Abstract
Background:
Avian infectious bronchitis (IB) is an infectious viral disease of chickens. The effective protection of chickens against many different infectious bronchitis virus (IBV) variants is not achieved unless the circulating genotypes in the region are identified and the cross-protection of the potential of vaccines in use is assessed.
Aims:
In a monitoring program of IBVs, a new genotype was identified in the north of Iran, 2019. This work was conducted to isolate and characterize this new IBV genotype.
Methods:
Tracheal tissues were collected from chickens showing signs of respiratory involvement. Specimens were homogenized and inoculated to the allantoic fluid of embryonated specific pathogen-free (SPF) eggs. Infectious bronchitis virus was detected using real time-polymerase chain reaction (RT-PCR). The hypervariable region of the IBV S1 gene was amplified for sequencing.
Results:
Positive samples were phylogenetically analyzed, and both positive isolates were clustered with Q1 IBV strains.
Conclusion:
This is the first report of the Q1 outbreak in Iran. More investigations are needed to find the role of Q1 IBV in the respiratory disease complex of chickens.
Key Words: Avian infectious bronchitis, Genotyping, Iran, Phylogenetic tree, Q1
Introduction
Infectious bronchitis (IB) is an infectious viral disease affecting different bird species (Cavanagh, 2007 ▶). The etiologic agent is an avian Gammacoronavirus infectious bronchitis virus (IBV) (Laconi et al., 2019 ▶). Different IBV variants have been emerging due to a high mutation rate and genetic recombination. This could endanger intensive poultry production throughout the world, in spite of using vaccines (Toffan et al., 2013 ▶). The effective protection of chickens is not achieved unless identifying the circulating genotypes in the region and assessing the cross-protection of vaccines in use (Chhabra et al., 2015 ▶). Different types of IBV have been circulating in Iran since 1994, and vaccines are used frequently. However, outbreaks happen regularly, and this probably originates from the emergence of new variants and poor protection induced by in use vaccines. To overcome this issue, continuous surveillance strategies are needed. In a monitoring program of IBVs, a new genotype was identified in the north of Iran. This work was conducted to isolate and characterize this new IBV type.
Materials and Methods
During December 2019, tracheal tissue samples were collected from two broiler farms located in the north region of Iran, Gilan province. Chicks were 35 days old. The mortality rate was 2% and birds showed respiratory involvement. Also, kidneys were enlarged and pale in necropsy. Tissue specimens from each farm were pooled, homogenized, and 10% (w/v) suspension made in phosphate buffered saline )PBS). Centrifugation was performed at 1500 g for 20 min at 4°C. The supernatant was used for inoculation of 9 to 11 day-old specific pathogen-free embryonated eggs. After incubation, the allantoic fluid was harvested.
RNA extraction and cDNA synthesis
RNA from allantoic fluid was extracted by the CinnaPure RNA extraction kit (SinaClon, Iran). The cDNA was synthesized by the RevertAid cDNA synthesis kit (Thermo Scientific, USA).
Real time-polymerase chain reaction (RT-PCR) for IBV detection
A RT-PCR, which amplifies 5´-UTR of the IBV genome, was used to detect the virus. Primers and probe were as follows: a forward primer (5´-GCT TTT GAG CCT AGC GTT-3´), a reverse primer (5´-GCC ATG TTG TCA CTG TCT ATT G-3´), and a TaqMan dual-labeled probe (5´-FAM-CAC CAC CAG AAC CTG TCA CCT C-BHQ1-3´). Primers and probe nucleotide sequences and the cycling condition were previously described by Najafi et al. (2016) ▶.
Nested PCR, sequencing, and phylogenetic analysis
Nested PCR targeting the hypervariable S1 region of the IBV was conducted, primers used in the first round of PCR included: SX1 (5´-CAC CTA GAG GTT TGY TWG CAT G-3´) and SX2 (5´-TCC ACC TCT ATA AAC ACC YTT AC-3´). Thermal cycling concluded an initial denaturation period at 94°C for 2 min and 35 cycles of denaturation at 94°C for 15 s, annealing at 58°C for 30 s, and polymerization at 72°C for 30 s. Final extension was performed at 72°C for 10 min. For nested PCR, a 1 µL aliquot of a 1:100 dilution of the first amplicon was subjected to a second round of amplification using the primers SX3 (5´-TAA TAC TGG YAA TTT TTC AGA TGG-3´) and SX4 (5´-AAT ACA GAT TGC TTA CAA CCA CC-3´) and the same cycling procedures (Najafi et al., 2016). Polymerase chain reaction amplicons were sent to Codon genetic group, Iran, for sequencing. Multiple sequence alignments were generated using Clustal W, and the alignments were subsequently used to construct distance matrices using the Kimura 2-parameter model implemented in Mega software version 7.0.26. Maximum likelihood trees were plotted with Mega, with a 1,000-fold bootstrap approach (Kumar et al., 2017 ▶).
Results
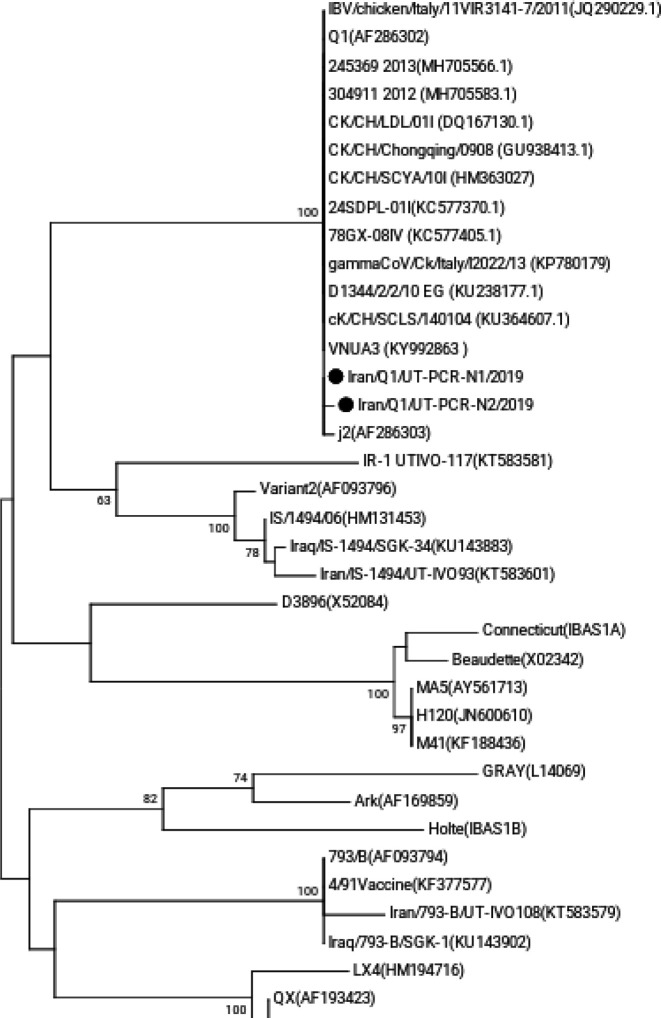
The allantoic fluid inoculated by specimens of both farms showed positive results in RT-PCR. Both positive samples were sequenced, and sequences were submitted in GenBank under accession numbers of MN841015 for Iran/Q1/UT-PCR-N1/2019 and MN841016 for Iran/Q1/UT-PCR-N2/2019. Comparisons of the hypervariable region of S1 nucleotide sequences revealed that IBVs isolated in this study were clustered with Q1 type IBVs (Fig. 1). The two isolates of this study showed the highest nucleotide homologies with the original Q1 identified in China in 1996, and with a Q1 IBV strain isolated in Vietnam in 2014 (VNUA3) and with another Q1 strain detected in Italy in 2013 (Table 1).
Fig. 1.
The maximum likelihood phylogenetic tree based on the hypervariable region of the S1 IBV glycoprotein. Infectious bronchitis viruses isolated in this study are noted by black circles
Table 1.
Sequence homology (%) matrix for IBV based on partial nucleotide sequences of S1 protein gene
| Virus isolate | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1- Iran/Q1/UT-PCR-N1/2019 | ||||||||||||||
| 2- Iran/Q1/UT-PCR-N2/2019 | 99.60 | |||||||||||||
| 3- VNUA3_(KY992863_) | 100.00 | 99.60 | ||||||||||||
| 4- CK/CH/SCLS/140104_(KU364607.1) | 100.00 | 99.60 | 100.00 | |||||||||||
| 5- gammaCoV/Ck/Italy/I2022/13_(KP780179) | 100.00 | 99.60 | 100.00 | 100.00 | ||||||||||
| 6- Q1(AF286302) | 100.00 | 99.60 | 100.00 | 100.00 | 100.00 | |||||||||
| 7- j2(AF286303) | 99.60 | 99.20 | 99.60 | 99.60 | 99.60 | 99.60 | ||||||||
| 8- Iraq/IS-1494/SGK-34(KU143883) | 82.87 | 82.87 | 82.87 | 82.87 | 82.87 | 82.87 | 82.47 | |||||||
| 9- Iran/IS-1494/UT-IVO93(KT583601) | 82.07 | 82.07 | 82.07 | 82.07 | 82.07 | 82.07 | 81.67 | 98.01 | ||||||
| 10- Iran/793-B/UT-GL55/2017 | 81.53 | 81.12 | 81.53 | 81.53 | 81.53 | 81.53 | 81.12 | 82.33 | 84.34 | |||||
| 11- IR-1_UTIVO-117(KT583581) | 81.27 | 80.88 | 81.27 | 81.27 | 81.27 | 81.27 | 81.27 | 86.45 | 85.26 | 81.53 | ||||
| 12- Iran/QX/UT-IVO105(KU143898) | 80.88 | 80.88 | 80.88 | 80.88 | 80.88 | 80.88 | 80.48 | 83.27 | 83.27 | 82.73 | 81.67 | |||
| 13- Iran/793-B/UT-GL57/2017 | 79.28 | 78.88 | 79.28 | 79.28 | 79.28 | 79.28 | 78.88 | 80.08 | 82.07 | 97.59 | 80.48 | 80.48 | ||
| 14- H120(JN600610) | 77.69 | 77.69 | 77.69 | 77.69 | 77.69 | 77.69 | 77.69 | 81.67 | 81.67 | 76.31 | 77.69 | 78.88 | 74.50 |
Discussion
The first isolation of IBV from Iranian poultry farms dates back to 1994 when Massachusetts IBVs were the major type founded in poultry farms of the country. The presence of a new serotype, 4/91, was reported in 2000 (Shoushtari et al., 2008 ▶). Akbari et al. (2004) ▶ detected 793/B and Massachusetts IBVs during an investigation performed between 1997 and 2003. 793/B was introduced as the predominant IBV type in 2004 (Shoushtari et al., 2008 ▶). In a more comprehensive work carried out between 2010 and 2014, different genotypes, including Mass, 793/B, IS720, variant 2, QX, IR-I, and IR-II, were reported (Hosseini et al., 2015 ▶). Subsequent investigation isolated Mass, 4/91, IS/720, variant 2, QX, and IR-I (Najafi et al., 2016 ▶). The next two-year genotyping project reported the presence of variant 2, 793/B, QX, and Mass with the domination of variant-2 type IBV (Hamadan et al., 2017 ▶). In 2017, D274 was detected in Iran for the first time. Variant-2 was the predominant type IBV, and 793/B, QX and mass occurred to a lesser extent (Ghalyanchilangeroudi et al., 2019 ▶). The last data on IBV phylogeny has not yet been published but reveals the presence of 4 genotypes of variant-2, QX, 793B, and Mass from across the country (data not published). Not only have more investigations been conducted to detect different IBV types in Iran in recent years, rapid replication, a high mutation rate, and genome recombination of the genome, and also inefficiency of used vaccination regimens to protect chickens have led to increasing incidence of different IBV types in the country.
The present study reports the first detection of the Q1 IBV genotype in Iran. Q1 was firstly detected from the proventriculus of layer chicks in China from 1996 to 1998 (Yu et al., 2001 ▶). Another Q1 virus, CH/CK/LDL/97I, known as a nephropathogenic strain, was isolated from the oviduct of layer hen in 2001 in China. Infected birds showed clinical signs of IB disease; furthermore, nephritis was characterized by pale and enlarged kidneys, with urate deposits in the tubules (Liu et al., 2006 ▶). An IBV isolated in 2009 in China also belonged to the Q1 genotype (NCBI). In early 2011, Q1 type viruses were isolated from Iraq, Jordan, and Saudi Arabia. In addition to respiratory involvement, some chicks represented nephritis and a drop in egg production (Ababneh et al., 2012 ▶). In the autumn of 2011, Q1 caused an outbreak in chicks of Northern Italy, and it was associated with kidney lesions, proventriculitis, and higher mortality (Toffan et al., 2013 ▶). Since then, this genotype has been regularly detected in Italy. Laconi et al. (2019) ▶ suggested a common origin between 642I IBV strains circulating in Italy since the 1990s and the Q1 genotype. Infectious bronchitis virus 624I was associated with kidney lesions and a decline in egg production in breeders and layers Laconi et al. (2019) ▶. Franzo et al. (2015) ▶ determined the complete genome of an Italian Q1 strain isolated from broilers showing respiratory involvement. Their isolate named gammaCoV/Ck/Italy/I2022/13 had the highest homology with a Chinese strain CK/CH/LDL/97i. Iran/Q1/UT-PCR-N1/2019 isolated in this study shared 100% homology with gammaCoV/Ck/Italy/I2022/13. In 2010 Kiss et al. (2016) ▶ isolated the D1344/2/2/10 strain, which showed 100% homology to the J2 strain of the Q1 genotype in Egypt. Q1 IBV genotype was isolated from poultry farms of Iran for the first time. Broilers were vaccinated with live H120 at one day of age and subsequently with IB88 at 14 days of age. This vaccination regime did not confer enough protection against the Q1 strain. Chhabra et al. (2015) ▶ suggested that combined vaccination of H120 and CR88 of 1-day-old chicks, followed by CR88 at 14 days of age, provides greater protection. Liu (2009) ▶ concluded that the homologous vaccine conferred complete protection of chicks against a Q1 strain and commercial vaccines did not provide enough respiratory protection. In conclusion, this new type of IBV which seems to be nephropathogenic has emerged in Iran, and since regular vaccination regimes cannot confer proper protection, more investigations are needed to try different vaccination regimes or to apply new effective vaccines.
Acknowledgment
We acknowledge NAVID MORGH Company for their support of this project.
Conflict of interest
The authors do not have any particular conflicts of interest to declare.
References
- Ababneh, M , Dalab, AE , Alsaad, S , Al-Zghoul, M Presence of infectious bronchitis virus strain CK/CH/LDL/97I in the Middle East. ISRN Vet. Sci. 2012;2012:1–6. doi: 10.5402/2012/201721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari, AG , Vasfi, MM , Keyvani, H Isolation and identification of infectious bronchitis viruses in poultry farms of Iran. J. Vet. Res. 2004;59:259–264. [Google Scholar]
- Cavanagh, D Coronavirus avian infectious bronchitis virus. Vet. Res. 2007;38:281–297. doi: 10.1051/vetres:2006055. [DOI] [PubMed] [Google Scholar]
- Chhabra, R , Forrester, A , Lemiere, S , Awad, F , Chantrey, J , Ganapathy, K Mucosal, cellular, and humoral immune responses induced by different live infectious bronchitis virus vaccination regimes and protection conferred against infectious bronchitis virus Q1 strain. Clin. Vaccine Immunol. 2015;22:1050–1059. doi: 10.1128/CVI.00368-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzo, G , Listorti, V , Naylor, CJ , Lupini, C , Laconi, A , Felice, V , Drigo, M , Catelli, E , Cecchinato, M Molecular investigation of a full-length genome of a Q1-like IBV strain isolated in Italy in 2013. Virus Res. 2015;210:77–80. doi: 10.1016/j.virusres.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghalyanchilangeroudi, A , Hosseini, H , Mehrabadi, MHF , Ghafouri, SA , Hamdan, AM , Ziafati, Z , Dizaji, RE , Mohammadi, P Genotyping of avian infectious bronchitis virus in Iran: Detection of D274 and changing in the genotypes rate. Comp. Immunol. Microbiol. Infect. Dis. 2019;65:110–115. doi: 10.1016/j.cimid.2019.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamadan, AM , Ghalyanchilangeroudi, A , Hashemzadeh, M , Hosseini, H , Karimi, V , Yahyaraeyat, R , Najafi, H Genotyping of avian infectious bronchitis viruses in Iran (2015-2017) reveals domination of IS-1494 like virus. Virus Res. 2017;240:101–106. doi: 10.1016/j.virusres.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini, H , Fard, MHB , Charkhkar, S , Morshed, R Epidemiology of avian infectious bronchitis virus genotypes in Iran (2010-2014) Avian Dis. 2015;59:431–435. doi: 10.1637/11091-041515-ResNote.1. [DOI] [PubMed] [Google Scholar]
- Kiss, I , Mató, T , Homonnay, Z , Tatár-Kis, T , Palya, V Successive occurrence of recombinant infectious bronchitis virus strains in restricted area of Middle East. Virus Evol. 2016;2:vew021. doi: 10.1093/ve/vew021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, S , Stecher, G , Tamura, K MEGA7: Molecular Evolutionary Genetics Analysis (en línea, programa informático) version 7 0 for bigger datasets. Mol. Biol. Evol. 2017;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laconi, A , Listorti, V , Franzo, G , Cecchinato, M , Naylor, C , Lupini, C , Catelli, E Molecular characterization of whole genome sequence of infectious bronchitis virus 624I genotype confirms the close relationship with Q1 genotype. Transbound. Emerg. Dis. 2019;66:207–216. doi: 10.1111/tbed.13000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, S , Zhang, Q , Chen, J , Han, Z , Liu, X , Feng, L , Shao, Y , Rong, J , Kong, X , Tong, G Genetic diversity of avian infectious bronchitis coronavirus strainsisolated in China between 1995 and 2004. Arch. Virol. 2006;151:1133–1148. doi: 10.1007/s00705-005-0695-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, S , Zhang, X , Wang, Y , Li, C , Liu, Q , Han, Z , Zhang, Q , Kong, X , Tong, G Evaluation of the protection conferred by commercial vaccines and attenuated heterologous isolates in China against the CK/CH/LDL/97I strain of infectious bronchitis coronavirus. Vet. J. 2009;179:130–136. doi: 10.1016/j.tvjl.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafi, H , Langeroudi, AG , Hashemzadeh, M , Karimi, V , Madadgar, O , Ghafouri, SA , Maghsoudlo, H , Farahani, RK Molecular characterization of infectious bronchitis viruses isolated from broiler chicken farms in Iran, 2014-2015. Arch. Virol. 2016;161:53–62. doi: 10.1007/s00705-015-2636-3. [DOI] [PubMed] [Google Scholar]
- Shoushtari, A , Toroghi, R , Momayez, R , Pourbakhsh, S 793/B type, the predominant circulating type of avian infectious bronchitis viruses 1999-2004 in Iran: a retrospective study. Arch. Razi Inst. 2008;63:1–5. [Google Scholar]
- Toffan, A , Bonci, M , Bano, L , Valastro, V , Vascellari, M , Capua, I , Terregino, C Diagnostic and clinical observation on the infectious bronchitis virus strain Q1 in Italy. Vet. Ital. 2013;49:347–355. doi: 10.12834/VetIt.1303.01. [DOI] [PubMed] [Google Scholar]
- Yu, L , Jiang, Y , Low, S , Wang, Z , Nam, SJ , Liu, W , Kwang, J Characterization of three infectious bronchitis virus isolates from China associated with proventriculus in vaccinated chickens. Avian Dis. 2001;45:416–424. [PubMed] [Google Scholar]