Abstract
Background:
Haemoproteus columbae is widely distributed in tropical and subtropical regions, causing pseudomalaria in pigeons.
Aims:
The current study aimed to characterize the phylogenetic position of H. columbae in pigeons in Sharkia province, Egypt, based on partial sequencing of the cytb gene as the conserved regions. The “DNA barcode” of the cytb gene helps in designing primers that can be used to amplify the same gene in the related haemosporidians. Methods: One hundered blood samples were collected from domestic pigeons to identify H. columbae by polymerase chain reaction (PCR) and detect its relationship with other related haemosporidians.
Results:
Weight losses of 60%, anemia 40%, low growth rates 26.67%, diarrhea 76.67%, dyspnea 66.67%, some neurological symptoms 33.33%, and death 16.67% were observed in the studied birds. Post-mortem examinations showed chocolate-brown appearance of the livers of the birds and congested parenchymatous organs. Microscopical examinations of Giemsa stained blood smears (n=100) revealed a 30% infection rate. The obtained infection percentages were more pronounced in males (35.71%) than females (16.66%) and more in adults (57.14%) than young pigeons (15.38%). The present sequence of H. columbae was deposited in GenBank under accession No.: MH345964 and shows 100% identity with other related Haemoproteus species in the Sao Paulo Zoo, Brazil (KU131585 and KU131583) and the UK (KX832581 and KX832586).
Conclusion:
This study concluded that the accurate diagnosis of H. coulmbae infection in pigeons by specific primers will help with the early treatment of affected cases, especially in the presence of the immature forms, and can thus avoid the noticed clinical signs and the induced pathological lesions mentioned in our study.
Key Words: Avian, Disease, Infection, Haemoproteus columbae, Pigeon
Introduction
Pigeons are considered to be potential carriers of zoonotic parasites because of their interactions with man and other domestic and wild birds. Haemoproteus columbae infection is widely distributed in tropical and subtropical regions (Springer, 1972 ▶), causing pseudomalaria or pigeon malaria which is fatal to young pigeons (Borkataki et al., 2015 ▶). Infected birds show loss of body weight, dullness, depression, dyspnoea, torticollis, diarrhea, anemia, anorexia, dehydration, and finally death (Nematollahi et al., 2012 ▶; Joshi et al., 2017 ▶). Haemoproteus columbae has subclinical pathogenic effects except in acute forms of infection, where heavy mortality has been recorded (Dey et al., 2010 ▶). In addition, previously infected pigeons become immunized against re-infection with H. columbae (Ahmed and Mohammed, 1978 ▶).
Haemoproteus columbae was studied for the first time by Kruse 1890 in Columba livia pigeons’ blood. Haemoproteus spp. are intracellular parasites transmitted by blood-sucking insects, including mosquitoes, biting midges, Pseudolynchia canariensis and tabanid flies (Al-Barwari and Saeed, 2012 ▶). Once the vector bites birds, the sporozoites are released in the bloodstream, invade endothelial cells of blood vessels of the lung, liver and spleen, and produce schizonts, which in turn produce numerous merozoites. They later penetrate red blood cells (RBCs) and transform into gametes. Then, the insect vector takes blood from infected birds and undergoes sexual reproduction producing oocysts. The oocysts rupture and release several sporozoites that invade the salivary gland and act as a focus for subsequent infection (Soulsby, 1982 ▶; Rupiper, 1998 ▶; Taylor et al., 2007 ▶).
The reproduction of Columba livia pigeons is of economic importance to Egypt as domestic pigeons, especially the younger ones (squabs), are mainly bred for their meat (Hussein and Abdelrahim, 2016 ▶). Therefore, this study was conducted to identify the clinical symptoms and pathological lesions caused by H. columbae in infected pigeons. In Egypt, domestic pigeons are considered a member of the poultry family. The population of raised pigeons reached more than 2,500,000 in 2003 (Lane, 2003 ▶) and later on about 11,300,000 in 2018 according to FAO. The early and accurate diagnosis of H. columbae would thus help with the effective treatment of infected pigeons in these populations.
The ability of H. columbae to infect domestic pigeon populations and risk their health has caused an economically marked decrease in reproductive success. Due to the pigeons’ role in transmitting infection with this parasite, and its significant impact on the overall status of the bird population, the current study aimed to determine the prevalence, describe pathological lesions in visceral organs and find the molecular characteristics and phylogenetic positions of H. columbae in pigeons in the Sharkia province, Egypt, based on partial sequencing of the cytb gene.
Materials and Methods
Bird collection and sampling
A total of 100 domestic pigeons were examined. These cases belonged to different localities admitted to the Veterinary Clinic, Faculty of Veterinary Medicine, Zagazig University, Sharkia province, Egypt. The examined pigeons included 70 males and 30 females, and their ages ranged between 2 months to 3 years. The blood samples were collected in tubes containing Ethylenediaminetetraacetic acid (EDTA) from the pigeons’ wing vein. A small drop of the blood (EDTA) sample was placed on a clean glass slide. A thin blood smear was then prepared and allowed to be air-dried and fixed with absolute methyl for 10 min. The sample was then stained with freshly prepared Giemsa stain and examined under an oil immersion lens to identify and differentiate macro and micro gametocytes of H. columbae according to the characters described by (Soulsby, 1982 ▶). The remained blood samples were kept at -20°C until DNA extraction.
This study was ethically approved by ZU-IACUC Committee, Zagazig University, Egypt (ZU-IACUC/2/F/6/2018).
Histopathology
The collected specimens from the liver, lung, and heart were fixed in a 10% buffered formalin solution, dehydrated in gradual alcohol (70-100%), cleared in xylene and embedded in paraffin. Five-micron thickness paraffin sections were prepared and stained with haematoxylin and eosin (H&E) dyes and examined microscopically (Suvarna et al., 2013 ▶).
Polymerase chain reaction (PCR) assay, sequencing, and phylogenetic analysis
DNA extraction from blood samples was performed using the QIAamp DNA Mini kit (Qiagen, Germany, GmbH). Amplification of the extracted DNA required a reaction mixture (25 µL) including 1.5 µL of Emerald Amp Max PCR Master Mix (Takara, Japan), 0.25 µL of 20 pmol of each primer (H. clom-F 5´-TTA GAT ACA TGC ATG CAA CTG GTG-3´ and H. clom-R 5´-TAG TAA TAA CAG TTG CAC CCC AG-3´, Bio Basic Canada Inc.), 5 µL of undiluted DNA and up to 25 µL nuclease-free water. The PCR cycling program included an initial denaturation at 94°C for 3 min, 30 cycles of denaturation at 94°C for 40 s, annealing at 60°C for 45 s, extension at 72°C for 1 min and final extension at 72°C for 5 min (Doosti et al., 2014 ▶).
The PCR products were purified using QIAquick PCR product extraction kit. (Qiagen, Valencia), sequenced using Bigdye Terminator V3.1 cycle sequencing kit (Perkin-Elmer) in an Applied Biosystems3130 genetic analyzer (HITACHI, Japan). The obtained sequences were aligned and compared with other associating sequences in the GenBank database by Basic Local Alignment Search Tool (BLAST®) tool (Altschul et al., 1990 ▶). The phylogenetic tree was created by the MegAlign module of Lasergene DNAStar (Thompson et al., 1994) and the phylogenetic analyses were performed using maximum likelihood in MEGA6 (Tamura et al., 2013 ▶). Sequence identity percentages were calculated using pairwise comparisons of the aligned sequence data.
Results
Clinical findings and PM lesions
The ante mortem (AM) examination of some pigeons revealed weight losses of 60%, anemia 40%, low growth rates 26.67%, diarrhea 76.67%, dyspnea 66.67%, and death 16.67% (Table 1). Neurological symptoms 33.33% were also recorded including movement of the head in circular or backward directions and torticollis episodes. Post-mortem examinations showed a chocolate-brown appearance of the liver with a septicemic picture (congestion of the parenchymatous organs) (Figs. 1A to C).
Table 1.
Infection percentages and noticed symptoms of examined pigeons according to age and sex
| Item | Ex. No. | Infect. % (No.) | Noticed symptoms % (Noticed No./Infected No.) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Weight losses | Anemia | Low growth | Diarrhea | Dyspnea | Neuro. | Death | |||
| Male | 70 | 35.71% (25) | 60% (15/25) |
40% (10/25) |
32% (8/25) |
80% (20/25) |
60% (15/25) |
24% (6/25) |
16% (4/25) |
| Female | 30 | 16.66% (5) | 60% (3/5) |
40% (2/5) |
0% (0/5) |
60% (3/5) |
100% (5/5) |
80% (4/5) |
20% (1/5) |
| Adult (7 months-3 years) | 35 | 57.14% (20) | 80% (16/20) |
35% (7/20) |
15% (3/20) |
90% (18/20) |
55% (11/20) |
25% (5/20) |
15% (3/20) |
| Young (2-6 months) | 65 | 15.38% (10) | 20% (2/10) |
50% (5/10) |
50% (5/10) |
50% (5/10) |
90% (9/10) |
50% (5/10) |
20% (2/10) |
| Total | 100 | 30% (30) | 60% (18/30) |
40% (12/30) |
26.67% (8/30) |
76.67% (23/30) |
66.67% (20/30) |
33.33% (10/30) |
16.67% (5/30) |
Ex.: Examined, Infect.: Infection, Neuro.: Neurological symptoms, and No.: Number
Fig. 1.
Clinical signs and post mortem lesion of pigeons suspected to be infected with Haemoproteus columbae. (A) Backward direction of pigeon’s head, (B) Torticollis, and (C) Chocolate brown appearance of the liver in the 6-month-old pigeon
Morphological identification
Infection percentages were found by microscopical examinations of Giemsa stained thin blood smears from 100 pigeons (Table 1).
Three forms of H. columbae blood stages were described as the following:
Immature forms
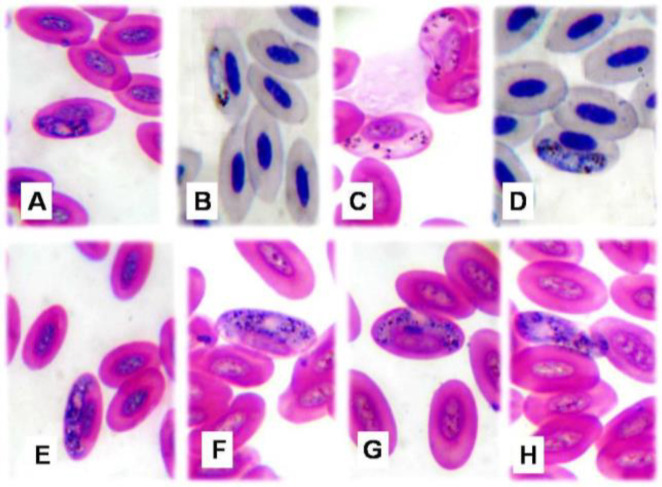
These forms reached 2.8-7.5 µm in length and 2.5-3.2 µm in width. They were situated lateral to the cell nucleus and not attached to the host cell membrane (Figs. 2A and B).
Fig. 2.
Microphotographs of Haemoproteus columbae (×1000). (A) Immature macrogametocyte, (B) Immature micro-gametocyte, (C) Mature microgametocyte broad at one end and narrow at the other, (D) Mature microgametocyte rounded at both poles, (E) Mature macrogametocyte encircled host cell nucleus, (F&G) Mature macrogametocyte displaced host cell nucleus to periphery, and (H) Extra-corpuscular form
Mature forms
These forms were partially encircled and might displace the host cell nucleus. Their ends might be curved at both poles or broad at one pole and narrow at the other. They included microgametocytes and macrogametocytes. Microgametocytes measured 8.1-11.3 µm long and 1.2-3 µm wide. Their granules were regularly arranged at both poles (Figs. 2C and D). Macrometocytes measured 12-14.2 µm long and 2.1-5.8 µm wide. The granules were irregularly scattered and colored brown to black (Figs. 2E-G).
Extra-corpuscular forms
These forms appeared elongated outside RBCs with a granular cytoplasm and dispersed granules. They measured 5.5-15 µm in length and 1.5 to 8 µm in width (Fig. 2H).
Histopathological changes
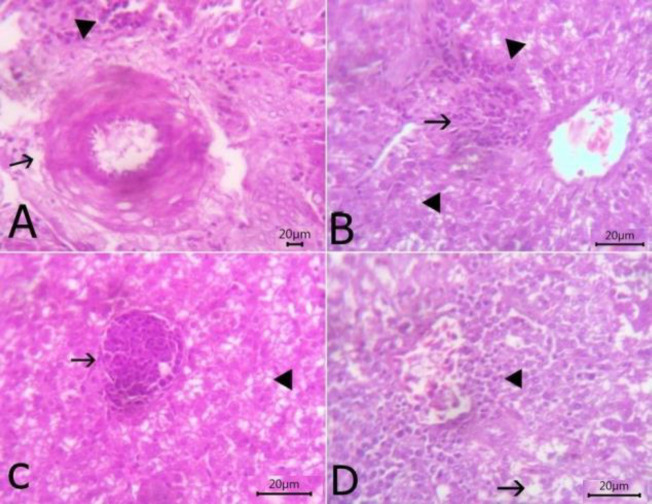
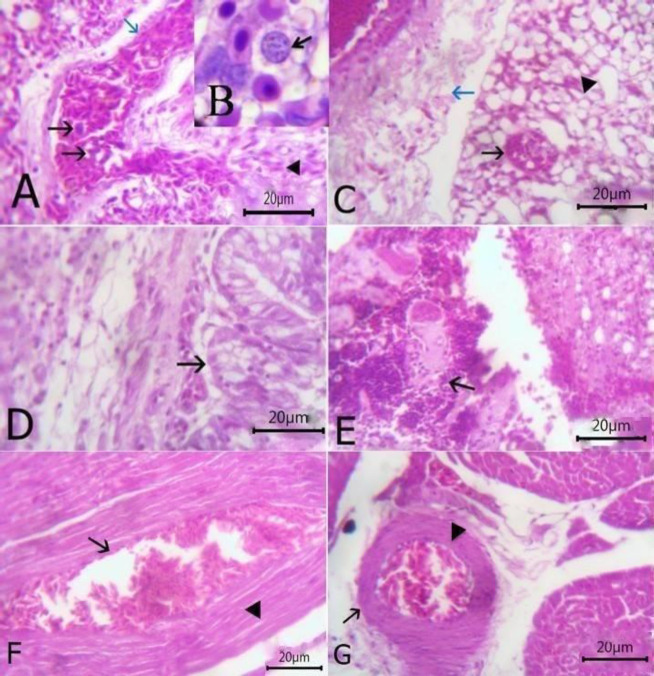
The liver exhibited slight congestion of the portal vein with thickening, hyalinization of tunica media, and perivascular edema beside few mononuclear cells infiltration (Fig. 3A). Vacuolar degeneration was noticed in hepatocytes with aninterstitial aggregation of round cells and fibroblasts (Fig. 3B). Round shaped thin-walled megaloschizonts of H. columbae were observed inside the hepatic parenchyma (Fig. 3C). Hydropic degeneration of hepatic cells and congestion of central vein with a perivascular aggregation of mononuclear inflammatory cells were also observed (Fig. 3D). The lungs revealed the presence of H. columbae schizonts within the pulmonary blood vessel which was dilated and congested with thickened interalveolar septa due to the presence of extravasated erythrocytes (Figs. 4A and B). Schizonts were also present inside the alveoli, destroying its wall with emphysematous alveoli. Perivascular edema was detected around congested blood vessels (Fig. 4C). Catarrhal bronchitis was observed, characterized by metaplasia of lining epithelium into goblet cells with the desquamation of lining epithelium and leukocytic infiltration (Fig. 4D). There was round cell granuloma, which consisted of a central area of caseous necrosis surround by mononuclear inflammatory cells (Fig. 4E). The heart showed extravasated erythrocytes among the cardiac muscles beside the degeneration of the myocardium (Fig. 4F). Congestion of blood vessels with hyaline thickening of walls and perivascular edema were also observed (Fig. 4G).
Fig. 3.
The histopathological lesions in liver sections stained with H&E. (A) The liver showing slight congestion of portal vein with thickening of the wall and perivascular edema (arrow) beside few mononuclear cells infiltration (arrowhead) (bare=20), (B) Liver showing vacuolar degeneration of hepatocytes (arrowhead) with interstitial aggregation of round cells and fibroblasts (arrow) (bare=20), (C) Liver showing round-shaped thin-walled megaloschizont of Haemoproteus columbae (arrow) and vacuolation of hepatocytes (arrowhead) (bare=20), and (D) Liver showing hydropic degeneration of hepatic cells (arrow) and congestion of central vein with a perivascular aggregation of mononuclear inflammatory cells (arrowhead) (bare=20)
Fig. 4.
(A) Lung section stained with H&E showing the presence of Haemoproteus columbae schizont within pulmonary blood vessel (arrow), dilated and congested (blue arrow) with presence extravasated erythrocytes (arrowhead) (bare=20), (B) Higher magnification of schizont (arrow), (C) Lung showing schizont inside the alveoli (arrow) with some emphysematous alveoli (arrowhead) and perivascular edema around congested blood vessels (blue arrow) (bare=20), (D) Lung showing catarrhal bronchitis (arrow) (bare=20), (E) Lung showing granuloma which consisting of the central area of caseous necrosis surrounded by mononuclear inflammatory cells (arrow) (bare=20), (F) Heart showing extravasated erythrocytes (arrow) among degenerated cardiac muscles (arrowhead) (bare=20), and (G) Heart showing congestion of blood vessels with hyaline thickening of its wall (arrow) and perivascular edema (arrowhead) (bare=20)
Molecular characterization (PCR assay), sequence polymorphism, and phylogenetic analysis
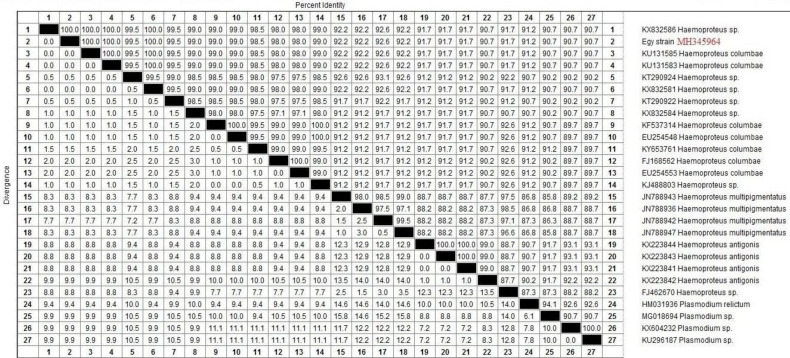
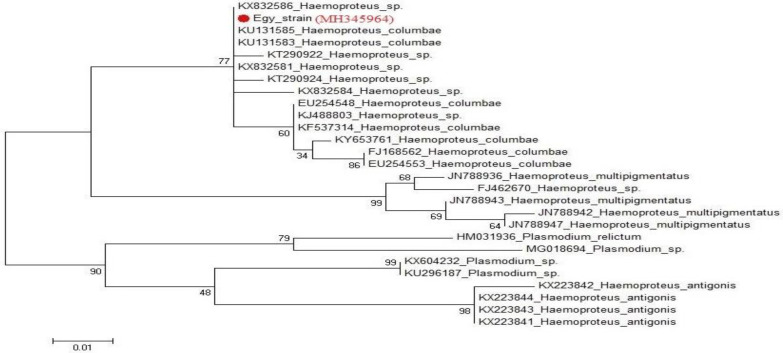
For PCR, the target sequence chosen for amplification was part of the mitochondrial cytb gene. Those variable regions had shown to be suitable genetic markers for distinguishing H. columbae. The length of their PCR products was 204 bp. The BlAST search results for H. columbae (accession No.: MH345964) in the current study revealed 100% identity with KU131585 and KU131583 in the Sao Paulo Zoo, Brazil, KX832581 and KX832586 in the UK, respectively. These sequences shared the same clade but the identity percentage reached 92.2% with Haemoproteus multipigmentatus (JN788943), 91.7% with Haemoproteus antigonis (KX223844) and 91.2% with Plasmodium relictum (HM031936). The phylogenetic tree based on cytb gene sequences showed that the currently studied H. columbae (MH345964) shared the same clade with H. multipigmentatus, while, H. antigonis and Plasmodium relictum had a separate clade (Figs. 5 and 6).
Fig. 5.
A sequence identity matrix of cytb gene (upper right triangle), reconstructed from protein sequences and showing the sequence distance (lower left triangle) for the Egy strain of Haemoproteus columbae (MH345964) with other related haemosporidian parasites in birds
Fig. 6.
Phylogenetic tree showing genetic relationship between cytb gene sequences of Haemoproteus columbae in this study (marked with red circle) and other sequences in the GenBank. The tree was generated maximum likelihood in MEGA6
Discussion
Infection with H. columbae is known as pseudomalaria because of its similarity with Plasmodium species (Friend and Franson, 1999 ▶). Most infections with H. columbae in pigeons were asymptomatic. However, young and immunocompromised pigeons might suffer from the disease. The clinical examination of pigeons in the present study revealed anorexia, depression, inability to fly, circling movements and episodes of torticollis. Postmortem examinations revealed livers with chocolate brown appearances and congested parenchymatous organs. These were similar to the observations recorded by (Varshney et al., 2014 ▶; Maharana and Kumar, 2017 ▶; Ortiz-Catedral et al., 2019 ▶) and the noticed in vivo gametocytes recorded by Coral et al. (2015) ▶.
Diagnosis of infected birds with H. columbae depended mainly upon the microscopical examination of Giemsa-stained blood smears. Gametocytes were only detected in RBCs. They are characterized by multiple, refractile, and golden brown pigment granules (haemozoin) arose from haemoglobin digestion (Friend and Franson, 1999 ▶). Gametocytes also appeared halter-shaped and some encircled the host cell nucleus, inducing cell distortion and nuclear displacement (Samani et al., 2013 ▶; Hussein and Abdelrahim, 2016 ▶). Only one gametocyte was detected inside the infected red cells of pigeons. This is considered as an indicator of mild infection, according to (Gicik and Arslan, 2001 ▶; Samani et al., 2013 ▶; Borkataki et al., 2015 ▶).
By examining microscopical Giemsa staining films, we recorded a 30% infection percentage (total) with H. columbae in pigeons in Sharkia province, Egypt. Similar rates were reported to be 26.7% by Martinez-Moreno et al. )1989) ▶ in Spain, 33% by Razmi and Andalibian (2006) ▶ in the Northeast of Iran, 37% by Msoffe et al. (2010) ▶ in Tanzania, 30% by Youssefi and Rahimi (2011) ▶ in Iran, 29.47% by Abed et al. (2014) ▶ in Iraq, and 29.4% by Scaglione et al. (2015) ▶ in Italy.
Higher rates were reported to be 43.2% by Gulander et al. (2002) ▶ in Turkey, 43.63% by Islam et al. (2014) ▶ in Chittagong district, Bangladesh, 50% by Borji et al. (2011 and 2012) ▶ in Iran, 55.63% by Gupta et al. (2011) ▶ in India, 57% in Ankara by Gicik and Arslan (2001) ▶, 57.2% in Qena, Egypt by Hussein and Abdelrahim (2016) ▶, 58.25% by Islam et al. (2014) ▶ Khulna district, Bangladesh, 61.33% by Borkataki et al. (2015) ▶ in Jammu district, India, 62% by Nematollahi et al. (2012) ▶ in Isfahan, Iran, 73% by Earle and Little (1993) ▶ in South Africa, 74% by Yunus and Arsalan (2001) ▶ in Turkey, 75% by Mushi et al. (1999) ▶ in Botswana, 76.5% by Dranzoa et al. (1999) ▶ in Uganda, and 80% in Sebele by Mushi et al. (2000) ▶.
Lower rates were reported to be 14% in Lapai, Nigeria by (Dadi-Mamud et al., 2012 ▶), 15.6% in Nigeria (Natala et al., 2009 ▶), 17.47% in North Iran (Youssefi et al., 2010 ▶), 18.8% in Turkey (Senlik et al., 2005 ▶), 22.3% in Bangladesh (Zahan et al., 2018 ▶), 22.7% in Tangail, Bangladesh (Abdul Momin et al., 2014 ▶), 23.18% in Iran (Doosti et al., 2014 ▶), and 24% in Southwest of Iran (Samani et al., 2013 ▶).
Variations in infection rates might have resulted from different geographic distribution, bird habitat and physiological differences, feeding habits, housing systems, abundance of insect vectors, and proper usage of insecticides against pigeon fly larvae in crevices of pigeon houses or in their nests.
Contrary to several studies (Earle and Little, 1993 ▶; Samani et al., 2013 ▶; Abed et al., 2014 ▶; Saikia et al., 2019 ▶), our obtained infection percentage was higher in male compared to female pigeons. Nevertheless, our findings matched those of Al-Barwari and Saeed, (2012) ▶, Hussein and Abdelrahim, (2016) ▶, and Zahan et al. (2018) ▶. Concerning the age factor, the obtained infection rate was more pronounced in adults than younger ages, and this was similar to Samani et al. (2013) ▶ and Zahan et al. (2018) ▶. On the other hand, Senlik et al. (2005) ▶ and Scaglione et al. (2015) ▶ could not detect a significant relationship between age or sex and infection rates. According to Jones (2006) ▶, not only environmental and genetic factors affect the resistance of birds to blood parasites, but age and sex of the birds, parasite strain, and stress also induce different infection rates. We also suggest that males were more susceptible to infection possibly due to their:
(a) Differences in appearance with females
(b) Mating habits, whereby each male mates with several females which might be infested with pigeon fly
(c) Testosterone which has an immunosuppressive role which hinders the clearance of the parasitic infection
Histopathological changes were exhibited within examined organs, including erosion of the blood vessels with lysis of their endothelial lining during the development of H. columbae schizonts in blood vessels of infected pigeons. In such a case, they infect another host they are directed towards through the endothelial cells of the blood vessels (Cottin et al., 1998 ▶). The liver revealed congestion of the portal vein with thickening, and the hyalinization of tunica media beside few mononuclear cell infiltrations. Vacuolar degeneration was noticed in hepatocytes with an interstitial aggregation of round cells. Round shaped thin-walled megaloschizonts of H. columbae were observed inside the hepatic parenchyma. These findings partially agreed with Hussein and Abdelrahim (2016) ▶ and Cepeda et al. (2019) ▶, who observed moderate and multifocal lymphoplasmocytic hepatitis with hepatocyte degeneration and vacuolated cytoplasm. In addition, H. columbae meronts were present inside the hepatic sinusoid. Microscopic changes in the lung showed H. columbae schizonts within the pulmonary blood vessel and inside the alveoli, destroying its wall with emphysematous alveoli. Round cell granuloma was observed, which consisted of the central area of the caseous necrosis surrounded by mononuclear inflammatory cells. These results partially agreed with those of Mubarak and Abed (2005) ▶ who reported that the pulmonary tissue was the main target for H. columbae schizonts in pulmonary blood vessels, and caused granulomatous pulmonary tissue reactions with emphysematous alveoli and other collapsed alveoli. The heart showed extravasated erythrocytes among cardiac muscles beside the degeneration of myocardium. These findings agreed with Olias et al. (2011) ▶ who observed necrosis and haemorrahge inside heart muscle fibers.
Recently, the cytb gene has been extensively used as a suitable target for the accurate identification of H. columbae. The conserved regions of the cytb gene “DNA barcode” help to design primers that can be used to amplify the same gene in the related species (Bensch et al., 2009 ▶). Many authors used PCR to identify and differentiate H. columbae affecting pigeons. In our study, the length of the PCR products was found to be 204 bp. This result was similar to those obtained by previous studies (Doosti et al., 2014 ▶; Maharana and Kumar, 2017 ▶; Saikia et al., 2019 ▶). The primers were also specifically used for the detection of H. columba in pigeons as previously described (Doosti et al., 2014 ▶; Maharana and Kumar, 2017 ▶; Saikia et al., 2019 ▶). On the other hand, the primers used by Hellgren et al. (2004) ▶ or Pacheco et al. (2011) ▶ could not specify the genus and species of the Haemosporidian infection (either Plasmodium sp. or Haemoproteus sp.).
Sequence analysis for the cytb gene revealed that the Egy strain of H. columbae (accession No.: MH345964) in domestic pigeons in Sharkia province, Egypt, shared 100% similarity with others in Brazil and the UK. Besides, the phylogenetic analysis in our study showed that H. columbae and H. multipigmentatus shared the same clade as those reported by Chagas et al. (2016) ▶ in São Paulo Zoo, Brazil.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Abdul Momin, M , Begum, N , Dey, AR , Paran, MS , Alam, MZ Prevalence of blood protozoa in poultry in Tangail, Bangladesh. J. Agri. Vet. Sci. 2014;7:55–60. [Google Scholar]
- Abed, AA , Naji, HA , Rhyaf, AG Investigation study of some parasites infected domestic pigeon (Columba livia domestica) in Al-Dewaniya city. J. Pharm. Bio. Sci. 2014;9:13–20. [Google Scholar]
- Ahmed, FE , Mohammed, AH Haemoproteus columbae: course of infection relapse and immunity to reinfection in the pigeon. Z. Parasitenkd. 1978;57:229–236. doi: 10.1007/BF00928036. [DOI] [PubMed] [Google Scholar]
- Al-Barwari, S , Saeed, I The parasitic communities of the rock pigeon Columbae livia from Iraq: component and importance. Turkiye. Parazitol. Derg. 2012;36:232–239. doi: 10.5152/tpd.2012.56. [DOI] [PubMed] [Google Scholar]
- Altschul, SF , Gish, W , Miller, W , Myers, EW , Lipman, DJ Basic local alignment search tool. J. Mol. Bio. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bensch, S , Hellgren, O , Pérez-Tris, J MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol. Ecol. Resour. 2009;9:1353–1358. doi: 10.1111/j.1755-0998.2009.02692.x. [DOI] [PubMed] [Google Scholar]
- Borji, H , Moghaddas, E , Razmi, GR , Azad, M A survey of ecto- and endo-parasites of domestic pigeons (Columba livia) in Mashhad, Iran. Iran. J. Vet. Sci. Techn. 2012;4:37–42. [Google Scholar]
- Borji, H , Moghaddas, E , Razmi, GR , Bami, MH , Mohri, M , Azad, M Prevalence of pigeon haemosporidians and effect of infection on biochemical factors in Iran. J. Parasit. Dis. 2011;2:199–201. doi: 10.1007/s12639-011-0056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkataki, S , Katoch, R , Goswami, P , Godara, R , Khajuria, JK , Yadav, A , Kour, R , Mir, I Incidence of Haemoproteus columbae in pigeons of Jammu district. J. Parasit. Dis. 2015;39:426–428. doi: 10.1007/s12639-013-0356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda, AS , Lotta-Arévalo, IA , Pinto-Osorio, DF , Macías-Zacipa, J , Valkiūnas, G , Barato, P , Matta, NE Experimental characterization of the complete life cycle of Haemoproteus columbae, with a description of a natural host-parasite system used to study this infection. Int. J. Parasitol. 2019;49:975–984. doi: 10.1016/j.ijpara.2019.07.003. [DOI] [PubMed] [Google Scholar]
- Chagas, CRF , Guimaraes, LO , Monteiro, EF , Valkiunas, G , Katayama, MV , Santos, SV , Guida, FJ , Simoes, RF , Kirchgatter, K Hemosporidian parasites of free-living birds in the Sao Paulo Zoo, Brazil. Parasitol. Res. 2016;115:1443–1452. doi: 10.1007/s00436-015-4878-0. [DOI] [PubMed] [Google Scholar]
- Coral, AA , Valkiunas, G , González, AD , Matta, NE In vitro development of Haemoproteus columbae (Haemosporida: Haemoproteidae), with perspectives for genomic studies of avian haemosporidian parasites. Exp. Parasitol. 2015;157:163–169. doi: 10.1016/j.exppara.2015.08.003. [DOI] [PubMed] [Google Scholar]
- Cottin, V , Donsbeck, AV , Revel, D , Loire, R , Cordier, JF Nonspecific interstitial pneumonia Individualization of a clinicopathologic entity in a series of 12 patients. Am. J. Respir. Crit. Care Med. 1998;158:1286–1293. doi: 10.1164/ajrccm.158.4.9802119. [DOI] [PubMed] [Google Scholar]
- Dadi-Mamud, NJ , Kabir, MA , Dibal, DM , Rajab, MH Study on the prevalence of Haemoparasites of pigeon (Columba livia) in Lapai-Nigeria. Inter. J. Applied Bio. Res. 2012;4:121–127. [Google Scholar]
- Dey, AR , Begum, N , Paul, SC , Noor, M , Islam, KM Prevalence and pathology of blood protozoa in pigeons reared at Mymensingh district, Bangladesh. Int. J. Bio. Res. 2010;2:25–29. [Google Scholar]
- Doosti, A , Ahmadi, R , Mohammadalipour, Z , Zohoor, A . Detection of Haemoproteus columbae in Iranian pigeons using PCR. Paper Presented at the International Conference on Biological. Civil and Environmental Engineering, Dubai (UAE); 2014. [Google Scholar]
- Dranzoa, C , Ocaido, M , Katete, P The ectogastro-intestinal and haemo-parasitesof live pigeons (Columba livia) in Kampala, Uganda. Avian Pathol. 1999;28:119–124. doi: 10.1080/03079459994830. [DOI] [PubMed] [Google Scholar]
- Earle, RA , Little, RM Haematozoa of feral rock doves and rock pigeons in mixed flocks. S. Afr. J. Wildl. Res. 1993;23:98–100. [Google Scholar]
- Friend, M , Franson, J . Parasitic diseases. In: Field manual of wildlife diseases: general field procedures and diseases of birds. 1st Edn. Reston, US Geological Survey; 1999. p. 425. [Google Scholar]
- Gicik, Y , Arslan, M Blood parasites of wild pigeons in Ankara District. Turk. J. Vet. Anim. Sci. 2001;25:169–172. [Google Scholar]
- Gulander, A , Tuzer, E , Cetinkaya, H Haemoproteus columbae infections and Pseudolynchia canariensis infestations in pigeons in Istanbul, Turkey. Istanbul Univ. Vet. Fak. Derg. Istanb. 2002;28:227–229. [Google Scholar]
- Gupta, DK , Jahan, N , Gupta, N New records of Haemoproteus and Plasmodium (Sporozoa: Haemosporida) of rock pigeon (Columba livia) in India. J. Parasit. Dis. 2011;35:155–168. doi: 10.1007/s12639-011-0044-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellgren, O , Waldenström, J , Bensch, S A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J. Parasitol. 2004;90:797–802. doi: 10.1645/GE-184R1. [DOI] [PubMed] [Google Scholar]
- Hussein, NM , Abdelrahim, EA Haemoproteus columbae infection and its histopathological effects on pigeons in Qena governorate, Egypt. J. Pharm. Bio. Sci. 2016;11:79–90. [Google Scholar]
- Islam, MS , Abdul Alim, M , Das, S , Ghosh, KK , Pervin, S , Lipi, A , Siddiki, AZ , Masuduzzaman, M , Hossain, MA Prevalence of Haemoproteus sp in domestic pigeon at Chittagong and Khulna District in Bangladesh. J. Adv. Parasitol. 2014;1:24–26. [Google Scholar]
- Jones, MP Selected infectious diseases of birds of prey. J. Exot. Pet. Med. 2006;15:5–17. [Google Scholar]
- Joshi, V , Dimri, U , Alam, S , Gopalakrishnan, A Buparvaquone therapy in a rock pigeon infected with Haemoproteus columbae showing torticollis. J. Parasit. Di. 2017;41:514–516. doi: 10.1007/s12639-016-0840-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane, EW . An account of the manners and of the customs modern Egyptian. 5th Edn. Egypt, American University in Cairo Press: 2003. p. 664. [Google Scholar]
- Maharana, BR , Kumar, B Pseudomalaria in a domestic pigeon: acase report. J. Parasit. Dis. 2017;41:295–297. doi: 10.1007/s12639-016-0764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Moreno, FJ , Martinez-Mareno, A , Becerra-Martell, C , Martinez-Cruz, MS Parasitic fauna of pigeons (Columba livia) in Cordoba Province, Spain. Rev. Iber. Parasitol. 1989;49:279–281. [Google Scholar]
- Msoffe, PLM , Muhairwa, AP , Chiwanga, GH A study of ecto- and endoparasites of domestic pigeon in Morogoro Municipality. Tanz. Afr. J. Agri. Res. 2010;5:264–267. [Google Scholar]
- Mubarak, M , Abed, GH Pathological changes of lung tissues of pigeons (Columba livia domestica) infected with Haemoproteus columbae (Haemosporina: Haemoproteidae) J. Bio. Sci. 2005;5:536–541. [Google Scholar]
- Mushi, EZ , Binta, MG , Chabo, RG , Mathaio, M , Ndebele, RT Haemoproteus columbae in domestic pigeons in Sebele, Gaborone, Botswana. Onderstepoort J. Vet. Res. 1999;66:29–32. [PubMed] [Google Scholar]
- Mushi, EZ , Binta, MG , Chaba, NRG , Panzirah, RR Parasites of domestic pigeon (Columbia livia domestica) in Sebele, Gaborone, Botswana. J. S. Afr. Vet. Assoc. 2000;7:249–250. [PubMed] [Google Scholar]
- Natala, AJ , Asemadahun, ND , Okubanjo, OO , Ulayi, BM , Owolabi, YH , Jato, ID , Yusuf, KH 2009) A survey of parasites of domesticated pigeon (Columba livia domestica) in Zaria, Nigeria. Int. J. Soft. Comput. 4:148–150. [Google Scholar]
- Nematollahi, A , Ebrahimi, M , Ahmadi, A , Himan, M Prevalence of Haemoproteus columbae and Trichomonas gallinae in pigeons (Columba domestica) in Isfahan, Iran. J. Parasit. Dis. 2012;36:141–142. doi: 10.1007/s12639-011-0082-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olias, P , Wegelin, M , Zenker, W , Freter, S , Gruber, AD , Klopfleisch, R Avian malaria deaths in parrots, Europe. Emerg. Infect. Dis. 2011;17:950. doi: 10.3201/eid1705.101618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Catedral, L , Brunton, D , Stidworthy, MF , Elsheikha, HM , Pennycott, T , Schulze, C , Braun, M , Wink, M , Gerlach, H , Pendl, H Haemoproteus minutus is highly virulent for Australasian and South American parrots. Parasit. Vect. 2019;12:40. doi: 10.1186/s13071-018-3255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco, MA , Battistuzzi, FU , Junge, RE , Cornejo, OE , Williams, CV , Landau, I , Rabetafika, L , Snounou, G , Jones-Engel, L , Escalante, AA Timing the origin of humanmalarias: the lemurpuzzle. BMC Evolution. Biol. 2011;11:299. doi: 10.1186/1471-2148-11-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razmi, G , Andalibian, E Survey on infestation to Haemoproteus columbae in pigeons of Mashhad. Pagh. Saza. 2006;71:95–96. [Google Scholar]
- Rupiper, DJ Diseases that affect race performance of homing pigeons Part II: Bacterial, fungal, and parasitic diseases. J. Avian Med. Surg. 1998;12:138–148. [Google Scholar]
- Saikia, M , Bhattacharjee, K , Sarmah, PC , Deka, DK , Tamuly, S , Kakati1, P , Konch, P Prevalence and molecular detection of blood protozoa in domestic pigeon. Int. J. Curr. Microbiol. App. Sci. 2019;8:1426–1436. [Google Scholar]
- Samani, AA , Kheirabadi, KP , Samani, AD Prevalence and rate of parasitemia of Haemoproteus columbae in Columba Livia domestica in Southwest of Iran. Iran. J. Parasitol. 2013;8:641–644. [PMC free article] [PubMed] [Google Scholar]
- Scaglione, FE , Pregel, P , Cannizzo, FT , Pérez-Rodríguez, AD , Ferroglio, E , Bollo, E Prevalence of new and known species of haemoparasites in feral pigeons in northwest Italy. Malar. J. 2015;14:1–5. doi: 10.1186/s12936-015-0617-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senlik, B , Gulegen, E , Akyol, V Prevalence and intensity of Haemoproteus columbae in domestic pigeons. Indian Vet. J. 2005;82:998–999. doi: 10.1556/AVet.53.2005.4.5. [DOI] [PubMed] [Google Scholar]
- Soulsby, EJL . Helminths, arthropods and protozoa of domesticated animals. 7th Edn. London: Baillière Tindall ; 1982. p. 215. [Google Scholar]
- Springer, WT . In: Blood and tissue protozoa. Hofstad, MS , editor. Ames, Iowa State University Press: 1972. p. 102. [Google Scholar]
- Suvarna, SK , Layton, C , Bancroft, JD . Bancroft’s theory and practice of histological techniques. 7th Edn. England, Churchill Livingstone, Elsevier: 2013. p. 85. [Google Scholar]
- Tamura, K , Stecher, G , Peterson, D , Filipski, A , Kumar, S Mega 6: molecular evolutionary genetics analysis version 60. Mol. Bio. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, MA , Coop, RL , Wall, RL . Veterinary parasitology. 3rd Edn. UK, Blackwell Publishing Ltd: 2007. p. 189. [Google Scholar]
- Thompson, JD , Higgins, DG , Gibson, TJ ClustalW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney, JP , Deshmukh, VV , Chaudhary, PS Pseudomalaria (Haemoproteus columbae) in pigeon shelter. Intas Polivet. 2014;15:176–177. [Google Scholar]
- Youssefi, MR , Rahimi, MT Haemoproteus columbae in Columba livia domestica of Three Areas in Iran in 2010. Glob.Vet. 2011;7:593–595. [Google Scholar]
- Youssefi, MR , Sadeghian, AG , Esfandiari, B Prevalence of H columbae infection in Columbia livia in North of Iran. World J. Zoo. 2010;5:275–277. [Google Scholar]
- Yunus, G , Arsalan, M Blood parasites of wild pigeons in Ankara. J. Vet. Anim. Sci. 2001;25:169–172. [Google Scholar]
- Zahan, T , Hossain, S , Dey, AD , Shahiduzzaman, M , Alam, MZ Prevalence and seasonal distribution of haemosporidian parasites in pigeons of My-mensingh and Rangpur districts, Bangladesh. Eurasian J. Vet. Sci. 2018;34:117–122. [Google Scholar]