (25.0%) | 80 (60.2%) | 9 (2.6%) | ||
| Demir 2020 [54] | Turkey | ROS | 40 | 44.9 | 20 (50%) | 3 (7.5%) | ||||
| Li 2020 [8] | China | ROS | 193 | 57 | 95 (49.2%) | 55 (28.5%) | 7 (3.6%) | |||
| Zhao 2020 [55] | China | ROS | 77 | 52 | 34 (44.2%) | 2 (2.6%) | ||||
| Xiao 2020 [56] | China | ROS | 287 | 62 | 160 (55.7%) | 55 (19.2%) | ||||
| Jiang 2020 [57] | China | ROS | 55 | 45 | 27 (55.7%) | 3 (5.5%) | ||||
| Zheng 2020 [58] | China | ROS | 34 | 66 | 23 (67.6%) | 7 (20.6%) | 5 (14.7%) | |||
| Rubin 2020 [59] | France | ROS | 71 | 61.2 | 55 (77.5%) | 57 (80.3%) | 57 (80.3%) | 10 (14.1%) | 10 (14.1%) | |
| Almazeedi 2020 [60] | Kuwait | ROS | 1096 | 41 | 888 (81.0%) | 14 (1.3%) | 5 (0.5%) | |||
| Yang 2020 [61] | China | ROS | 463 | 60 | 231 (49.9%) | 35 (7.6%) | 19 (28.8%) | 10 (2,2%) | 9 (13.6%) | |
| Ayed 2020 [62] | Kuwait | ROS | 103 | 53 | 88 (85.4%) | 21 (20.4%) | 21 (20.4%) | |||
| Gaetano 2020 [63] | Italy | ROS | 307 | 65.2 | 219 (71.3%) | 69 (22.4%) | 24 (39.3%) | 5 (1.6%) | ||
| Shi 2020 [64] | China | ROS | 134 | 46 | 69 (51.5%) | 3 (2.2%) | 1 (0.7%) | |||
| Lubetzky 2020 [65] | USA | ROS | 39 | 59 | 31 (79.5%) | 20 (51.3%) | 5 (12.8%) | |||
| Chan 2020 [66] | USA | ROS | 3235 | 66.5 | 1868 (57.7%) | 1406(43.5%) | 280 (8.6%) | |||
| Pongpirul 2020 [67] | Thailand | ROS | 193 | 37 | 113 (58.5%) | 7 (6.3%) | 5 (83.3%) | |||
| Xu 2020 [68] | China | ROS | 45 | 56.7 | 29 (64.4%) | 7 (15.6%) | 4 (8.9%) | |||
| Yan 2020 [69] | China | ROS | 168 | 51 | 81 (48.2%) | 6 (3.6%) |
ROS: retrospective observational study; POS: prospective observational study; AKI: acute kidney injury; RRT: renal replacement therapy; ICU: intensive care unit;
Table 2.
Newcastle-Ottawa score of the included studies.
| Study | Exposed cohortepresentative | Non exposed cohort selected from same source | Exposure ascertained | Outcome of study was not present at start of the study | Comparability | Adequate assessment | Follow up was long enough | Adequate follow-up | Quality score |
|---|---|---|---|---|---|---|---|---|---|
| Wang 2020 | * | * | * | * | * | * | * | ******* | |
| Wang 2020 | * | * | * | * | * | * | * | * | ******** |
| Richardson 2020 | * | * | * | * | * | * | * | ******* | |
| Huang 2020 | * | * | * | * | * | * | * | ******* | |
| Cheng 2020 | * | * | * | * | * | * | ****** | ||
| Chen 2020 | * | * | * | * | * | * | * | ******* | |
| Guan 2020 | * | * | * | * | * | * | ****** | ||
| Alberici 2020 | * | * | * | * | * | ***** | |||
| Yang 2020 | * | * | * | * | * | * | * | ******* | |
| Zhou 2020 | * | * | * | * | * | * | * | ******* | |
| Chen 2020 | * | * | * | * | * | ***** | |||
| Arentz 2020 | * | * | * | * | * | ***** | |||
| Banerjee 2020 | * | * | * | * | **** | ||||
| Deng 2020 | * | * | * | * | * | * | ****** | ||
| Guo 2020 | * | * | * | * | **** | ||||
| Lei 2020 | * | * | * | * | * | * | * | ******* | |
| Shi 2020 | * | * | * | * | * | ***** | |||
| Wang 2020 | * | * | * | * | * | * | * | ******* | |
| Zhang 2020 | * | * | * | ** | * | * | ****** | ||
| Zhang 2020 | * | * | * | * | * | ***** | |||
| Hu 2020 | * | * | * | * | * | * | ****** | ||
| Brill 2020 | * | * | ** | * | * | * | * | ******* | |
| Cheung 2020 | * | * | * | ** | * | ***** | |||
| Salacup 2020 | * | * | * | * | * | * | ****** | ||
| Piva 2020 | * | * | * | * | * | ***** | |||
| Argenziano 2020 | * | * | * | * | * | * | * | ******* | |
| Anish 2020 | * | * | * | * | **** | ||||
| Aggarwal 2020 | * | ** | * | * | * | * | ****** | ||
| Haydar 2020 | * | * | * | * | **** | ||||
| Yi 2020 | * | * | * | * | * | ***** | |||
| Wan 2020 | * | ** | * | * | * | * | ****** | ||
| Nowak 2020 | * | * | * | ** | * | * | * | ******* | |
| Goyal 2020 | * | * | * | ** | * | * | ****** | ||
| Hardy 2020 | * | * | * | * | **** | ||||
| Crespo 2020 | * | * | * | * | * | * | ****** | ||
| Borobia 2020 | * | * | * | * | * | * | * | ******* | |
| Cravedi 2020 | * | * | * | * | * | * | * | ******* | |
| Husain-Syed 2020 | * | * | * | * | * | * | * | * | ******** |
| Nair 2020 | * | * | * | * | * | ***** | |||
| Akalin 2020 | * | * | * | * | **** | ||||
| Wang 2020 | * | * | * | * | ** | * | * | ******* | |
| Demir 2020 | * | * | * | * | * | ***** | |||
| Li 2020 | * | * | * | * | * | * | ****** | ||
| Zhao 2020 | * | * | * | * | * | * | ****** | ||
| Xiao 2020 | * | * | * | * | * | ***** | |||
| Jiang 2020 | * | * | * | * | * | * | ****** | ||
| Zheng 2020 | * | * | * | * | * | * | * | ******* | |
| Rubin 2020 | * | ** | * | * | ** | * | * | ****** | |
| Almazeedi 2020 | * | * | * | * | * | ***** | |||
| Yang 2020 | * | * | * | * | * | * | * | ******* | |
| Ayed 2020 | * | * | * | * | * | **** | |||
| Gaetano 2020 | * | * | * | * | * | * | * | ******* | |
| Shi 2020 | * | * | * | ** | * | * | ****** | ||
| Lubetzky 2020 | * | * | * | * | * | * | ****** | ||
| Chan 2020 | * | * | * | * | * | * | ******** | ||
| Pongpirul 2020 | * | * | * | ** | * | * | * | ******* | |
| Xu 2020 | * | * | * | * | * | * | * | ******* | |
| Yan 2020 | * | * | * | * | * | * | ****** |
3.2. Clinical outcomes
3.2.1. Acute kidney injury
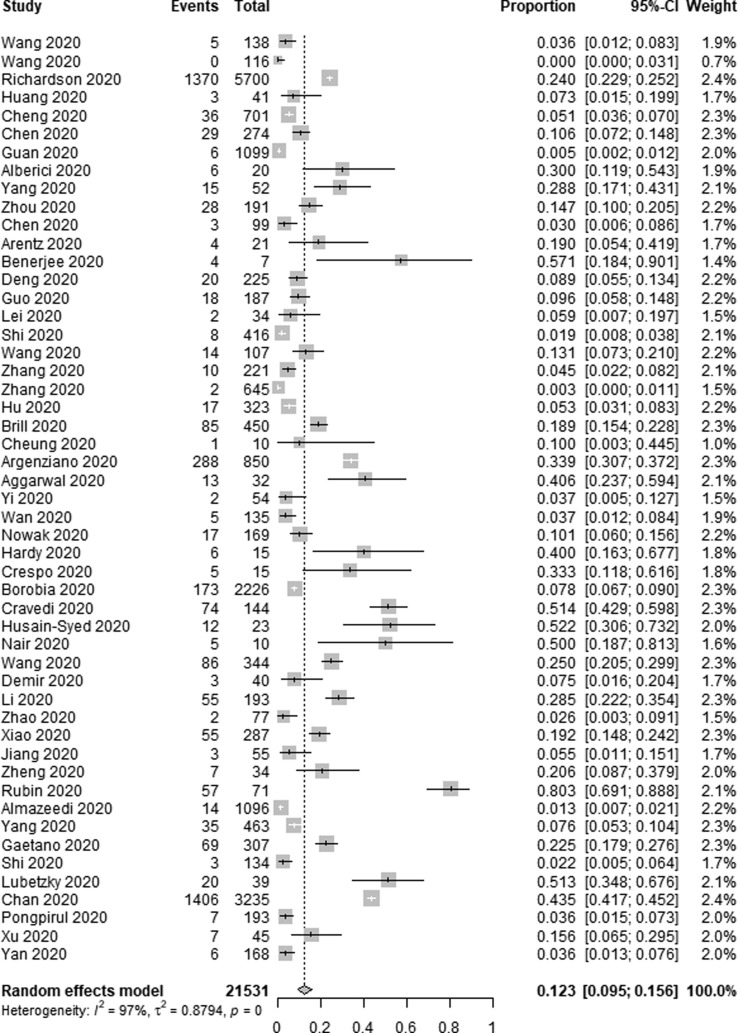
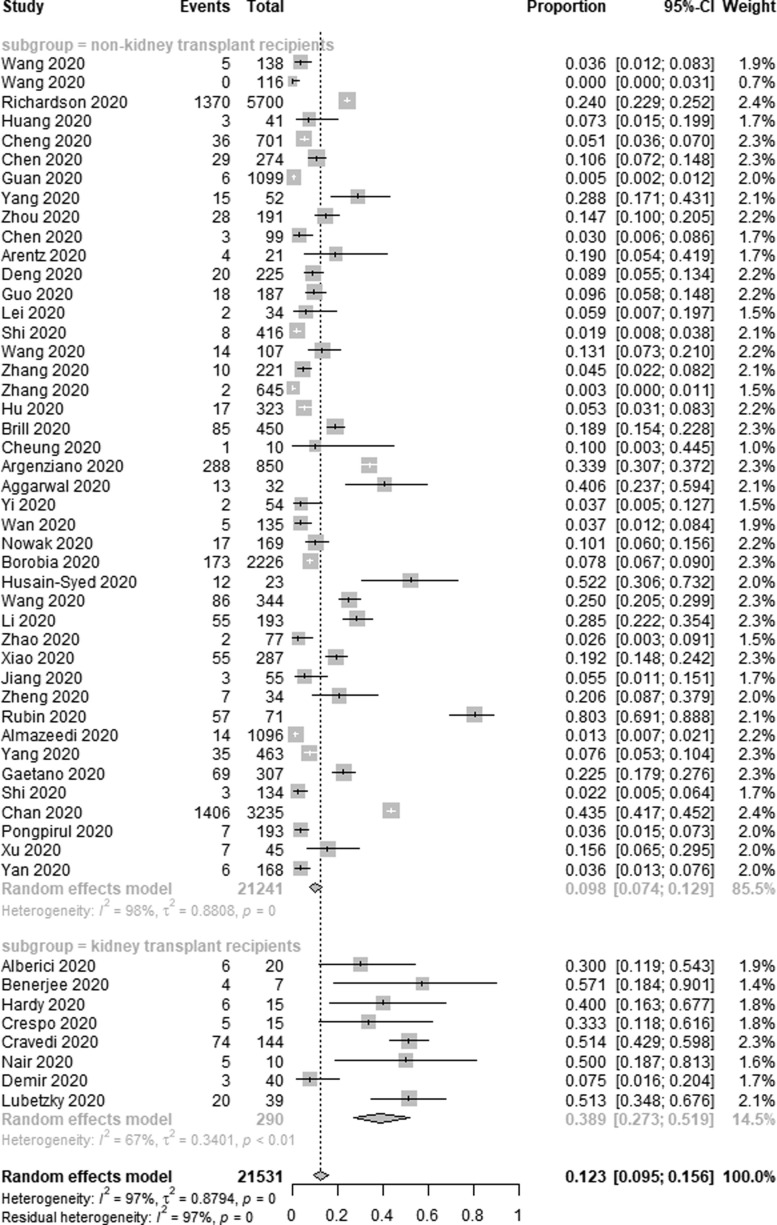
Incidence of AKI among COVID-19 patients was reported in 51 of the included studies. Of the 21,531 patients included in these studies, 4121 developed AKI, with a pooled AKI incidence rate of 12.3% among COVID-19 patients (95% CI 9.5–15.6%, I2 = 97%) (Fig. 2a ). In an effort to identify sources of heterogeneity among included studies, a sensitivity analysis was conducted which failed to identify the drivers of such heterogeneity. As AKI incidence was highest among kidney transplant recipients, we additionally conducted a subgroup analysis, which revealed that the incidence of AKI among transplant patients was 38.9% (95% CI 27.3–51.9%, I2 = 67%), whereas among non-transplant patients this incidence rate was 9.8% (95% CI 7.4–12.9%, I2 = 98%) (Fig. 2b ).
Fig. 2a.
Forest plot depicting the incidence of AKI in COVID-19 patients.
Fig. 2b.
Subgroup analysis depicting the incidence of AKI in COVID-19 patients.
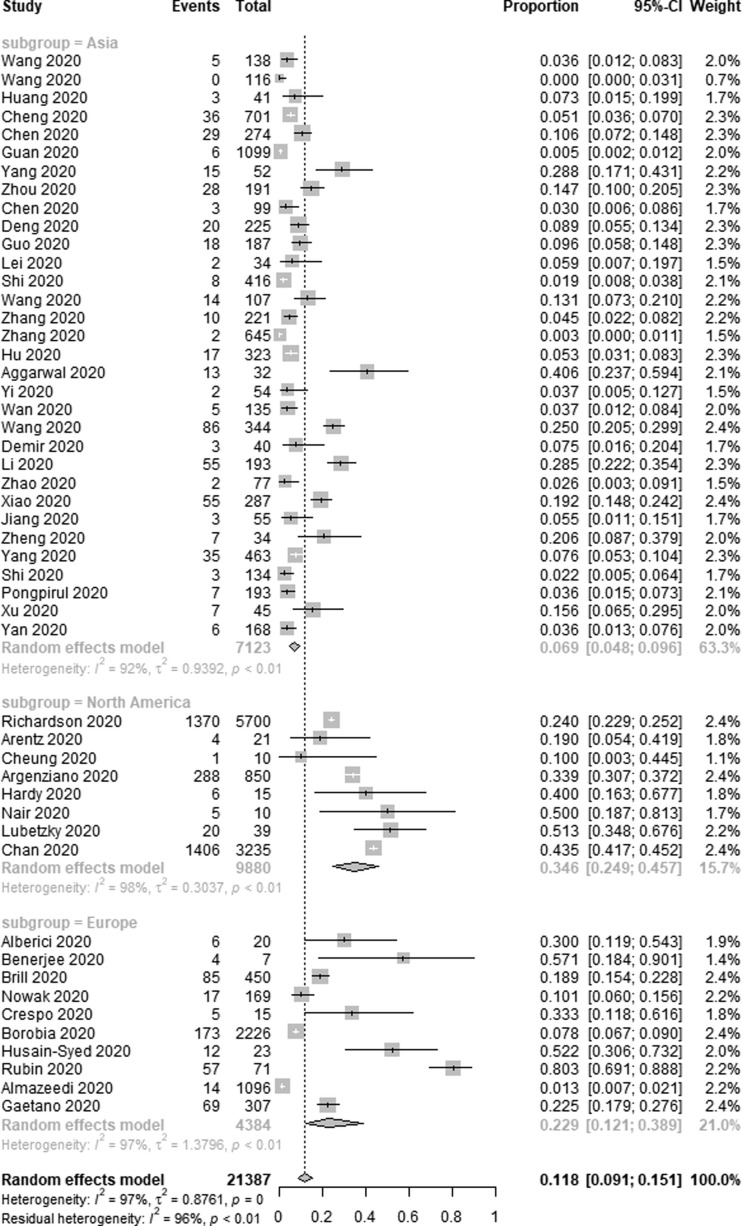
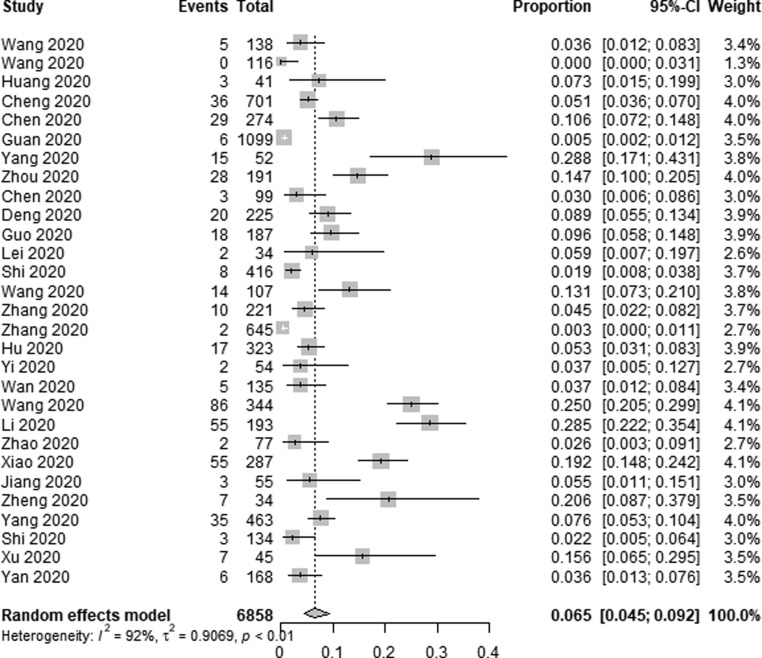
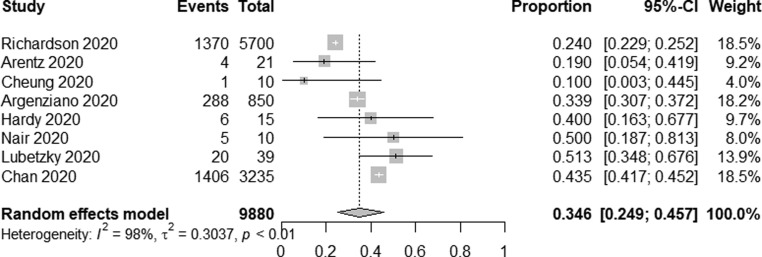
Three continents were included in our study: Asia, North America and Europe, and we conducted a subgroup analysis according to different continents, the AKI incidence was lower in Asia [6.9% (95% CI 4.8–9.6%, I2 = 92%)] compared to Europe [22.9% (95% CI 12.1–38.9%, I2 = 97%)] and North America [34.6% (95% CI 24.9–45.7%, I2 = 98%)] (Fig. 2c ). Meanwhile, most of the studies came from China and the USA, the AKI incidence was lower in 6858 Chinese patients [6.5% (95% CI 4.5–9.2%, I2 = 92%)] compared to 9880 American patients [34.6% (95% CI 24.9–45.7%, I2 = 98%)] (Fig. 2d ) (Fig. 2e ).
Fig. 2c.
Subgroup analysis depicting the incidence of AKI between different continents in COVID-19 patients.
Fig. 2d.
Forest plot depicting the incidence of AKI in COVID-19 patients in China.
Fig. 2e.
Forest plot depicting the incidence of AKI in COVID-19 patients in the USA.
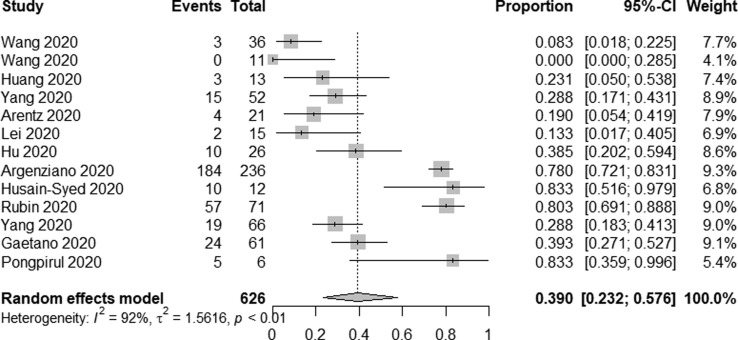
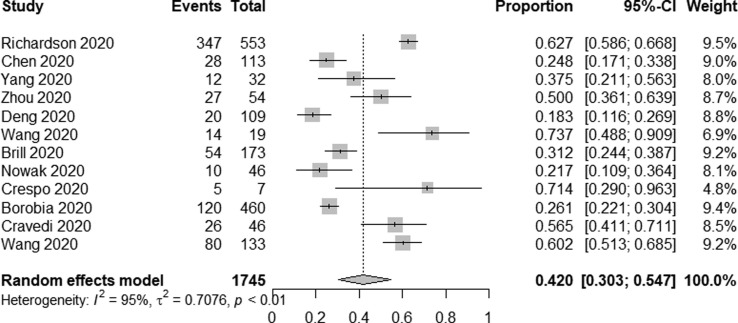
In total, 13 studies reported on AKI incidence among ICU patients. Overall, AKI occurred in 312/565 patients admitted to the ICU for an overall pooled incidence rate of 39.0% (95% CI 23.2–57.6%, I2 = 92%) (Fig. 2f ). A total of 12 studies reported on AKI incidence among deceased patients, of whom 743/1745 exhibited signs of AKI for an incidence rate of 42.0% (95% CI 30.3–54.7%, I2 = 95%) (Fig. 2g ). Furthermore, in seven studies [17], [50], [51], [54], [59], [63], [66], AKI patients were divided into three stages according to the Kidney Disease: Improving Global Outcomes (KDIGO) guideline, among 1588 AKI patients, the proportions of stage 1, 2 and 3 AKI were 36.3%, 20.7%, 43.0%, respectively.
Fig. 2f.
Forest plot depicting the incidence of AKI in intensive care unit Patients.
Fig. 2g.
Forest plot depicting the incidence of AKI in deaths.
3.2.2. Renal replacement therapy
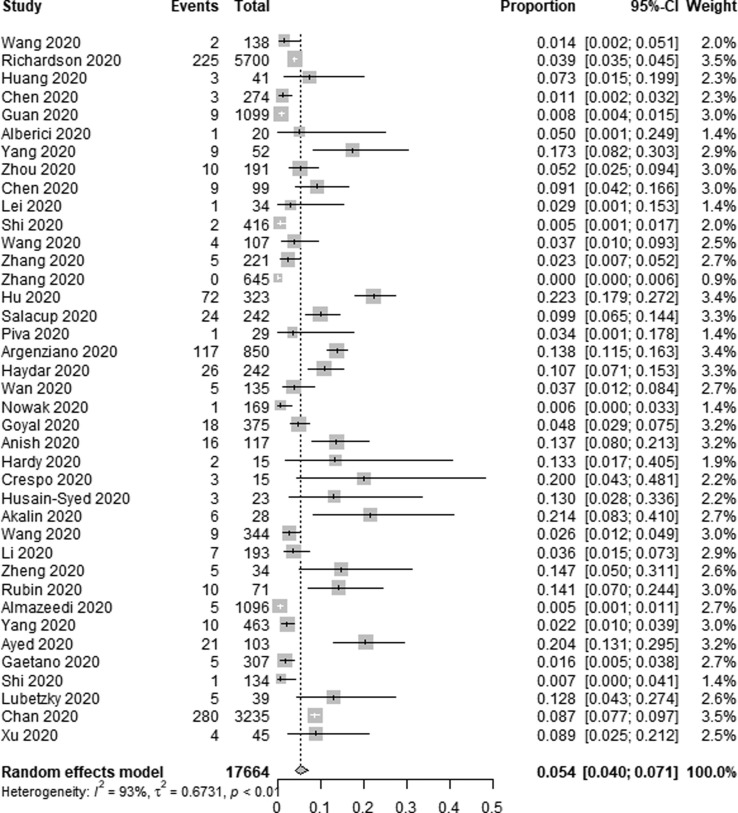
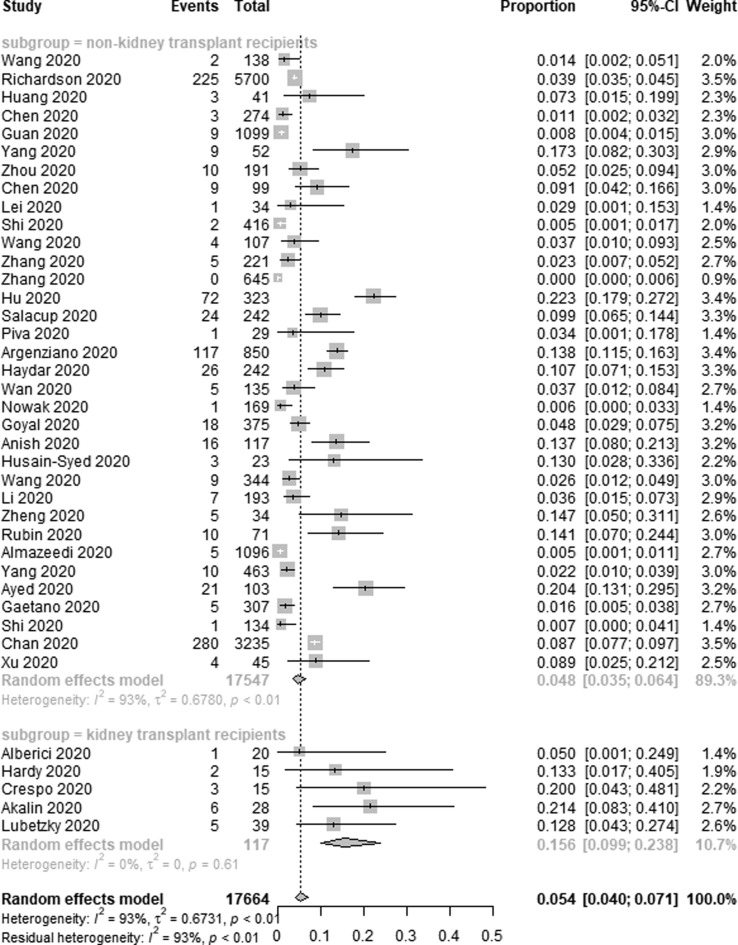
Data pertaining to the use of RRT among COVID-19 patients was reported in 39 of the studies included in the present meta-analysis. In total, RRT was used to treat 939 out of 17,664 COVID-19 patients in these studies, for a pooled application rate of 5.4% (95% CI 4.0–7.1%, I2 = 93%) (Fig. 3a ). Sensitivity analyses did not result in any meaningful changes in the high heterogeneity observed among the included studies. Subgroup analyses revealed that RRT was used in 15.6% of transplant patients (95% CI 9.9–23.8%, I2 = 0%) and in 4.8% of non-transplant patients (95% CI 3.5–6.4%, I2 = 93%) (Fig. 3b ).
Fig. 3a.
Forest plot depicting the incidence of RRT use in COVID-19 patients.
Fig. 3b.
Subgroup analysis depicting the incidence of RRT use in COVID-19 patients.
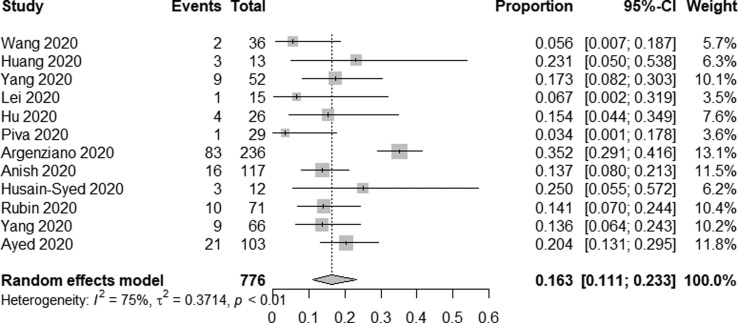
In total, 12 studies reported on RRT use among COVID-19 patients admitted to the ICU. Overall, RRT use was reported for 162/776 of these patients for a pooled incidence rate of 16.3% (95% CI 11.1–23.3%, I2 = 75%) (Fig. 3c ).
Fig. 3c.
Forest plot depicting the incidence of RRT use in intensive care unit Patients.
4. Discussion
We found that rates of AKI (12.3%) and RRT use (5.4%) were high among COVID-19 patients, the AKI incidence was lower in Asia (6.9%) compared to Europe (22.9%) and North America (34.6%). We also found that patients admitted to the ICU exhibited very high rates of AKI (39.0%) and RRT use (16.3%). This meta-analysis also reported the rates of AKI (38.9%) and RRT use (15.6%) among kidney transplant patients. While we were unable to specifically establish rates of mortality among AKI patients, we did determine that AKI was common among patients that died of COVID-19 (42.0%).
AKI development in COVID-19 patients may be driven by several mechanisms. For one, the virus may directly infect and damage renal cells. SARS-CoV-2 utilizes ACE2 as a cell entry receptor [70], and in humans, ACE2 is expressed on proximal tubular cells and podocytes [71], [72]. A postmortem analysis of postmortem renal histopathology in 26 COVID-19 patients conducted in China detected the presence of coronavirus particles within proximal tubular cells and podocytes, with these particles coinciding with diffuse acute proximal tubular injury and occasional podocyte vacuolation [73]. In a systematic review involving 11 studies, the positive rate of SARS‐CoV‐2 viral RNA in urine of 195 patients was 5.74% [10]. SARS‐CoV‐2 viral particles also have been detected in patient's blood, with an average period of one week between virus detection in the blood and AKI, suggesting that direct renal infection may be a key process driving the incidence of this dangerous condition [16], [19], [74], [75]. Another study found that SARS-CoV-2 may also be able to enter target cells using CD147, which is highly expressed in the kidneys, as a cell surface receptor [76], [77]. In critically ill patients, sepsis is also thought to be the primary driver of AKI incidence [78], and is common in deceased COVID-19 patients [18], [22]. Indeed, up to 20% of severe hospitalized COVID-19 patients develop viral sepsis and acute respiratory distress syndrome (ARDS) [79]. In individuals suffering from sepsis, hypoxia-related acute tubular necrosis (ATN) and severe hyperinflammation can drive AKI development [80]. Such hyperinflammation is closely associated with cytokine release syndrome (CRS), which results in intrarenal inflammation and increased vascular permeability [81]. CRS has frequently been detected among COVID-19 patients, with particularly elevated levels of IL-6 having bene detected in affected individuals [16], [82]. Organ crosstalk may also govern AKI pathogenesis. Indeed, ARDS and associated hypoxemia, inflammation, and the need for mechanical ventilation can result in the degradation of kidney hemodynamics and functionality [83]. Annat et al [84] found that continuous positive pressure ventilation (CPPV) was sufficient to decrease urine output, glomerular filtration rate (GFR), and renal blood flow (RBF), potentially resulting in AKI. Drug- or hyperventilation-related rhabdomyolysis can also result in tubular toxicity, and analyses of COVID-19 patient renal histopathology have suggested that rhabdomyolysis may be a disease-related complication as evidenced by the presence of pigmented casts within tubules and increased levels of creatine phosphokinase [73]. Ultimately, a number of pathogenic factors are likely to contribute to the incidence and severity of AKI in individuals suffering from COVID-19.
There are multiple limitations to the present study. For one, some of these outcomes were associated with high heterogeneity that may be attributable to the diverse COVID-19 incidence rates and treatment approaches in different countries, as well as to differences in study design and mutation-related changes in viral virulence. In addition, the definition of AKI was not always clear as it was generally not the main study outcome. As such, only pre-print studies that explicitly define AKI and published studies were included in this analysis. Furthermore, the majority of the studies included in this meta-analysis were conducted in China, and their applicability to other regions remains to be established.
In summary, AKI frequently occurs among patients suffering from COVID-19 and is most common among severely ill patients and among those that ultimately succumb to this disease. It is thus essential that the incidence of AKI be identified as quickly as possible in these patients so that RRT and other treatments can be initiated as appropriate in an effort. We believe that the present study will underscore the severity of AKI rates among COVID-19 patients, while emphasizing the need for further clinical studies of this severe complication.
Funding
None.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.intimp.2020.107159.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Drosten C., Günther S., Preiser W., van der Werf S., Brodt H.R., Becker S., et al. Identification of a Novel Coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348(20):1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 2.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 3.Munster V.J., Koopmans M., van Doremalen N., van Riel D., de Wit E. Novel Coronavirus emerging in China -key questions for impact assessment. N. Engl. J. Med. 2020;3828(8) doi: 10.1056/NEJMp2000929. [DOI] [PubMed] [Google Scholar]
- 4.COVID-19 Data Repository by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. https://github.com/CSSEGISandData/COVID-19. 2020 [accessed 31 July 2020].
- 5.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu K.H., Tsang W.K., Tang C.S., Lam M.F., Lai F.M., To K.F., et al. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int. 2005;67(2):698–705. doi: 10.1111/j.1523-1755.2005.67130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu X., Chen P., Wang J., Feng J., Zhou H., Li X., et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life Sci. 2020;63(3):457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Z., Wu M., Yao J.W., Guo J., Liao X., Song S.J., et al. Caution on kidney dysfunctions of COVID-19 patients. medRxiv. 2020 doi: 10.2139/ssrn.3559601. [DOI] [Google Scholar]
- 9.Ali H., Daoud A., Mohamed M.M., Salim S.A., Yessayan L., Baharani J., et al. Survival rate in acute kidney injury superimposed COVID-19 patients: a systematic review and meta-analysis. Ren. Fail. 2020;42(1):393–397. doi: 10.1080/0886022X.2020.1756323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan V.W., Chiu P.K., Yee C.H., Yuan Y., Ng C.F., Teoh J.Y. A systematic review on COVID-19: urological manifestations, viral RNA detection and special considerations in urological conditions. World J. Urol. 2020;10223 doi: 10.1007/s00345-020-03246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vrabel M. Preferred reporting items for systematic reviews and meta-analyses. Oncol. Nurs. Forum. 2015;42(5):552–554. doi: 10.1188/15.ONF.552-554. [DOI] [PubMed] [Google Scholar]
- 12.Donna F.S., Jesse A.B., Sally C.M., Ingram O., Williamson G.D., Drummond R., et al. Meta-analysis of observational studies in epidemiology. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 13.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected Pneumonia in Wuhan, China. JAMA. 2020;323(11) doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L.W., Li X., Chen H., Yan S.N., Li D., Li Y., et al. Coronavirus disease 19 infection does not result in acute kidney injury: an analysis of 116 hospitalized patients from Wuhan, China. Am. J. Nephrol. 2020;51(5):343–348. doi: 10.1159/000507471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323(20) doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang C.L., Wang Y.M., Li X.W., Ren L.L., Zhao J.P., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng Y.C., Luo R., Wang K., Zhang M., Wang Z.X., Dong L., et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen T., Wu D., Chen H.L., Yan W.M., Yang D.L., Chen G., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ (online) 2020;368:368. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alberici F., Delbarba E., Manenti C., Econimo L., Valerio F., Pola A., et al. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int. 2020;97(6):1083–1088. doi: 10.1016/j.kint.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respiratory Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arentz M., Yim E., Klaff L., Lokhandwala S., Riedo F.X., Chong M., et al. Characteristics and outcomes of 21 critically Ill patients with COVID-19 in Washington State. JAMA. 2020 doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banerjee D., Popoola J., Shah S., Ster I.C., Quan V., Phanish M. COVID-19 infection in kidney transplant recipients. Kidney Int. 2020;97(6):1076–1082. doi: 10.1016/j.kint.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng Y., Liu W., Liu K., Fang Y.Y., Shang J., Zhou L., et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 in Wuhan, China: a retrospective study. Chin. Med. J. 2020;133(11):1261–1267. doi: 10.1097/CM9.0000000000000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA. Cardiology. 2020;5(7) doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lei S., Jiang F., Su W., Chen C., Chen J., Mei W., et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. E Clin. Med. 2020;21 doi: 10.1016/j.eclinm.2020.100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA. Cardiology. 2020;5(7) doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang D.W., Yin Y.M., Hu C., Liu X., Zhang X.G., Zhou S.L., et al. Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan, China. Crit. Care. 2020;24(1) doi: 10.1186/s13054-020-02895-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang G., Hu C., Luo L., Fang F., Chen Y., Li J., et al. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J. Clin. Virol. 2020;127 doi: 10.1016/j.jcv.2020.104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X.L., Cai H., Hu J.H., Lian J.S., Gu J.Q., Zhang S.Y., et al. Epidemiological, clinical characteristics of cases of SARS-CoV-2 infection with abnormal imaging findings. Int. J. Infect. Dis. 2020;94:81–87. doi: 10.1016/j.ijid.2020.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu L., Chen S., Fu Y., Gao Z., Long H., Wang J.M., et al. Risk factors associated with clinical outcomes in 323 COVID-19 hospitalized patients in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brill S.E., Jarvis H.C., Ozcan E., Burns T.L.P., Warraich R.A., Amani L.J., et al. COVID-19: a retrospective cohort study with focus on the over-80s and hospital-onset disease. BMC Med. 2020;18(1) doi: 10.1186/s12916-020-01665-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheung Z.B., Forsh D.A. Early outcomes after hip fracture surgery in COVID-19 patients in New York City. J. Orthopaedics. 2020;21:291–296. doi: 10.1016/j.jor.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salacup G., Lo K.B., Gul F., Peterson E., Joy R.D., Bhargav R., et al. Characteristics and clinical outcomes of COVID-19 patients in an underserved-inner city population: A single tertiary center cohort. J. Med. Virol. 2020 doi: 10.1002/jmv.26252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piva S., Filippini M., Turla F., Cattaneo S., Margola A., De Fulviis S., et al. Clinical presentation and initial management critically ill patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in Brescia, Italy. J. Critical Care. 2020;58:29–33. doi: 10.1016/j.jcrc.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Argenziano M.G., Bruce S.L., Slater C.L., Tiao J.R., Baldwin M.R., Barr R.G., et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020 doi: 10.1136/bmj.m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitra A.R., Fergusson N.A., Lloyd-Smith E., Wormsbecker A., Foster D., Karpov A., et al. Baseline characteristics and outcomes of patients with COVID-19 admitted to intensive care units in Vancouver, Canada: a case series. Can. Med. Assoc. J. 2020;192(26):E694–E701. doi: 10.1503/cmaj.200794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aggarwal A., Shrivastava A., Kumar A., Ali A. Clinical and epidemiological features of SARS-CoV-2 patients in SARI ward of a tertiary care centre in New Delhi. J. Assoc. Physicians India. 2020;68 [PubMed] [Google Scholar]
- 41.Haydar A., Lo K.B., Goyal A., Gul F., Peterson E., Bhargav R., et al. Palliative care utilization among patients with COVID-19 in an underserved population: a single-center retrospective study. J. Pain Symptom Manage. 2020;60(2):e18–e21. doi: 10.1016/j.jpainsymman.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yi H., Lu F., Jin X., Chen R., Liu B., Dong X., et al. Clinical characteristics outcomes of coronavirus disease 2019 infections among diabetics: a retrospective and multicenter study in China. J. Diabetes. 2020 doi: 10.1111/1753-0407.13098. [DOI] [PubMed] [Google Scholar]
- 43.Wan S., Xiang Y., Fang W., Zheng Y., Li B., Hu Y., et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J. Med. Virol. 2020;92(7):797–806. doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nowak B., Szymański P., Pańkowski I., Szarowska A., Życińska K., Rogowski W., et al. Clinical characteristics and short-term outcomes of coronavirus disease 2019: retrospective, single-center experience of designated hospital in Poland. Polish. Arch. Intern. Med. 2020 doi: 10.20452/pamw.15361. [DOI] [PubMed] [Google Scholar]
- 45.Goyal P., Choi J.J., Pinheiro L.C., et al. Clinical characteristics of Covid-19 in New York City. N. Engl. J. Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Early Description of Coronavirus 2019 Disease in Kidney Transplant Recipients in New York. J. Am. Soc. Nephrol. 31(6) (2020) 1150–1156. https://doi.org/10.1681/ASN.2020030375. [DOI] [PMC free article] [PubMed]
- 47.Crespo M., Pérez-Sáez M.J., Redondo-Pachón D., Llinàs-Mallol L., Montero M.M., Villar-García J., et al. COVID-19 in elderly kidney transplant recipients. Am. J. Transplant. 2020 doi: 10.1111/AJT.16096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Borobia A., Carcas A., Arnalich F., Álvarez-Sala R., Monserrat-Villatoro J., Quintana M., et al. A cohort of patients with COVID-19 in a major teaching hospital in Europe. J. Clin. Med. 2020;9(6) doi: 10.3390/jcm9061733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cravedi P., Suraj S.M., Azzi Y., Haverly M., Farouk S., Perez-Saez M.J., et al. COVID-19 and kidney transplantation: results from the TANGO international transplant consortium. Am. J. Transplant. 2020 doi: 10.1111/ajt.16185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Husain-Syed F., Wilhelm J., Kassoumeh S., Birk H.W., Herold S., Vadasz I., et al. Acute kidney injury and urinary biomarkers in hospitalized patients with coronavirus disease-2019. Nephrol. Dial. Transplant. 2020;35(7):1271–1274. doi: 10.1093/ndt/gfaa162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nair V., Jandovitz N., Hirsch J.S., Nair G., Abate M., Bhaskaran M., et al. COVID-19 in kidney transplant recipients. Am. J. Transplant. 2020;20(7):1819–1825. doi: 10.1111/ajt.15967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Enver A., Yorg A., Rachel B., Harish S., Michael P., Vagish H. Covid-19 and kidney transplantation. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2011117. [DOI] [Google Scholar]
- 53.Wang Y., Lu X.F., Li Y.S., Chen H., Chen T.G., Su N., et al. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am. J. Respir. Crit. Care Med. 2020;201(11):1430–1434. doi: 10.1164/rccm.202003-0736LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Demir E., Uyar M., Parmaksiz E., Sinangil A., Yelken B., Dirim A.B., et al. COVID-19 in kidney transplant recipients: A multicenter experience in Istanbul. Transpl. Infect. Dis. 2020 doi: 10.1111/tid.13371. e13371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao W., Yu S.K., Zha X.Y., Wang N., Pang Q.M., Li D.Z., et al. Clinical characteristics and durations of hospitalized patients with COVID-19 in Beijing: a retrospective cohort study. medRxiv. 2020 doi: 10.1101/2020.03.13.20035436. [DOI] [Google Scholar]
- 56.Xiao G.H., Hu H.B., Wu F., Sha T., Huang Q.B., Li H.J., et al. Acute kidney injury in patients hospitalized with COVID-19 in Wuhan, China: A single-center retrospective observational study. medRxiv. 2020 doi: 10.1101/2020.04.06.20055194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang X.F., Tao J.X., Wu H., Wang Y.X., Zhao W., Zhou M., et al. Clinical features and management of severe COVID-19: A retrospective study in Wuxi, Jiangsu Province, China. medRxiv. 2020 doi: 10.1101/2020.04.10.20060335. [DOI] [Google Scholar]
- 58.Zheng Y., Sun L.J., Xu M., Pan J., Zhang Y.T., Fang X.L., et al. Clinical characteristics of 34 COVID-19 patients admitted to ICU in Hangzhou, China. medRxiv. 2020 doi: 10.1101/2020.04.12.20062604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.S. Rubin, A. Orieux, R. Prevel, A. Garric, M.-L. Bats, S. Dabernat, et al. Characterisation of acute kidney injury in critically Ill patients with severe coronavirus disease-2019 (COVID-19). medRxiv 2020. https://doi.org/10.1101/2020.05.06.20069872.2020. [DOI] [PMC free article] [PubMed]
- 60.Almazeedi S., Al Youha S., Jamal M.H., Al-Haddad M., Al-Muhaini A., Al-Ghimlas F., et al. Clinical characteristics, risk factors and outcomes among the first consecutive 1,096 patients diagnosed with COVID-19: The Kuwait Experience. medRxiv. 2020 doi: 10.1101/2020.05.09.20096495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang S., Ma L., Wang Y.-L., Wang Q., Tong Q., Chen M., et al. Risk factors for critical-ill events of patients with COVID-19 in Wuhan, China: a retrospective cohort study. medRxiv. 2020 doi: 10.1101/2020.06.14.20130765. [DOI] [Google Scholar]
- 62.M. Ayed, A. Borahmah, A. Yazdani, A. Sultan, A. Mossad, H. Rawdhan, Assessment of clinical characteristics and mortality-associated factors in COVID-19 Critical cases in Kuwait, medRxiv 2020. https://doi.org/10.1101/2020.06.17.20134007. [DOI] [PMC free article] [PubMed]
- 63.G. Alfano, A. Ferrari, F. Fontana, G. Mori, R. Magistroni, M. Marianna, et al., Incidence, risk factors and mortality outcome in patients with acute kidney injury in COVID-19: a single-center observational study, medRxiv 2020. https://doi.org/10.1101/2020.06.24.20138230.
- 64.Shi P.Y., Ren G.X., Yang J., Li Z.Q., Deng S.J., Li M., et al. Clinical characteristics of imported and second-generation COVID-19 cases outside Wuhan, China: A multicenter retrospective study. medRxiv. 2020 doi: 10.1101/2020.04.19.20071472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lubetzky M., Aull M., Craig-Shapiro R., Lee J., Lee J., Sultan S., et al. Kidney allograft recipients diagnosed with coronavirus disease-2019: a single center report. medRxiv. 2020 doi: 10.1101/2020.04.30.20086462. [DOI] [Google Scholar]
- 66.L.L. Chan, K. Chaudhary, A. Saha, K. Chauhan, A. Vaid, M. Baweja, et al. Acute kidney injury in hospitalized patients with COVID-19. medRxiv 2020. https://doi.org/10.1101/2020.05.04.20090944.
- 67.W.A. Pongpirul, S. Wiboonchutikul, L. Charoenpong, N. Panitantum, A. Vachiraphan, S. Uttayamakul, et al. Clinical course and potential predicting factors of pneumonia of adult patients with coronavirus disease 2019 (COVID-19): A retrospective observational analysis of 193 confirmed cases in Thailand. medRxiv 2020. https://doi.org/10.1101/2020.06.24.20139642. [DOI] [PMC free article] [PubMed]
- 68.Xu Y.H., Xu Z.H., Liu X.S., Cai L.H., Zheng H.C., Huang Y.B., et al. Clinical findings in critically ill patients infected with SARS-CoV-2 in Guangdong Province, China: a multi-center, retrospective, observational study. medRxiv. 2020 doi: 10.1101/2020.03.03.20030668. [DOI] [Google Scholar]
- 69.Yan S.J., Song X.Y., Lin F., Zhu H.Y., Wang X.Z., Li M., et al. Clinical characteristics of coronavirus disease 2019 in Hainan China. medRxiv. 2020 doi: 10.1101/2020.03.19.20038539. [DOI] [Google Scholar]
- 70.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e278. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zou X., Chen K., Zou J.W., Han P.Y., Hao J., Han Z.G. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020;14(2):185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Monteil V., Kwon H., Prado P., Hagelkrüys A., Wimmer R.A., Stahl M., et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181(4):905–913.e907. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Su H., Yang M., Wan C., Yi L.X., Tang F., Zhu H.Y., et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(1):219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peng L., Liu J., Xu W.X., Luo Q.M., Chen D.B., Lei Z.Y., et al. SARS-CoV-2 can be detected in urine, blood, anal swabs, and oropharyngeal swabs specimens. J. Med. Virol. 2020 doi: 10.1002/jmv.25936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang W.L., Xu Y., Gao R., Lu R.J., Han K., Wu G.Z., et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA J. Am. Med. Assoc. 2020;323(18) doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang K., Chen W., Zhou Y.S., Lian J.Q., Zhang Z., Du P., et al. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. medRxiv. 2020 doi: 10.1101/2020.03.14.988345. [DOI] [Google Scholar]
- 77.Kosugi T., Maeda K., Sato W., Maruyama S., Kadomatsu K. CD147 (EMMPRIN/Basigin) in kidney diseases: from an inflammation and immune system viewpoint. Nephrol. Dial. Transplant. 2015;30(7):1097–1103. doi: 10.1093/ndt/gfu302. [DOI] [PubMed] [Google Scholar]
- 78.Poukkanen M., Vaara S.T., Pettila V., Kaukonen K.-M., Korhonen A.-M., Hovilehto S., et al. Acute kidney injury in patients with severe sepsis in Finnish Intensive Care Units. Acta Anaesthesiol. Scand. 2013;57(7):863–872. doi: 10.1111/aas.12133. [DOI] [PubMed] [Google Scholar]
- 79.Prescott H.C., Girard T.D. Recovery from severe COVID-19 leveraging the lessons of survival from sepsis. JAMA. 2020 doi: 10.1001/jama.2020.14103. on 08/22/2020. [DOI] [PubMed] [Google Scholar]
- 80.Aslan A., van den Heuvel M.C., Stegeman C.A., Popa E.R., Leliveld A.M., Molema G., et al. Kidney histopathology in lethal human sepsis. Crit. Care. 2018;22(1):359. doi: 10.1186/s13054-018-2287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tisoncik J.R., Korth M.J., Simmons C.P., Farrar J., Martin T.R., Katze M.G. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 2012;76(1):16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.David S., Inbal Z.L., Lori R., Shane R., Carl F.W., Garry A.N. Levels of the TNF-related cytokine LIGHT increase in hospitalized COVID-19 patients with cytokine release syndrome and ARDS. Clin. Sci. Epidemiol. 2020;5(4) doi: 10.1128/mSphere.00699-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Darmon M., Clec'h C., Adrie C., Argaud L., Allaouchiche B., Azoulay E., et al. Acute respiratory distress syndrome and risk of AKI among critically ill patients. Clin. J. Am. Soc. Nephrol. 2014;9(8):1347–1353. doi: 10.2215/CJN.08300813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Annat G., Viale J.P., Xuan B.B. Effect of PEEP ventilation on renal function, plasma renin, aldosterone, neurophysins and urinary ADH, and prostaglandins. Anesthesiology. 1983;582(2) doi: 10.1097/00000542-198302000-00006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.