Abstract
Background:
Salmonella, a bacterial genus of more than 2500 serotypes is considered as the most significant foodborne pathogen causing infections in humans and animals. Increased antimicrobial resistance and persistence of antimicrobial residues in food matrices warrants the need for alternative infection management strategies.
Aims:
The present study aimed to isolate and evaluate the lytic activity of bacteriophage against Salmonella.
Methods:
Twenty-eight Salmonella isolates obtained from the poultry sources were screened for antibiotic sensitivity. Poultry slaughterhouse wastewater was used for the isolation of phage. Host range and random amplified polymorphic DNA (RAPD) are vital tools used for differentiating the phage.
Results:
The isolates showed a high degree of resistance to nalidixic acid (71%), tetracycline (71%), nitrofurantoin (50%), and ampicillin (43%). Five lytic phages are specific for Salmonella spp. were isolated and characterized by RAPD. In the colony forming unit (CFU) reduction assay, the highest activity of phage was observed at 0.01 multiplicity of infection (MOI) within 2 h after the addition of phage. PSE5 at 0.01 MOI was administered to Salmonella Enteritidis seeded on the surface of the chicken egg by immersion method. The results indicated that administration of phage reduced recoverable Salmonella by 2 × 106 CFU/ml relative to the phage-excluded control.
Conclusion:
The results presented here suggested the application of the bacteriophage treatment has the potential to be used as an alternative strategy to prevent Salmonella infection in poultry farms to prevent vertical transmission of the pathogen.
Key Words: Antibiotic resistance, Phage therapy, Salmonella
Introduction
Salmonella is a Gram-negative, rod shaped, facultative anaerobic genus belonging to the family Enterobacteriaceae. Salmonella infections spread via faecal-oral route and can be transmitted by the consumption of contaminated food of animal origin such as poultry, egg, milk products and water (Buncic and Sofos, 2012 ▶; Andino and Hanning, 2015 ▶). Salmonella is the common cause of foodborne illness termed as salmonellosis. The prevention of salmonellosis is quite challenging due to its complex epidemiology and wide mode of transmission. Presently, the treatment of salmonellosis with conventional antibiotics is handicapped. The incidence of multi-drug resistance brings an additional vital dimension to the challenge of Salmonella associated infections.
Bacteriophages are considered as the potent biocontrol agent for food safety applications or as therapeutic agents due to their specificity and self-replicating property with no adverse effects on beneficial microflora and human cells (Babickova and Gardlik, 2015 ▶). Biocontrol of various food pathogens has been studied in chicken, pig skin, sprout seeds lettuce and vegetables (Ye et al., 2010 ▶; Adriaenssens et al., 2012 ▶; Campos et al., 2019 ▶). US food safety and inspection service permits the use of Salmonella-specific phage against bacterial contamination in live poultry before processing (Huff et al., 2002 ▶; Zhang et al., 2019 ▶). Hence, the present study was designed for the successful application of bacteriophage in poultry. In this study, lytic phages against Salmonella were evaluated for their biocontrol ability on the food matrix of egg.
Materials and Methods
Bacterial isolates
Twenty-eight isolates obtained from poultry were identified using standard biochemical test’s like Gram staining, cytochrome oxidase, catalase, oxidation fermentation, triple sugar iron agar and urease tests. Further, isolates were confirmed as Salmonella by polymerase chain reaction (PCR) targeting invasion associated (invA) gene. The susceptibility to different antibiotics as commonly described, nalidixic acid (30 mcg), tetracycline (30 mcg), nitrofurantoin (300 mcg), ampicillin (10 mcg), meropenem (10 mcg), co-trimoxazole (25 mcg), piperacillin/tazobactam (100/10 mcg), ciprofloxacin (5 mcg) cefotaxime (30 mcg), kanamycin (30 mcg), streptomycin (10 mcg), imipenem (10 mcg), and gentamycin (10 mcg) were evaluated by the disc-diffusion method on Muller-Hinton agar as recommended by the Clinical and Laboratory Standards Institute (CLSI) guidelines.
Bacteriophages
The effluent from the local commercial chicken market was used for the isolation of phages. Bacteriophages were isolated by enrichment method. All the twenty-eight bacterial isolates were used as baits. Twenty-five ml of effluent was centrifuged at 10000 g for 10 min, and the supernatant obtained was filtered through 0.22 μm syringe filter. The filtrate was used as a source of bacteriophage.
Bacteriophage activity in the supernatant was tested by spot assay by placing 5 μL of the supernatant on a lawn of Salmonella culture. Plates were incubated at 30°C overnight and observed for plaques. Plaques were purified by single plaque purification and titre of phage’s was determined by soft agar overlay technique (Adams, 1959 ▶). The isolated bacteriophages were further propagated on their respective host.
Host range and random amplified polymorphic DNA (RAPD)
Twenty-eight Salmonella spp. were used for this experiment. On nutrient agar plate, the bacterial lawn was made and allowed to dry. 5 μL of all the phage lysate was spotted on the lawn and incubated at 30°C overnight and observed for the appearance of clear zones. Tested bacteria were considered as either sensitive or resistant to the phage depending on the appearance of plaque. Bacteriophage DNA was extracted by zinc chloride precipitation followed by phenol extraction as described by Su et al. (2004) ▶.
Molecular typing of bacteriophage was performed using RAPD PCR analysis with RAPD5 (5´-AAC GCG CAA C-3´) primer. The reaction mixture (30 µL) consisted of nuclease free water (21.5 µL), 10X buffer with 2.5 mM MgCl2 (3 µL), 10 mM/µL dNTP (1 µL), 10 pmol of primer, and 2 µL DNA template. The program used was as follows: 3 min at 94°C followed by 35 cycles of 50 s at 94°C, 36 s at 36°C, and 30 min at 72°C followed by a final extension at 72°C for 5 min. Polymerase chain reaction products were then electrophoresed on 1% agarose gel and visualized by ethidium bromide staining.
In vitro assessment of the lytic activity of bacteriophages
Colony forming unit ( CFU) reduction assay
Bacterial culture was inoculated to 250 ml broth and the turbidity of the inoculum was adjusted to 0.5 Mac Farland standard (Schneider et al., 2018). Spread plating on nutrient agar was performed to determine the initial cell density. The culture was distributed into conical flasks labelled as control, 10 multiplicity of infection (MOI), 1 MOI, 0.1 MOI, and 0.01 MOI in duplicates. To each flask different quantity of phage were added to attain the respective MOI. Sampling was done every 0, 4, 8, 12, and 16 h and 100 μL of culture was spread onto nutrient agar plate to determine viable cell count. Plates were incubated at 37°C overnight followed by enumerating the colonies.
Control of Salmonella on eggs
Eggs (n=40) were purchased from the local market. The eggs were washed, and surface disinfected with 70% ethanol. Sterile eggs were divided into 4 experimental groups with 10 eggs in each group. Group I and II were inoculated with Salmonella by surface application of 1 ml of Salmonella culture adjusted to 4 × 105 CFU/ml. After 30 min, the eggs were treated with 0.01 MOI of phage PSE5 and SM buffer (100 mM NaCl, 8 mM MgSO4•7H2O, 50 mM Tris-Cl (pH = 7.5), 1 L distilled water), respectively by immersing the eggs in 500 ml of phage/SM buffer for 30 min. Group III and IV served as phage and negative control, respectively. Eggs were further incubated at 37°C for up to 2 h. Viable count of bacteria and phage was performed at time zero and various time points (30, 60, and 120 min).
Results
All 28 isolates were resistant to at least one of the antibiotics tested. Among the different antibiotics used maximum resistance to nalidixic acid and tetracycline (71%), nitrofurantoin (50%), and ampicillin (43%) was observed (Fig. 1).
Fig. 1.
Graphical representation of antibiotic susceptibility test. IPM: Imipenem, PIT: Piperacillin/Tazobactum, MRP: Meropenem, S: Streptomycin, K: Kanamycin, CTX: Co-trimoxazole, NIT: Nitrofurantoin, AMP: Ampicillin, CIP: Ciprofloxacin, COT: Co-trimoxazole, TE: Tetracycline, GEN: Gentamycin, NA: Nalidixic acid
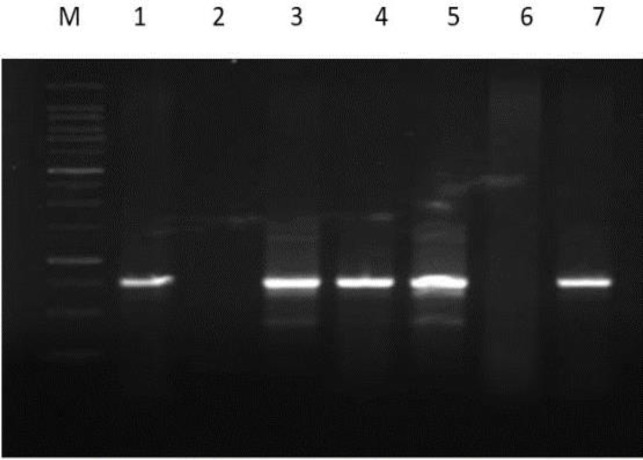
In this study, a total of five phages Salmonella Enteritidis phage 1 (PSE1), PSE2, PSE3, PSE4, and PSE5 were isolated from chicken market effluent. Phages were differentiated based on their plaque morphology (Fig. 2) and RAPD analysis. Results of RAPD clearly indicate that phage PSE3, and PSE4 belonged to the same clade. PSE1 did not show any amplification with RAPD primer PM5 (Fig. 3). Out of 5 phages isolated, PSE5 showed broad host range by being lytic on 82% (23 of 28) of the Salmonella isolates. Due to the excellent lytic activity of PSE5, it was chosen for further control studies.
Fig. 2.
Random amplified polymorphic DNA. Lane M: 100 bp marker, Lane 1: Positive control, Lane 2: Negative control, Lanes 3 and 5: PSE3, PSE5 showed similar pattern, Lanes 4 and 7: PSE2, PSE4 showed different pattern, and Lane 6: PSE1 showed no amplification
Fig. 3.

Representative image of plates showing plaques (plaques or clear zones were observed when overlaid with phage (PSE3) indicating the lysis of bacteria)
In the CFU drop assay, drop curve represents the duplication cycle of the bacteriophage which was performed for PSE5 phage. 0.01 MOI showed a gradual increase in the bacterial count after 2 h thereby, increase in the count soon after other MOI like 10, 1, 0.1 did not show any visible decrease in the bacterial count (Fig. 4).
Fig. 4.
Reduction of Salmonella spp. counts at different time intervals in the presence of different concentrations of bacteriophage
In the control study, egg infected with 4 × 105 CFU/ml of Salmonella Enteritidis were treated with phages at 0.01 MOI. Phages were able to reduce the load of bacteria by 99.9% i.e., 3 log reduction within 30 min and retain such low numbers up to 1 h (Fig. 5).
Fig. 5.
Effectiveness of phage on the eggs treated with Salmonella
Discussion
Persistence of Salmonella in milk products, fruit juice, tomatoes, fish, shrimp, frog legs, yeast, and vegetables have been reported. Among all the food sources, poultry and poultry-associated products are commonly recognized as the most remarkable vehicles for human Salmonella infections (Campos et al., 2019 ▶).
Antimicrobials used in the veterinary settings are considered as a crucial factor in the emergence of drug resistant Salmonella, and multidrug resistance was commonly seen in isolates from food animals rather than from human clinical cases (Briers et al., 2014 ▶). To overcome this, phage therapy is considered an alternative environment friendly method to combat antibiotic resistant bacteria. Phage’s that present a broad host range exhibited great potential to control pathogens in the food sample. Hence, in this study phage which showed excellent lytic activity and broad host range were chosen for CFU drop assay and biocontrol study.
The emergence of antibiotic-resistant bacteria has led to understanding the importance of the isolation and characterization of phage. Several studies used transmission electron microscopy and genome sequencing to study the phage morphology and to identify the genes of antimicrobial significance respectively (Ashwale et al., 2019 ▶; Besler et al., 2020 ▶).
The lytic ability of phage PSE5 upon infection of Salmonella host was further studied at various MOIs. Inoculation of 0.01 MOI was sufficient to prevent a noticeable change in the count of the initial inoculum up to 2 h of incubation. From these results, it is clear that amplified phages can continually inhibit the growth of Salmonella at applied concentration. Low MOI ratio is beneficial for the commercial feasibility of large-scale application since it reduces the cost of preparation, purification and application of phage.
Several studies proved that application of phages to the contaminated surface of food reduces the number of bacteria and ensures food safety (Sukumaran et al., 2016 ▶; El-Dougdoug et al., 2019 ▶). When a lytic phage enters a host cell, it multiplies and lyse the cell there is an outburst of viral particles. Thus, with every host cell lysis there is an increase in the phage numbers. However, high titer of phage is sufficient to lyse bacteria without multiplication. In lysis without multiplication, phages adsorb to the surface of the host cell at high MOI (>100) and puncture the host cell at multiple cell surface regions leading to host death as against a lysis with multiplication. Shibinay (2016) ▶ studied the effectiveness of Escherichia coli and Salmonella phages in reducing the number of E. coli and Salmonella on cucumber and egg at low temperature, respectively.
Phage PSE(5) can be considered as appropriate applicant phage for phage treatment since it was active against antibiotic resistant bacteria and also showed the lytic activity on the egg infected with Salmonella. Data obtained in this study supports the possibility of application of phages as the biocontrol agent in food industry. Biocontrol strategy using single type phage is more economical than cocktail which contains several types of bacteriophages (Hooton et al., 2011 ▶; Endersen et al., 2014 ▶; Huang et al., 2018 ▶). It is essential to characterize the phage completely and ensure that it does not carry any virulence or antimicrobial resistance genes which may be done either by whole genome sequencing or by PCR based detection of specific virulence factors observed in that particular pathogen.
Based on the results obtained, it was concluded that phages can be used to reduce the microbial load in poultry industry. This study also provides insight into preventive and control strategy against Salmonella infection in the poultry industry.
Acknowledgment
Financial support received from the Nitte University Centre for Science Education and Research, NITTE (Deemed to be University), toward this research work is gratefully acknowledged.
Conflict of interest
There is no conflict of interest towards the study.
References
- Adams, MH . Enumeration of bacteriophage particles. Bacteriophages. London: Interscience Publishers; 1959. pp. 27–37. [Google Scholar]
- Adriaenssens, EM , vanVaerenbergh, J , Vandenheuvel, D , Dunon, V , Ceyssens, PJ , deProft, M , Kropinski, AM , Noben, JP , Maes, M , Lavigne, R T4-related bacteriophage LIME stone isolates for the control of soft rot on potato caused by Dickeya solani. PLoS One. 2012;7:e33227. doi: 10.1371/journal.pone.0033227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andino, A , Hanning, I Salmonella enterica: survival, colonization, and virulence differences among serovars. Sci. World J. 2015 doi: 10.1155/2015/520179. doi: 0.1155/2015/520179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwale, JK , Rohde, M , Rohde, C , Bunk, B , Sproer, C , Boga, HI , Klenik, HP , Wittmann, J Isolation, characterization and analysis of bacteriophages from the haloalkaline lake Elmenteita, Kenya. PLOS One. 2019;10:e0004813. doi: 10.1371/journal.pone.0215734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babickova, J , Gardlik, R Pathological and therapeutic interactions between bacteriophages, microbes and the host in inflammatory bowel disease. World J. Gastroenterol. 2015;21:11321–11330. doi: 10.3748/wjg.v21.i40.11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besler, I , Sazinas, P , Harrison, C , Gannon, L , Redowell, T , Michniewski, S , Hooton, SP , Milard, A Genome sequence and characterization of Coliphage vB_Eco_SLUR29. 2020;Phage. 1:38–44. doi: 10.1089/phage.2019.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briers, Y , Walmagh, M , Van Puyenbroeck, V , Cornelissen, A , Cenens, W , Aertsen, A , Oliveira, H , Azeredo, J , Verween, G , Pirnay, J , Miller, S , Volckaert, G , Lavigne, R Engineered endolysin-based “Artilysins” to combat multidrug-resistant Gram-negative pathogens. mBio. 2014;5:e01379–14. doi: 10.1128/mBio.01379-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buncic, S , Sofos, J Interventions to control Salmonella contamination during poultry, cattle and pig slaughter. Food Res. Int. 2012;45:641–655. [Google Scholar]
- Campos, J , Mourão, J , Peixe, L , Antunes, P Non-typhoidal Salmonella in the pig production chain: A comprehensive analysis of its impact on human health. Pathogens. 2019:8–1. doi: 10.3390/pathogens8010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum-Cianflone, NF Salmonellosis and the gastrointestinal tract: more than just peanut butter. Curr. Gastroenterol. Rep. 2008;10:424–431. doi: 10.1007/s11894-008-0079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Dougdoug, NK , Cucic, S , Abdelhamid, AG , Brovko, L , Kropinski, AM , Griffiths, MW , Anany, H Control of Salmonella Newport on cherry tomato using a cocktail of lytic bacteriophages. Int. J. Food Microbiol. 2019;293:60–71. doi: 10.1016/j.ijfoodmicro.2019.01.003. [DOI] [PubMed] [Google Scholar]
- Endersen, L , O’Mahony, J , Hill, C , Ross, RP , McAuliffe, O , Coffey, A Phage therapy in the food industry. Annu. Rev. Food Sci. Technol. 2014;5:327–349. doi: 10.1146/annurev-food-030713-092415. [DOI] [PubMed] [Google Scholar]
- Hooton, SPT , Atterbury, RJ , Connerton, IF Application of a bacteriophage cocktail to reduce Salmonella Typhimurium U288 contamination on pig skin. Int. J. Food Microbiol. 2011;151:157–163. doi: 10.1016/j.ijfoodmicro.2011.08.015. [DOI] [PubMed] [Google Scholar]
- Huang, C , Virk, SM , Shi, J , Zhou, Y , Willias, SP , Morsy, MK , Abdelnabby, HE , Liu, J , Wang, X , Li, J Isolation, characterization, and application of bacteriophage LPSE1 against Salmonella enterica in ready to eat (RTE) foods. Front. Microbiol. 2018;9 doi: 10.3389/fmicb.2018.01046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff, WE , Huff, GR , Rath, NC , Balog, JM , Donoghue, AM Prevention of Escherichia coli infection in broiler chickens with a bacteriophage aerosol spray. Poult. Sci. 2002;81:1486–1491. doi: 10.1093/ps/81.10.1486. [DOI] [PubMed] [Google Scholar]
- Shibiny, AE Bio-control of E coli and Salmonella in foods using bacteriophage to improve food safety. World J. Dairy Food Sci. 2016;11:150–155. [Google Scholar]
- Su, LH , Chiu, CH , Chu, C , Ou, JT Antimicrobial resistance in nontyphoid Salmonella serotypes: a global challenge. Clin. Infect. Dis. 2004;39:546–551. doi: 10.1086/422726. [DOI] [PubMed] [Google Scholar]
- Sukumaran, AT , Nannapaneni, R , Kiess, A , Sharma, CS Reduction of Salmonella on chicken breastfillets stored under aerobic or modified atmosphere packaging by the application of lytic bacteriophage preparation SalmoFreshTM. Poult. Sci. 2016;95:668–675. doi: 10.3382/ps/pev332. [DOI] [PubMed] [Google Scholar]
- Thung, TY , Krishanthi Jayarukshi Kumari Premarathne, JM , San Chang, W , Loo, YY , Chin, YZ , Kuan, CH , Tan, CW , Basri, DF , Jasimah Wan Mohamed Radzi, CW , Radu, S Use of a lytic bacteriophage to control Salmonella Enteritidis in retail food. LWT. 2017;78:222–225. [Google Scholar]
- Ye, J , Kostrzynska, M , Dunfield, K , Warriner, K Control of Salmonella on sprouting mung bean and alfalfa seeds by using a biocontrol preparation based on antagonistic bacteria and lytic bacteriophages. J. Food Prot. 2010;73:9–17. doi: 10.4315/0362-028x-73.1.9. [DOI] [PubMed] [Google Scholar]
- Zhang, X , Niu, YD , Nan, Y , Stanford, K , Holley, R , McAllister, T , Narvaez-Bravo, C SalmoFresh effectiveness in controlling Salmonella on romaine lettuce, mung bean sprouts and seeds. Int. J. Food Microbiol. 2019;305:108250. doi: 10.1016/j.ijfoodmicro.2019.108250. [DOI] [PubMed] [Google Scholar]