The pathogenesis of allergies has long been described as the interaction of the environment as well as the genetic susceptibility and immune system of an individual. Allergen sensitization, which involves allergen entry through the disrupted skin barrier, antigen processing, and presentation to the immune system that later induces mast cell activation and the T helper 2 (Th2) immune response, is regarded as the early event in the development of atopic dermatitis (AD). High circulating neuropeptides and nerve growth factors, the presence of neuropeptide-expressing neurons in AD skin lesions, and scratching behavior observed in patients with AD provide evidence that the nervous system also plays a key role in the manifestation of AD.1,2 However, our understanding of nociceptive sensory neurons that reside in the dermis of patients with AD has not been limited to their primary functions in sensing and transmitting pain and itch signals or initiating defensive responses such as scratching and removal. Several groups have unfolded the pathological role of nociceptors and neuropeptides in regulating AD-related innate lymphoid cell 2 in allergic inflammation.3,4 The interaction between the nervous system and immune cells was also reported in fungal-infected skin inflammation and psoriasis but not in AD.5,6 Serhan et al., for the first time, revealed the detailed mechanism of how allergens trigger peripheral nociceptive sensory neurons to release neuropeptides and eventually signal mast cells in the skin to degranulate and induce Th2 inflammation through a nonhistaminergic itch pathway in the early stage of AD development.7
House dust mites (HDMs) are the most potent indoor allergens and are associated with the manifestation of AD. HDMs can be taken up through the skin in various ways. Epithelial cells or dendritic cells can capture HDM proteases via protease-activated receptors and pattern-recognition receptors. HDMs can also enter via the disruption of the tight junctions and transmembrane adhesion molecules of epithelial cells by their proteolytic activities.8 The entered antigen is then presented to antigen-presenting cells, mainly dendritic cells, to signal downstream inflammatory responses in various allergic diseases. Along with HDM, bacterial Staphylococcus aureus (S. aureus) colonization on the skin is commonly found on the skin lesions of individuals with AD. Acute infection of S. aureus directly activates nociceptors to release neuropeptides, which modulate the local inflammatory response.9 Nevertheless, whether the Th2-related allergen HDM possesses a similar capacity in signaling nociceptors to induce neuropeptides for the downstream inflammatory response in AD is unclear.
Neuropeptides, such as substance P (SP), calcitonin gene-related peptide, neuropeptide Y, and somatostatin, are a group of heterogeneous molecules produced by afferent sensory neurons of the peripheral nervous system. Among them, SP is the inducer of vasodilation, local inflammation, and cellular proliferation in skin that is upregulated in the skin lesions of patients with AD.1,10 SP is also upregulated in the plasma of patients with AD and is positively correlated with their AD severity, suggesting the systemic involvement of SP in AD.2,11 On the other hand, immunohistochemical staining revealed that SP-positive mast cells were in close apposition to the SP-positive nerve fibers in the skin.12 SP can induce mast cell proliferation, differentiation, activation, and degranulation.13 Moreover, SP can also be rapidly degraded by neuropeptide-degrading enzymes released by mast cells.13 This evidence indicates the close interaction between the neuropeptide SP and mast cells in AD.12
Upon the cross-linking of anti-IgE antibody to the IgE receptor on mast cells, mast cells can actively degranulate mediators such as histamine and serotonin to mediate local histaminergic pruritus and downstream hypersensitivity.11,14 Although histamine has been the most common pruritic agent for studying itch, other histamine-independent pruritogens, such as the pruritogenic cytokine IL-31, were also found to possess pruritic properties.15,16 A recent study revealed that activation of mast cells through their Mas-related G protein-coupled receptor MRGPRB2 receptors promoted the release of nonhistaminergic pruritogens, such as tryptase, that trigger an alternative itch mechanism distinct from histamine in allergic contact dermatitis.14 The significant upregulation of tryptase-positive mast cells in itchy AD skin suggested that this nonhistaminergic itchiness may also play a pathological role in AD development.17
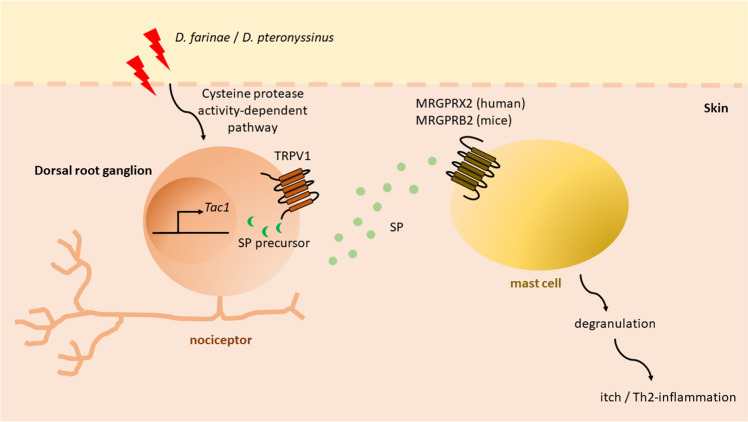
Serhan et al. demonstrated that the common allergen HDM strains Dermatophagoides farina (D. farina) and potentially Dermatophagoides pteronyssinus (D. pteronyssinus), which possess cysteine protease activities, could exclusively trigger the secretion of neuropeptide SP from the ion channel transient receptor potential vanilloid 1 (TRPV1)- and Tac1-expressing nociceptor in the dermis (Fig. 1). The SP then signals the MRGPRB2 of mast cells adjacent to the nociceptor and induces a type 2 inflammatory response. In contrast to activation via the canonical immunoglobulin (Ig)E-Fc epsilon RI region, activation of mast cells through their cationic molecule receptors, i.e., MRGPRB2 in mice or MRGPRX2 in humans, induces the release of mediators such as tryptase β2 that excite a specific itch-sensory neuron population, giving rise to a distinctive itch that is histamine-independent.14 Such activation was more prominent when both D. farina and the bacterial staphylococcal endotoxin B from S. aureus were simultaneously used as stimulants.7 Using the two antigens that can be commonly found on the skin lesions of patients with AD, their study mimics the Th2 inflammatory response of patients with moderate-to-severe AD induced by concurrent allergen triggers and bacterial infection on the skin.
Fig. 1.
A cluster formed between the nociceptor and mast cell in the skin upon house dust mite activation in the early development of type 2 allergic skin inflammation. Allergen house dust mites (D. farinae and D. pteronyssinus) directly activate Tac1-expressing and neuropeptide-producing nociceptive sensory neurons, i.e., peptidergic nociceptors, by degrading epithelial junctions by cysteine proteolytic activity. The gene Tac1 encodes the neuropeptide substance p (SP) precursor. The SP produced is secreted from the ion channel TRPV1+ nociceptor. The secreted SP is then taken up by adjacent mast cells via the cationic molecule receptor MRGPRB2 in mice. This triggers the degranulation of mast cells and drives itchy signals and the development of type 2 skin inflammation that resembles the features of AD in humans
Although the current study does not elucidate the mediators and cytokines that are released by SP-activated mast cells upon HDM activation, AD-related pruritic mediators, such as tryptase and IL-31, which can both be released by mast cells during degranulation, may be potential intermediate players in the induction of itchiness and Th2 inflammation.18 IL-31 can enhance the release of the central itch mediator brain-derived natriuretic peptide and hence orchestrate the release of cytokines and chemokines from skin cells to induce the itch signal.19 Moreover, the IL-31 receptor IL-31RA is a functional receptor expressed on TRPV1+ neurons in both humans and mice.20 We have found that IL-31 can also activate AD effector cell eosinophils and dermal fibroblasts in vitro, thereby suggesting that it is involved in Th2-related inflammatory responses.21 Investigation of these mediators in the immune and nervous systems may be beneficial to our current understanding of the chronic itch observed in patients with AD. In addition, further experiments are essential to unravel the precise mechanism of how the bacterial staphylococcal endotoxin B of S. aureus takes part in this model.
In conclusion, Serhan et al. elucidated the capacity of sensory neurons to detect HDM allergens and their potential interaction with mast cells in the early development of AD. As antihistamines may not always be effective in treating pruritus in chronic skin inflammation,18 the work by Serhan et al. pointed out that SP might be a promising therapeutic target for the prevention and treatment of AD. The neuron–mast cell cluster identified in their study also indicates a new direction to examine in other allergic disorders.
Competing interests
The authors declare no competing interests.
References
- 1.Ostlere LS, Cowen T, Rustin MH. Clin. Exp. Dermatol. 1995;20:462–467. doi: 10.1111/j.1365-2230.1995.tb01378.x. [DOI] [PubMed] [Google Scholar]
- 2.Toyoda M, et al. Br. J. Dermatol. 2002;147:71–79. doi: 10.1046/j.1365-2133.2002.04803.x. [DOI] [PubMed] [Google Scholar]
- 3.Wallrapp A, et al. Nature. 2017;549:351–356. doi: 10.1038/nature24029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moriyama S, et al. Science. 2018;359:1056–1061. doi: 10.1126/science.aan4829. [DOI] [PubMed] [Google Scholar]
- 5.Kashem SW, et al. Immunity. 2015;43:515–526. doi: 10.1016/j.immuni.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riol-Blanco L, et al. Nature. 2014;510:157–161. doi: 10.1038/nature13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serhan N, et al. Nat. Immunol. 2019;20:1435–1443. doi: 10.1038/s41590-019-0493-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scheurer S, Toda M, Vieths S. Clin. Exp. Allergy. 2015;45:1150–1161. doi: 10.1111/cea.12571. [DOI] [PubMed] [Google Scholar]
- 9.Chiu IM, et al. Nature. 2013;501:52–57. doi: 10.1038/nature12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vidal Yucha SE, Tamamoto KA, Kaplan DL. Cell Prolif. 2019;52:e12677. doi: 10.1111/cpr.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salomon J, Baran E. J. Eur. Acad. Dermatol. Venereol. 2008;22:223–228. doi: 10.1111/j.1468-3083.2007.02399.x. [DOI] [PubMed] [Google Scholar]
- 12.Toyoda M, Makino T, Kagoura M, Morohashi M. Arch. Dermatol. Res. 2000;292:418–421. doi: 10.1007/s004030000149. [DOI] [PubMed] [Google Scholar]
- 13.Scholzen T, et al. Exp. Dermatol. 1998;7:81–96. doi: 10.1111/j.1600-0625.1998.tb00307.x. [DOI] [PubMed] [Google Scholar]
- 14.Meixiong J, et al. Immunity. 2019;50:1163–1171 e1165. doi: 10.1016/j.immuni.2019.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LaMotte RH, Dong X, Ringkamp M. Nat. Rev. Neurosci. 2014;15:19–31. doi: 10.1038/nrn3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nocchi L, et al. Nat. Biomed. Eng. 2019;3:114–125. doi: 10.1038/s41551-018-0328-5. [DOI] [PubMed] [Google Scholar]
- 17.Nattkemper LA, et al. J. Investig. Dermatol. 2018;138:1311–1317. doi: 10.1016/j.jid.2017.12.029. [DOI] [PubMed] [Google Scholar]
- 18.Siiskonen H, Harvima I. Front. Cell Neurosci. 2019;13:422. doi: 10.3389/fncel.2019.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meng J, et al. J. Allergy Clin. Immunol. 2018;141:1677–1689 e1678. doi: 10.1016/j.jaci.2017.12.1002. [DOI] [PubMed] [Google Scholar]
- 20.Cevikbas F, et al. J. Allergy Clin. Immunol. 2014;133:448–460. doi: 10.1016/j.jaci.2013.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong CK, et al. PLoS ONE. 2012;7:e29815. doi: 10.1371/journal.pone.0029815. [DOI] [PMC free article] [PubMed] [Google Scholar]