Abstract
Here, we report our studies of immune-mediated regulation of Zika virus (ZIKV), herpes simplex virus 1 (HSV-1), and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in the human cornea. We find that ZIKV can be transmitted via corneal transplantation in mice. However, in human corneal explants, we report that ZIKV does not replicate efficiently and that SARS-CoV-2 does not replicate at all. Additionally, we demonstrate that type III interferon (IFN-λ) and its receptor (IFNλR1) are expressed in the corneal epithelium. Treatment of human corneal explants with IFN-λ, and treatment of mice with IFN-λ eye drops, upregulates antiviral interferon-stimulated genes. In human corneal explants, blockade of IFNλR1 enhances replication of ZIKV and HSV-1 but not SARS-CoV-2. In addition to an antiviral role for IFNλR1 in the cornea, our results suggest that the human cornea does not support SARS-CoV-2 infection despite expression of ACE2, a SARS-CoV-2 receptor, in the human corneal epithelium.
Keywords: SARS-CoV-2, coronavirus, herpes simplex virus, Zika virus, eye, cornea, COVID-19, interferon lambda, type III interferon
Graphical Abstract
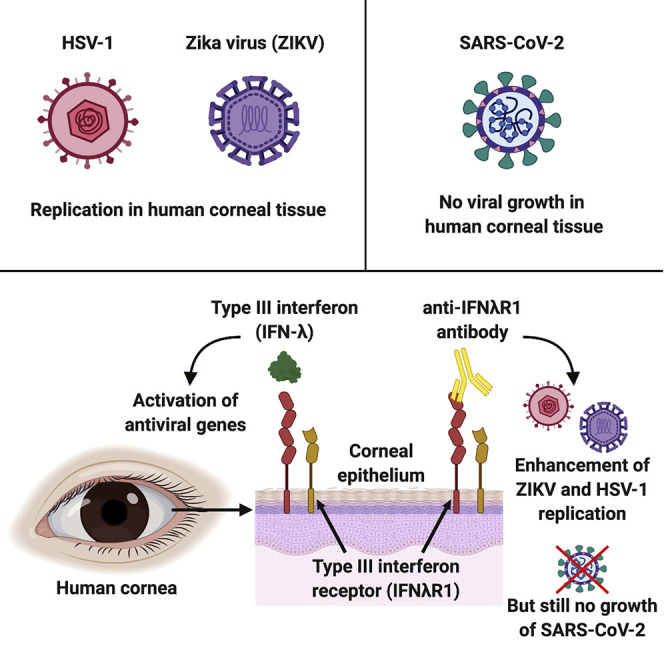
Miner et al. report that type III interferon (IFN-λ) and its receptor (IFNλR1) restrict herpes simplex virus 1 (HSV-1) and Zika virus (ZIKV) growth in the human cornea. Human corneal explants did not support severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection, even after blockade of the type III IFN receptor.
Introduction
The Zika virus (ZIKV) epidemic and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic have led to speculation that these viruses may, in some cases, enter the body through the eyes. Other viruses, including herpes simplex virus 1 (HSV-1), are well known to efficiently replicate in the cornea. Indeed, corneal infection with HSV-1 is a major cause of corneal damage and vision loss (Farooq and Shukla, 2012). However, the immune mechanisms that regulate infection of the human cornea are incompletely understood.
During viral infections, type I and type III interferons (IFNs) induce expression of IFN-stimulated genes (ISGs) that restrict viral replication and spread (Hoffmann et al., 2015; Lazear et al., 2015; Miner et al., 2016a, 2016b). Whereas the type I IFN receptor (IFNAR1) is expressed ubiquitously, the type III IFN receptor (IFNλR1; IL28RA) has a more restricted expression pattern, leading to antiviral effects that are especially notable at barrier surfaces, including the placenta, gut, urogenital tract, and blood-brain barrier (Bayer et al., 2016; Jagger et al., 2017; Lazear et al., 2015; Nice et al., 2015). A role for IFNλR1 signaling in the human cornea has not previously been described.
HSV-1 infection of the human cornea requires emergency treatment, including antiviral and corticosteroid therapy to help prevent corneal opacification and to preserve vision (Farooq and Shukla, 2012). HSV-1 keratitis alone is responsible for ∼40,000 new cases of visual impairment and blindness globally each year (Farooq and Shukla, 2012). Other viruses, including flaviviruses (e.g., ZIKV) also trigger ocular inflammation (e.g., conjunctivitis, uveitis) and can be detected in the vitreous humor in rare cases (de Paula Freitas et al., 2017; Heck et al., 2017; Kodati et al., 2017; Miner et al., 2016b). Additionally, SARS-CoV-2 RNA has been detected in tears (Fang et al., 2020; Karimi et al., 2020; Wu et al., 2020), and it has been widely hypothesized that the eye, including the cornea and conjunctiva, may serve as a route of entry for SARS-CoV-2.
ZIKV does not replicate efficiently in immunocompetent mice (Grant et al., 2016). Therefore, in our prior studies of ZIKV pathogenesis in the eye, it was necessary to study immunodeficient Ifnar1 −/− animals (Miner et al., 2016b). We found that ZIKV invades and replicates in the eyes of mice and that ZIKV RNA was detectable in the cornea. However, we did not test whether viral RNA found in the cornea was associated with infectious virus (Miner et al., 2016b). Subsequently, humans with ZIKV infection were reported to have ZIKV RNA and infectious ZIKV in conjunctival swabs (Sun et al., 2017). ZIKV-induced uveitis and conjunctivitis also occur in humans (de Paula Freitas et al., 2017; Kodati et al., 2017), suggesting that ZIKV can infect the eye and cause ocular inflammation.
Here, we report that HSV-1 and ZIKV, but not SARS-CoV-2, can replicate in human corneal explants. We found that type III IFN (also known as IFN-λ) and its receptor (IFNλR1) are expressed and functional in the human and mouse cornea. Using a model of corneal transplantation in mice, we found that ZIKV is only rarely transmitted by corneal transplantation in immunodeficient AG129 mice that lack the type I and type II IFN receptors. This led us to hypothesize that IFN-λ may play a role in controlling infection in the cornea. Indeed, we found that IFN-λ and IFNλR1 are expressed within and exhibit antiviral activity in cultured human corneal explants. Furthermore, we found that antiviral ISGs are constitutively upregulated in the epithelium of human corneal explants and that treatment of mice with IFN-λ eye drops causes IFNλR1-dependent upregulation of antiviral ISGs. Blockade of IFNλR1 in human corneal explants led to enhanced replication of ZIKV and a ∼10-fold increase in replication of HSV-1. In contrast, blockade of IFNλR1 did not result in permissiveness of human corneal tissue to infection with SARS-CoV-2. Thus, IFNλR1 restricts HSV-1 and ZIKV infection in human corneal tissue. Furthermore, resistance of human corneal tissue to SARS-CoV-2 infection is likely regulated by a distinct antiviral pathway.
Results and Discussion
ZIKV Transmission by Corneal Transplantation in Mice
Although we previously detected ZIKV RNA in mouse corneas after subcutaneous inoculation, we did not test whether this viral RNA represents infectious viral particles (Miner et al., 2016b). AG129 mice, which lack both type I and type II IFN receptors (van den Broek et al., 1995), are highly susceptible to ZIKV. Indeed, even a single infectious particle is sufficient to cause death in AG129 animals (Aliota et al., 2016). To test whether infectious virus was present in mouse corneas following subcutaneous inoculation with ZIKV, we intraperitoneally inoculated 8-week-old AG129 animals with corneal homogenates after microdissection of eyes from Ifnar1 −/− mice 7 days after subcutaneous inoculation with a clinical ZIKV isolate from Brazil. Three of 11 AG129 mice inoculated with corneal homogenates developed ZIKV encephalitis and succumbed to infection (Figure 1 A). Thus, infectious ZIKV is associated with the corneas of Ifnar1 −/− mice following subcutaneous footpad inoculation.
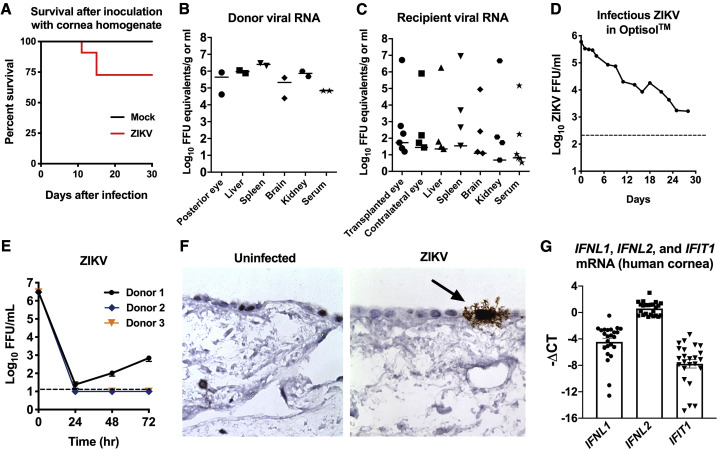
Figure 1.
ZIKV Can Be Transmitted via Corneal Transplantation in AG129 Mice but Infects Human Corneal Explants Inefficiently
(A) Survival of AG129 mice inoculated with Ifnar1−/− corneal homogenates 7 days after subcutaneous inoculation with ZIKV (n = 11) or uninfected Ifnar1−/− cornea control homogenates (n = 4).
(B and C) ZIKV mRNA expression in tissues was quantitated using qRT-PCR. Tissues were harvested from cornea donors (n = 2) and recipient (n = 6) mice on days 6 and 7 after infection.
(D) ZIKV (6 × 105 FFU) was stored in cornea storage medium (Optisol) at 4°C for 28 days. Infectious virus was measured by focus forming assay at the indicated time points.
(E) Human donor corneas (n = 4 technical replicates per donor from three independent donor corneas) were inoculated with 3 × 106 FFU/mL of ZIKV (Brazil strain), and infectious virus was quantitated at the indicated time points by focus forming assay. Data represent the mean ± SEM.
(F) RNA ISH and histological analysis was performed on uninfected (left panel) and ZIKV-infected (right panel) human corneal explants in order to determine virus tropism. The black arrow indicates infected corneal epithelial cells.
(G) Baseline expression of IFNL1, IFNL2, and IFIT1 was measured using qRT-PCR in three human donor corneas in independent experiments. Data represent the mean ΔCT ± SEM relative to expression of GAPDH. Each cornea was sectioned to produce eight technical replicates per experiment (n = 24) from three biological replicates in independent experiments.
Transmission of viruses by solid organ transplantation is a major clinical concern that requires careful safeguards (Heck et al., 2017; Nogueira et al., 2017). Furthermore, ZIKV transmission via solid organ transplantation has been previously described in humans (Nogueira et al., 2017). To test whether ZIKV transmission might be possible after corneal transplantation in animals, we performed corneal transplantation experiments in AG129 mice. Donor AG129 mice were subcutaneously inoculated with 1,000 FFU of ZIKV and euthanized 7 days after infection, followed by tissue harvesting and microdissection of corneas. We confirmed the presence of 105–106 FFU equivalents of ZIKV in the posterior eye, liver, spleen, brain, kidney, and serum of infected donor animals (Figure 1B). The donor corneas were washed extensively in PBS and subsequently transplanted into naive AG129 recipient mice. Six days after transplantation, recipient mice were euthanized, and ZIKV RNA in multiple organs was quantitated using qRT-PCR. Although viral RNA was detectable in multiple organs at low levels in all recipient animals, severe infection of multiple organs occurred in just one of the six recipient mice (Figure 1C). Thus, ZIKV can cause systemic infection following corneal transplantation in mice, but this occurs infrequently. Next, to evaluate whether ZIKV remains stable and infectious in cornea storage medium, we tested the stability of ZIKV in Optisol. ZIKV (6 × 105 FFU per tube) was stored in Optisol at 4°C for 1 month, followed by quantitation of infectious virus by focus forming assay. By day 28, more than 103 FFU/mL of infectious ZIKV was still present under these conditions (Figure 1D), indicating that infectious ZIKV can persist in conditions typical for storage of human donor corneas.
ZIKV Infects the Human Corneal Epithelium but Does Not Replicate Efficiently in Human Corneal Explants
To test whether ZIKV replicates in human corneal tissue, we performed viral growth curve studies by inoculating human corneal explants with 3 × 106 FFU/mL of ZIKV, followed by focus forming assays over the course of 72 h (Figures 1E and F). ZIKV replicated inefficiently in just one of three human cornea donor samples (Figure 1E), suggesting that the human cornea is somewhat resistant to ZIKV infection. To define ZIKV tropism in the human cornea, we performed RNA in situ hybridization (ISH) of ZIKV-infected corneal explants 24 h after infection (Figure 1F). Histological analysis revealed that ZIKV preferentially infects a small number of corneal epithelial cells, without appreciably penetrating into deeper layers of the human cornea. We hypothesized that constitutive IFN signaling may be controlling viral infection in the human corneal explants, so we measured IFN and ISG expression in human corneal explants cultured in the absence of virus. We observed expression of type III IFNs (IFNL1, IFNL2) and IFIT1 (an ISG) mRNA, even in the absence of infection (Figure 1G). As IFNL1 and IFNL2 expression is regulated by IRF3, we hypothesized that either cytosolic DNA- or RNA-sensing pathways may upregulate IFNL1 or IFNL2 via IRF3 downstream of TBK1 signaling induced by endogenous retroelements. To test this hypothesis, we treated corneas with an antiretroviral drug (AZT) or TBK1 inhibitor (MRT67307), but neither of these inhibitors affected basal expression of IFNL1 or IFNL2 (Figure S1), suggesting that basal expression of IFNL1 and IFNL2 are regulated independently of TBK1-mediated phosphorylation of IRF3.
Treatment of Mouse Corneal Explants with IFN-λ Upregulates Expression of ISGs
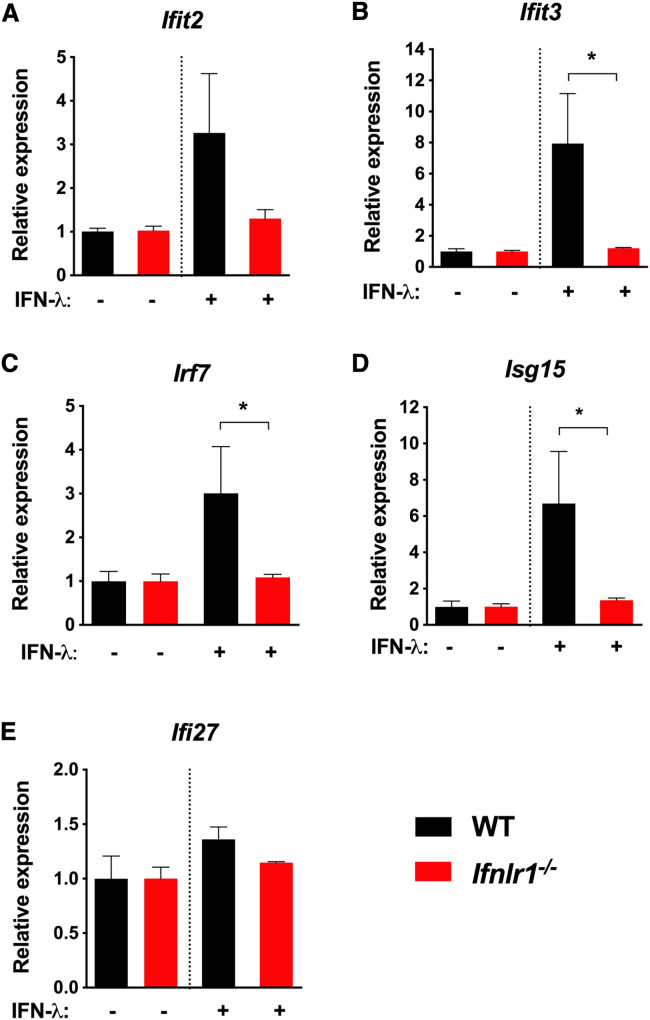
As IFN-λ restricts infection at some barrier surfaces, we reasoned that topical treatment with IFN-λ eye drops might upregulate ISG expression in mice specifically in the cornea. To test this hypothesis, we administered two doses of 1 μg topical recombinant IFN-λ eye drops or PBS control to wild-type (WT) and Ifnlr1 −/− mice, followed by microdissection of corneas and quantitation of ISG expression using qRT-PCR. In WT mice, but not Ifnlr1 −/− mice, treatment with IFN-λ caused upregulation of multiple ISGs, including Ifit2, Ifit3, and Isg15, but not Ifi27 (Figures 2 A–2E). These results indicate that topical IFN-λ induces antiviral gene expression in the mouse cornea under physiological conditions.
Figure 2.
IFN-λ Eye Drops Induce ISG Expression in WT but Not Ifnlr1−/− Corneas
Adult WT and Ifnlr1−/− animals were treated with recombinant mouse IFN-λ (1 μg) in 10 μL PBS or PBS vehicle control. Mice were treated again 3 h later and then euthanized for corneal microdissection 6 h after the first treatment. Corneas were homogenized in PBS, and mRNA was isolated for gene expression analysis. ISG expression in corneas was quantitated using qRT-PCR (n = 3–5 mice per group). Results represent the mean ± SEM of data pooled from two independent experiments. Results were analyzed using the Mann-Whitney test. ∗p < 0.05.
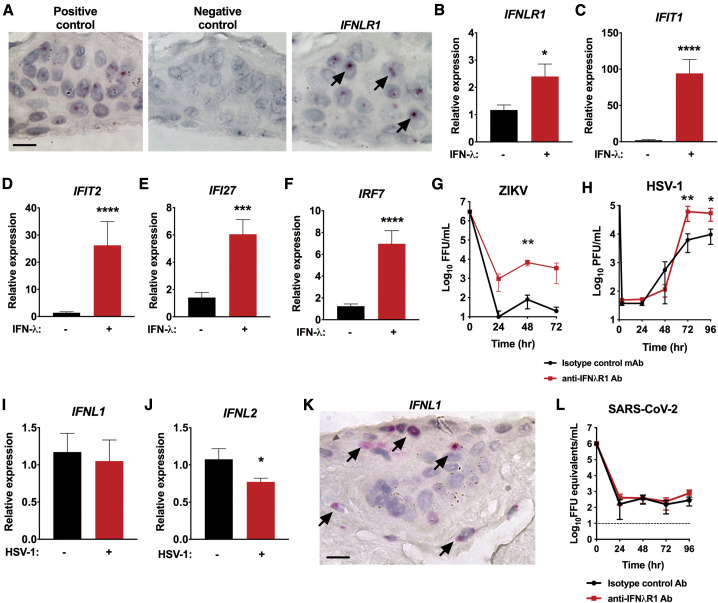
To determine whether IFN-λ promotes antiviral immunity in the human cornea, we began by performing RNA ISH and discovered that IFNλR1 is expressed specifically in the human corneal epithelium (Figure 3 A), which are target cells for viruses known to cause corneal pathology, including HSV-1 (Oh, 1976). Treatment of human corneal explants with 4 × 104 U/mL of IFN-λ for 6 h led to a ∼2-fold increase in IFNLR1 mRNA (Figure 3B), as well as a ∼50-fold increase in expression of IFIT1 and a ∼25-fold increase in IFIT2 (Figures 3C and 3D). IFI27 and IRF7 also were upregulated by approximately ∼3-fold to 5-fold after treatment with IFN-λ (Figures 3E and 3F). Thus, IFN-λ induces expression of antiviral ISGs in the human cornea.
Figure 3.
The IFN-λ Receptor (IFNλR1) Regulates Antiviral ISG Expression and Restricts HSV-1 Growth in Human Corneal Explants
(A) RNA ISH and histological analysis were performed on human donor corneas to determine IFNLR1 mRNA expression in the corneal epithelium. Panels shown are positive control housekeeping gene (left), negative control probe (middle), and IFNLR1 (right). Scale bar, 10 μm. Data are representative of three independent experiments with unique donor corneas. Black arrows indicate positive staining of cells for IFNLR1.
(B–F) Human donor corneas were treated with IFN-λ or PBS vehicle control, and ISG expression from human corneal explants was quantitated using qRT-PCR. Data represent the mean ± SEM of 12 samples, including 4 cornea segments per donor from 3 donor corneas. Results were pooled from two independent experiments.
(G) ZIKV replication in a representative human donor cornea sample treated with anti-IFNλR1 or isotype control antibody. Infectious units of ZIKV were quantitated by focus forming assay. ZIKV replicated in only 2 of 5 human cornea samples under these conditions. Results were analyzed using two-way ANOVA.
(H) HSV-1 replication in human donor corneas treated with anti-IFNλR1 or isotype control antibody. Infectious units of HSV-1 were quantitated at the 3 h eclipse phase and every 24 h thereafter by plaque assay. Data represent the mean ± SEM of n = 12 replicates, including 4 technical replicates per group from 3 corneas. Results in (H) were pooled from three independent experiments and were analyzed using two-way ANOVA.
(I and J) IFNL1 and IFNL2 mRNA expression levels were quantitated using qRT-PCR 24 h after infection with HSV-1. Data represent the mean ± SEM of mock-infected (n = 3) and infected (n = 3) human donor corneas with 4 cornea segments per donor in two independent experiments.
(K) RNA ISH and histological analysis were performed on human donor corneas to determine IFNL1 mRNA expression in the cornea. Scale bar, 10 μm. Data are representative of 10 samples from seven independent donors. Samples from two donors repeatedly did not stain for positive control (or target) and were excluded. Black arrows indicate positive staining of cells for IFNL1.
(L) SARS-CoV-2 replication in human donor corneas samples treated with anti-IFNλR1 or isotype control antibody. SARS-CoV-2 mRNA expression was measured using qRT-PCR. Data represent the mean ± SEM of n = 7–11 replicates, with two to four technical replicates per group from 3 donor corneas. Results in (L) were pooled from two independent experiments and were analyzed by two-way ANOVA.
Results in (B)–(F), (I), and (J) were pooled from two independent experiments and were analyzed using the Mann-Whitney test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001. See also Figure S1.
To test whether IFN-λ protects against infection with ZIKV, we pre-treated human donor corneas for 1 h with either anti-IFNλR1 monoclonal antibody (mAb) or a control mAb (Kotenko et al., 2003) and then performed viral growth curve analysis as described in Figure 1. In two of five samples, blockade of the IFNλR1 resulted in an approximately 100-fold greater ZIKV replication (Figure 3G). However, in other growth curve experiments of ZIKV in the human cornea, viral replication was not detected (data not shown). This suggests that the role of IFNλR1 in controlling ZIKV infection is variable in distinct donors.
Next, as HSV-1 is well established as a corneal pathogen in humans, we inoculated human corneal explants with 106 PFU/mL of HSV-1 in the presence of the same blocking anti-IFNλR1 mAb or control mAb (Kotenko et al., 2003). This led to consistently enhanced replication of HSV-1 in all infected human donor samples, with approximately 10-fold higher levels of infectious virus by 72 h after infection (6.1 × 104 PFU/mL in anti-IFNλR1 treated corneas versus 6.3 × 103 PFU/mL in control mAb-treated corneas, p < 0.005) (Figure 3H). HSV-1 blocks the type I IFN response in order to facilitate viral replication (Yuan et al., 2018), so we reasoned that HSV-1 may similarly antagonize IFN-λ production or signaling. Quantitation of IFNL1 and IFNL2 mRNA with and without HSV-1 revealed that HSV-1 infection reduces expression of IFNL2 within the first 24 h after infection (Figures 3I and 3J). RNA ISH demonstrated that IFNL1 was expressed primarily within the corneal epithelial cells, similar to what was observed for the IFNLR1 (Figure 3K). Thus, IFN-λ and IFNλR1 are expressed in the human corneal epithelium, and signaling through IFNλR1 restricts replication of HSV-1 in human corneal explants.
It has been widely speculated that SARS-CoV-2 may infect the eye (Xia et al., 2020), although it is unusual for patients with SARS-CoV-2 to develop ocular symptoms. The possibility of corneal infection is supported by the fact that ACE2, a receptor for SARS-CoV-2, is highly expressed in the human corneal epithelium (Collin et al., 2020; Zhou et al., 2020). Therefore, we tested whether a clinical isolate of SARS-CoV-2 could replicate in human corneal tissue. However, we detected no evidence of SARS-CoV-2 replication using qRT-PCR in seven independent donor samples. The same negative results were obtained by plaque assay (data not shown). Positive controls were used in all experiments, including conformation that HSV-1 could replicate in portions of the very same donor corneas. Unlike what was observed with HSV-1 and ZIKV, blockade of IFNλR1 did not make the human corneal tissue more permissive to infection with SARS-CoV-2 (Figure 3L). As the corneal explants also include a small amount of conjunctival tissue, our findings suggest that the human cornea and conjunctiva may not support infection with SARS-CoV-2 and that antiviral immunity against SARS-CoV-2 in the cornea is mediated by a pathway other than IFNλR1 signaling. Nevertheless, further study of human patient and autopsy samples will be necessary to confirm that the human cornea and conjunctiva are resistant to infection with SARS-CoV-2.
Studies of antiviral immunity at barrier surfaces previously demonstrated that IFN-λ regulates permeability of the blood-brain barrier (Lazear et al., 2015), protects the gut against enteric virus infection (Nice et al., 2015), and promotes antiviral immunity at the placental barrier (Jagger et al., 2017). We discovered that the human corneal barrier also is a site of action for IFN-λ and IFNλR1 and that IFNλR1 signaling inhibits replication of HSV-1 in human corneal explants. Additionally, we found that IFN-λ eye drops upregulate antiviral ISGs in WT but not in Ifnlr1 −/− corneas in mice, revealing a capacity of IFN-λ to exert a topical effect. As the IFN-λ antiviral pathway was not previously described to have activity in the human eye, our discoveries may lead to further exploration of the role of IFN-λ and IFNλR1 during ocular infection and inflammatory eye disease pathogenesis in humans.
IFNλR1 signaling likely occurs in corneal epithelial cells. In support of this conclusion, we detected expression of IFNLR1 and IFNL1 mRNA within the human corneal epithelium. Corneal epithelial cells also are a primary target for HSV-1 infection of the cornea (Farooq and Shukla, 2012; Oh, 1976), and we found that blockade of IFNλR1 signaling led to more severe HSV-1 infection of human corneal explants, indicating that IFN-λ signaling in the cornea mediates protection against herpesvirus infection. As our experiments only tested the role of IFN-λ during infection of human corneas, future studies in mice may allow us to examine whether similar anti-viral effects occur in animal models. Although prior studies of the mouse cornea have not examined the effect of IFNλR1 blockade or genetic deletion, treatment of mice with recombinant IL-28A (IFN-λ) affected immune responses and infection in a model of stromal keratitis (Jaggi et al., 2018), providing further support for the idea that IFN-λ is relevant in the eyes of mice. Future studies in mouse models using Cre-lox alleles for IFNλR1 and IFN-λ2/3 will allow us to define the cell type-specific effects of IFN-λ and IFNλR1 during corneal inflammation and infection.
We previously found that ZIKV infects the corneas of mice lacking IFNAR1 (Ifnar1 −/− mice) (Miner et al., 2016b). Although we confirmed that ZIKV can be transmitted by corneal transplantation in immunodeficient mice, severe systemic infection occurred in only one of six animals. Furthermore, ZIKV replicated inefficiently in human corneal explants, suggesting that the cornea is unlikely to be a major target of ZIKV infection despite what has been observed in animal models. Nevertheless, ZIKV RNA has previously been detected in the vitreous humor of a human donor sample (Heck et al., 2017) and in human conjunctival swabs (Sun et al., 2017), suggesting that ZIKV can occasionally be harbored in or associated with human corneas. As even one infection occurring as a consequence of corneal transplantation could be harmful, there is a need to continue screening donated corneas for arboviruses, especially in the context of virus outbreaks.
We discovered that HSV-1 has the capacity to antagonize production of IFN-λ2 in the human cornea, implying that immune evasion of IFN-λ may contribute to HSV-1 disease pathogenesis. Prior cell culture studies demonstrated that IFN-λ inhibits HSV-1 replication in astrocytes and neurons by stimulating the expression of endogenous type I IFN and ISGs (Li et al., 2011). Our study confirms that IFNλR1 signaling restricts HSV-1 in human samples and also reveals a role for this pathway in the human eye.
In addition to expression in corneal epithelial cells, IFNλR1 is expressed on neutrophils, which are known to infiltrate and damage the cornea during HSV-1 infection (Thomas et al., 1997). Remarkably, in addition to controlling infection, IFN-λ can ameliorate inflammatory responses mediated by neutrophils (Blazek et al., 2015). Thus, our work may inform further studies of disease pathogenesis in animals, as well as therapies targeting IFNλR1. This may eventually lead to prophylactic or therapeutic administration of IFN-λ to control infection or to dampen destructive immune responses in the cornea.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse anti-human interferon lambda receptor 1 neutralizing mAb | PBL assay science | Cat# 21885-1 Unable to locate RRID |
| Mouse IgG1 kappa isotype control mAb | Sigma-Aldrich | Cat# M7894, RRID:AB_1163632 |
| Bacterial and Virus Strains | ||
| Herpes simplex virus-1 strain 17+ | Bertke et al., 2013 | N/A |
| Zika virus, strain Paraiba 2015 (Brazil, 2015) | Magnani et al., 2018 | N/A |
| SARS-CoV-2 (strain 2019 n-CoV/USA_WA1/2020) | CDC/BEI Resources | NR52281 |
| Biological Samples | ||
| Human corneas | Mid-America Transplant Institute | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Recombinant Human IL-29/IFN Lambda 1, HCE | PBL assay science | Cat# 11725-1 |
| Recombinant Mouse IL-28B/IFN Lambda 3 | PBL assay science | Cat# 12820-1 |
| Critical Commercial Assays | ||
| RNeasy Mini Kit | QIAGEN | Cat# 74104 |
| TaqMan RNA-to-Ct 1-Step Kit | ThermoFisher | Cat# 4392653 |
| Experimental Models: Cell Lines | ||
| Vero CCL81 cells | ATCC | CCL81; RRID: CVCL_0059 |
| Vero E6 cells | ATCC | CRL-1586; RRID: CVCL_0574 |
| Experimental Models: Organisms/Strains | ||
| Mouse: Ifnar1−/− | Jackson Laboratory | Cat# JAX:032048, RRID:IMSR_JAX:032048 |
| Mouse: AG129 | Marshall BioResources | AG129 |
| Mouse: Ifnlr1−/− | Baldridge et al., 2017 | N/A |
| Oligonucleotides | ||
| SARS-CoV-2 N F: 50 -ATGCTGCAATCGTGCTACAA-3 | Hassan et al., 2020 | N/A |
| SARS-CoV-2 N R: 50 -GACTGCCGCCTCTGCTC-30 | Hassan et al., 2020 | N/A |
| SARS-CoV-2 N Probe: 50 -/56-FAM/TCAAGGAAC/ZEN/ AACATTGCCAA/3IABkFQ/-30 | Hassan et al., 2020 | N/A |
| RNAscope® Probe- V-ZIKV | Advanced Cell Diagnostics | Cat# 467771 |
| RNAscope® Probe- Hs-IFNLR1 | Advanced Cell Diagnostics | Cat# 494771 |
| RNAscope® Probe- Hs-IFNL1 | Advanced Cell Diagnostics | Cat# 412341 |
| IFNL1 PrimeTime qPCR Assay | Integrated DNA Technologies | Hs.PT.56a.21113836.g |
| IFNL2 PrimeTime qPCR Assay | Integrated DNA Technologies | Hs.PT.56a.38564463 |
| IFNLR1 PrimeTime qPCR Assay | Integrated DNA Technologies | Hs00417120_m1 |
| IFIT1 PrimeTime qPCR Assay | Integrated DNA Technologies | Hs.PT.56a.20769090.g |
| IFIT2 PrimeTime qPCR Assay | Integrated DNA Technologies | Hs.PT.58.1099131 |
| IRF7 PrimeTime qPCR Assay | Integrated DNA Technologies | Hs.PT.58.24613215.g |
| IFI27 PrimeTime qPCR Assay | ThermoFisher | Hs01086373_g1 |
| GAPDH PrimeTime qPCR Assay | Integrated DNA Technologies | Hs.PT.39a.22214936 |
| Ifit1 PrimeTime qPCR Assay | Integrated DNA Technologies | Mm.PT.58.32674307 |
| Ifit2 PrimeTime qPCR Assay | Integrated DNA Technologies | Mm.PT.58.45910930.g |
| Ifitm3 PrimeTime qPCR Assay | Integrated DNA Technologies | Mm.PT.56a.29591636 |
| Irf7 PrimeTime qPCR Assay | ThermoFisher | Mm00516793 |
| Isg15 PrimeTime qPCR Assay | Integrated DNA Technologies | Mm.PT.58.41476392 |
| Ifi27 PrimeTime qPCR Assay | ThermoFisher | Mm00835449_g1 |
| Gapdh PrimeTime qPCR Assay | Integrated DNA Technologies | Mm.PT.39a.1 |
| Software and Algorithms | ||
| GraphPad Prism 8 | GraphPad | https://www.graphpad.com |
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Jonathan J. Miner (miner@wustl.edu).
Materials Availability
This study did not generate new unique reagents. Commercially available reagents are indicated in the Key Resources Table.
Data Availability
This study did not generate/analyze datasets/code.
Experimental Model and Subject Details
Cell lines
Vero CCL81 cells were used to passage and titrate ZIKV and HSV-1. Vero E6 cells were used to passage and titrate SARS-CoV-2. Both cell lines were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin/streptomycin, 10mM HEPES buffer, 1mM sodium pyruvate, and 1 x non-essential amino acids. Cell lines were kept at 37°C in a humidified atmosphere at 5% CO2.
Viruses
ZIKV (Brazil strain) was a passage 3 stock derived from a clinical isolate from Paraiba, Brazil (Magnani et al., 2018). Viral titers were measured by focus forming assay as previously described (Miner et al., 2016a). ZIKV was passaged in Vero CCL81 cells. HSV-1 strain 17+ also was passaged in Vero CCL81 cells and provided by T. Margolis (Washington University, Saint Louis, MO) (Bertke et al., 2013). SARS-CoV-2 (2019 n-CoV/USA_WA1/2020) was obtained from the Centers for Disease Control and Prevention (gift of Natalie Thornburg) and was passaged in Vero CCL81 cells. SARS-CoV-2 titers were measured by qRT-PCR and by infectious plaque assay in Vero E6 (CRL-1586) cells (Hassan et al., 2020).
Mouse experiments
All protocols for animal studies were approved by the Institutional Animal Care and Use Committees at the Washington University School of Medicine (assurance no. A-3381-01). Mice were housed in specific pathogen-free mouse facilities at the Washington University School of Medicine. AG129 and Ifnar1 −/− mice were purchased from the Jackson laboratory. Ifnlr1 −/− mice were previously described (Baldridge et al., 2017) and provided by M.T. Baldridge (Washington University, Saint Louis, MO). Power analysis was conducted for Institutional Animal Care and Use Committee-approved in vivo studies in order to determine the number of animals needed per experimental group. At least two independent experiments were conducted to replicate findings. No outliers were excluded from analyses. Approximately equal numbers of both sexes were studied. The age and number of animals used for each experiment is listed in the figure legends.
Ifnar1−/− mice were inoculated with 103 FFU of a pathogenic clinical ZIKV isolate from Brazil via subcutaneous footpad inoculation. Corneas were harvested 7 days post-inoculation and homogenized. Eight-week-old AG129 mice were then inoculated intraperitoneally with corneal homogenates in 100 μL of PBS or mock-infected cornea control samples. Mice were monitored for 30 days to assess survival. For corneal transplantation studies, AG129 mice were inoculated subcutaneously with 103 FFU of ZIKV. Donor mice were euthanized 7 days post-infection and corneas were microdissected and washed repeatedly in PBS, and then transplanted to AG129 recipients as previously described (He et al., 1991). Recipient mice were euthanized 7 days post-transplantation, followed by dissection of tissues, homogenization, and quantitation of viral RNA by qRT-PCR using virus-specific primers and probes as previously described (Miner et al., 2016a).
Method Details
Human cornea infections and virus stability measurements
De-identified human donor corneas were obtained from Mid-America Transplant Institute and consisted of cornea samples found to be unsuitable for transplant. Experiments were performed on a total of 25 donor corneas (ages of donors were 20-, 21-, 23-, 28-, 32-, 35-, 40-, 40-, 40-, 40-, 40-, 51-, 53-, 55-, 55-, 55-, 56-, 56-, 64-, 66-, 66-, 67-, 67-, 69-, and 74-years-old) from both genders. This work was IRB-exempt since it involved de-identified human tissue from deceased organ donors.
For ZIKV replication studies, human corneal explants were inoculated with 3 × 106 FFU/ml of ZIKV. Corneas were washed 6 times in 50 mL of PBS, and growth curve analysis was performed by focus forming assay as previously described (Miner et al., 2016a). For growth curve experiments examining HSV-1, ZIKV, and SARS-CoV-2 replication in the presence and absence of IFNλR1 blockade, anti-IFNλR1 mAb (PBL assay science, catalog # 21885-1) and isotype control antibody (Cat# M7894, RRID:AB_1163632), were used at a concentration of 10 μg/ml with fresh antibody introduced every 24 hours. In 2 of 5 samples, the initial HSV-1 infection failed, and so these samples were excluded. For SARS-CoV-2 infection studies, the inoculation dose was 1 × 106 PFU/ml, followed by washing steps as described for HSV-1 and ZIKV. Seven human donor samples were evaluated for SARS-CoV-2 infection, three of which were evaluated in the presence of anti-IFNλR1 mAb or control mAb. Treatment of human corneas with recombinant human IL-29/IFNL1 (PBL assay science, catalog #11725-1) was performed at a concentration of 4.35 x104 U/ml for 6 hours. Cornea storage medium (Optisol™) was obtained from the Washington University Department of Ophthalmology. Persistence of infectious ZIKV in cornea storage medium was assessed by introducing 6 × 105 FFU of ZIKV into cornea storage medium at 4°C. Levels of infectious ZIKV were quantitated by focus forming assay every two days for 28 days.
RNA in situ hybridization (ISH) and ISG expression
Human corneas were sectioned and RNA ISH was performed with RNAscope® 2.5 (Advanced Cell Diagnostics) according to the manufacturer’s instructions, using virus-specific probes for ZIKV as previously described (Miner et al., 2016b). To visualize IFNLR1 and IFNL1 mRNA expression in uninfected human corneas, samples were sectioned and RNA ISH was performed with specific probes for the IFNLR1 gene using ACD Bio probe number 494771 RNAscope ® 2.5 (Advanced Cell Diagnostics) Probe-Hs-IFNLR1. RNA Scope 2.5 HD red was used per manufacturer’s instructions.
Gene expression analysis and IFN-λ treatment of mice
Total RNA was extracted from microdissected corneal homogenates using the RNAeasy kit (QIAGEN) according to the manufacturer’s instructions. To test whether ISG upregulation was dependent on IFNλR1, we administered two doses of 1 μg of mouse IL28B/IFN-λ3 in PBS (PBL assay science, catalog #12820-1) or PBS control to WT and Ifnlr1 −/− mice. Doses were 3 hours apart. Three hours after the 2nd treatment, mice were euthanized and corneas were harvested. qRT-PCR was performed using TaqMan RNA-to-Ct 1-Step kit (Applied Biosystems) according to the manufacturer’s instructions. Results were calculated using the ΔΔCt method, normalizing to GAPDH. Primers were obtained from Integrated Device Technology (IDT).
Human cornea inhibitor experiments
Human corneas were cut into 8 pieces per cornea and placed individually into 24-well plates with 0.3mL media containing inhibitor or vehicle control. Inhibitors used were 10μM MRT67307 (Selleckchem S7948) and 50μM azidothymidine (R&D Systems 4150/50). For each donor, 4 pieces from one cornea were incubated with the inhibitor and the remaining 4 pieces with vehicle for 6 hours at 37°C. Cornea pieces were stored at −80°C. After thawing, pieces were transferred to gentleMACS C tubes containing 1mL DMEM + 2% FCS + collagenase (Sigma, C0130) + dispase I (Sigma D4818) and incubated with shaking at 37°C for 2 hours. Tissues were dissociated using a gentleMACS Dissociator (Miltenyi). Dissociated tissue was then filtered over 70μm mesh filters, centrifuged at 300 x g for 10 min at 4°C, and pellets were resuspended in 250uL RLT buffer (QIAGEN). RNA was isolated using the RNeasy mini kit (QIAGEN) according to the manufacturer’s protocol. qRT-PCR was performed using a TaqMan RNA-to-C T 1-step kit (Applied Biosystems). Results were calculated using the threshold cycle (ΔΔC T) method, normalizing to GAPDH. Primers were obtained from Integrated Device Technology, Inc. (IDT).
Quantification and Statistical Analysis
All data were analyzed using GraphPad Prism software by Mann-Whitney test or two-way ANOVA, as specified in the figure legends.
Acknowledgments
We acknowledge the Washington University Morphology and Imaging Core for assistance with tissue processing and staining. We thank Dr. Todd Margolis at Washington University for advice on studies of HSV-1, and Dr. Jerry Y. Niederkorn’s laboratory at University of Texas Southwestern Medical Center in Dallas for their expert advice regarding mouse corneal transplantation. The graphical abstract was created at biorender.com. D.J.P. is supported by the Washington University Chancellors Graduate Fellowship Program and the Initiative to Maximize Student Development. The Miner laboratory is supported by grants from the NIH (K08 AR070918, R21 EY027870, and R01 AI143982) and the Department of Defense (W81XWH-17-1-0111). The Apte laboratory is supported by grants from the NIH (R01 EY027870 and 5P30 002687) and the Starr Foundation. Additional support to the Miner and Apte laboratories is provided by MidAmerica Transplant. The Philips laboratory is supported by grants from the NIH (R01 AI087682, R01 AI130454, and R21 AI155380). NIH/National Center for Advancing Translational Sciences (NCATS) grant UL1 TR002345 also supported this work. Other support to the Apte laboratory is provided by an unrestricted grant to the Department of Ophthalmology and Visual Sciences from Research to Prevent Blindness and the Jeffery Fort Innovation Fund.
Author Contributions
D.J.P., P.C., A.S., A.M.M., Z.D., W.Q., and E.S.K. performed experiments. J.J.M., D.J.P., A.S., A.M.M., Z.D., and E.S.K. analyzed data. C.M.G. and E.R.W. analyzed data and wrote portions of the initial manuscript and edited subsequent versions of the manuscript. J.J.M., D.J.P., and E.R.W. wrote the final complete version of the manuscript. J.J.M., J.A.P., and R.S.A. conceived the project, analyzed data, and edited the final version of the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: November 3, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.celrep.2020.108339.
Supplemental Information
References
- Aliota M.T., Caine E.A., Walker E.C., Larkin K.E., Camacho E., Osorio J.E. Characterization of lethal Zika virus infection in AG129 mice. PLoS Negl. Trop. Dis. 2016;10:e0004682. doi: 10.1371/journal.pntd.0004682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldridge M.T., Lee S., Brown J.J., McAllister N., Urbanek K., Dermody T.S., Nice T.J., Virgin H.W. Expression of Ifnlr1 on intestinal epithelial cells is critical to the antiviral effects of interferon lambda against norovirus and reovirus. J. Virol. 2017;91:e02079-16. doi: 10.1128/JVI.02079-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer A., Lennemann N.J., Ouyang Y., Bramley J.C., Morosky S., Marques E.T., Jr., Cherry S., Sadovsky Y., Coyne C.B. Type III interferons produced by human placental trophoblasts confer protection against Zika virus infection. Cell Host Microbe. 2016;19:705–712. doi: 10.1016/j.chom.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertke A.S., Ma A., Margolis M.S., Margolis T.P. Different mechanisms regulate productive herpes simplex virus 1 (HSV-1) and HSV-2 infections in adult trigeminal neurons. J. Virol. 2013;87:6512–6516. doi: 10.1128/JVI.00383-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazek K., Eames H.L., Weiss M., Byrne A.J., Perocheau D., Pease J.E., Doyle S., McCann F., Williams R.O., Udalova I.A. IFN-λ resolves inflammation via suppression of neutrophil infiltration and IL-1β production. J. Exp. Med. 2015;212:845–853. doi: 10.1084/jem.20140995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin J., Queen R., Zerti D., Dorgau B., Georgiou M., Djidrovski I., Hussain R., Coxhead J.M., Joseph A., Rooney P., et al. Co-expression of SARS-CoV-2 entry genes in the superficial adult human conjunctival, limbal and corneal epithelium suggests an additional route of entry via the ocular surface. Ocul. Surf. 2020 doi: 10.1016/j.jtos.2020.05.013. Published online June 3, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paula Freitas B., Ventura C.V., Maia M., Belfort R., Jr. Zika virus and the eye. Curr. Opin. Ophthalmol. 2017;28:595–599. doi: 10.1097/ICU.0000000000000420. [DOI] [PubMed] [Google Scholar]
- Fang Z., Zhang Y., Hang C., Ai J., Li S., Zhang W. Comparisons of viral shedding time of SARS-CoV-2 of different samples in ICU and non-ICU patients. J. Infect. 2020;81:147–178. doi: 10.1016/j.jinf.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq A.V., Shukla D. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv. Ophthalmol. 2012;57:448–462. doi: 10.1016/j.survophthal.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant A., Ponia S.S., Tripathi S., Balasubramaniam V., Miorin L., Sourisseau M., Schwarz M.C., Sánchez-Seco M.P., Evans M.J., Best S.M., García-Sastre A. Zika virus targets human STAT2 to inhibit type I interferon signaling. Cell Host Microbe. 2016;19:882–890. doi: 10.1016/j.chom.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan A.O., Case J.B., Winkler E.S., Thackray L.B., Kafai N.M., Bailey A.L., McCune B.T., Fox J.M., Chen R.E., Alsoussi W.B., et al. A SARS-CoV-2 infection model in mice demonstrates protection by neutralizing antibodies. Cell. 2020;182:744–753.e4. doi: 10.1016/j.cell.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y.G., Ross J., Niederkorn J.Y. Promotion of murine orthotopic corneal allograft survival by systemic administration of anti-CD4 monoclonal antibody. Invest. Ophthalmol. Vis. Sci. 1991;32:2723–2728. [PubMed] [Google Scholar]
- Heck E., Cavanagh H.D., Robertson D.M. Zika virus RNA in an asymptomatic donor’s vitreous: risk of transmission? Am. J. Transplant. 2017;17:2227–2228. doi: 10.1111/ajt.14334. [DOI] [PubMed] [Google Scholar]
- Hoffmann H.H., Schneider W.M., Rice C.M. Interferons and viruses: an evolutionary arms race of molecular interactions. Trends Immunol. 2015;36:124–138. doi: 10.1016/j.it.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagger B.W., Miner J.J., Cao B., Arora N., Smith A.M., Kovacs A., Mysorekar I.U., Coyne C.B., Diamond M.S. Gestational stage and IFN-λ signaling regulate ZIKV infection in utero. Cell Host Microbe. 2017;22:366–376.e3. doi: 10.1016/j.chom.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggi U., Bhela S., Rouse B.T. Role of interferon lambda (IL-28A) in herpes stromal keratitis. J. Immunol. Res. Ther. 2018;3:135–144. [Google Scholar]
- Karimi S., Arabi A., Shahraki T., Safi S. Detection of severe acute respiratory syndrome coronavirus-2 in the tears of patients with coronavirus disease 2019. Eye (Lond.) 2020;34:1220–1223. doi: 10.1038/s41433-020-0965-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodati S., Palmore T.N., Spellman F.A., Cunningham D., Weistrop B., Sen H.N. Bilateral posterior uveitis associated with Zika virus infection. Lancet. 2017;389:125–126. doi: 10.1016/S0140-6736(16)32518-1. [DOI] [PubMed] [Google Scholar]
- Kotenko S.V., Gallagher G., Baurin V.V., Lewis-Antes A., Shen M., Shah N.K., Langer J.A., Sheikh F., Dickensheets H., Donnelly R.P. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat. Immunol. 2003;4:69–77. doi: 10.1038/ni875. [DOI] [PubMed] [Google Scholar]
- Lazear H.M., Daniels B.P., Pinto A.K., Huang A.C., Vick S.C., Doyle S.E., Gale M., Jr., Klein R.S., Diamond M.S. Interferon-λ restricts West Nile virus neuroinvasion by tightening the blood-brain barrier. Sci. Transl. Med. 2015;7:284ra59. doi: 10.1126/scitranslmed.aaa4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Hu S., Zhou L., Ye L., Wang X., Ho J., Ho W. Interferon lambda inhibits herpes simplex virus type I infection of human astrocytes and neurons. Glia. 2011;59:58–67. doi: 10.1002/glia.21076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnani D.M., Rogers T.F., Maness N.J., Grubaugh N.D., Beutler N., Bailey V.K., Gonzalez-Nieto L., Gutman M.J., Pedreño-Lopez N., Kwal J.M., et al. Fetal demise and failed antibody therapy during Zika virus infection of pregnant macaques. Nat. Commun. 2018;9:1624. doi: 10.1038/s41467-018-04056-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner J.J., Cao B., Govero J., Smith A.M., Fernandez E., Cabrera O.H., Garber C., Noll M., Klein R.S., Noguchi K.K., et al. Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell. 2016;165:1081–1091. doi: 10.1016/j.cell.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner J.J., Sene A., Richner J.M., Smith A.M., Santeford A., Ban N., Weger-Lucarelli J., Manzella F., Rückert C., Govero J., et al. Zika virus infection in mice causes panuveitis with shedding of virus in tears. Cell Rep. 2016;16:3208–3218. doi: 10.1016/j.celrep.2016.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nice T.J., Baldridge M.T., McCune B.T., Norman J.M., Lazear H.M., Artyomov M., Diamond M.S., Virgin H.W. Interferon-λ cures persistent murine norovirus infection in the absence of adaptive immunity. Science. 2015;347:269–273. doi: 10.1126/science.1258100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira M.L., Estofolete C.F., Terzian A.C., Mascarin do Vale E.P., da Silva R.C., da Silva R.F., Ramalho H.J., Fernandes Charpiot I.M., Vasilakis N., Abbud-Filho M. Zika virus infection and solid organ transplantation: a new challenge. Am. J. Transplant. 2017;17:791–795. doi: 10.1111/ajt.14047. [DOI] [PubMed] [Google Scholar]
- Oh J.O. Type 1 and type 2 herpes simplex virus in corneal cell cultures. Surv. Ophthalmol. 1976;21:160–164. doi: 10.1016/0039-6257(76)90093-x. [DOI] [PubMed] [Google Scholar]
- Sun J., Wu D., Zhong H., Guan D., Zhang H., Tan Q., Zhou H., Zhang M., Ning D., Zhang B., et al. Returning ex-patriot Chinese to Guangdong, China, increase the risk for local transmission of Zika virus. J. Infect. 2017;75:356–367. doi: 10.1016/j.jinf.2017.07.001. [DOI] [PubMed] [Google Scholar]
- Thomas J., Gangappa S., Kanangat S., Rouse B.T. On the essential involvement of neutrophils in the immunopathologic disease: herpetic stromal keratitis. J. Immunol. 1997;158:1383–1391. [PubMed] [Google Scholar]
- van den Broek M.F., Müller U., Huang S., Aguet M., Zinkernagel R.M. Antiviral defense in mice lacking both alpha/beta and gamma interferon receptors. J. Virol. 1995;69:4792–4796. doi: 10.1128/jvi.69.8.4792-4796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P., Duan F., Luo C., Liu Q., Qu X., Liang L., Wu K. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020;138:575–578. doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J., Tong J., Liu M., Shen Y., Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J. Med. Virol. 2020;92:589–594. doi: 10.1002/jmv.25725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H., You J., You H., Zheng C. Herpes simplex virus 1 UL36USP antagonizes type I interferon-mediated antiviral innate immunity. J. Virol. 2018;92:e01161-18. doi: 10.1128/JVI.01161-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Xu Z., Castiglione G.M., Soiberman U.S., Eberhart C.G., Duh E.J. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection. Ocul. Surf. 2020;18:537–544. doi: 10.1016/j.jtos.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate/analyze datasets/code.