Macrophages play a key role in the body’s immune functions, including tumor surveillance. Recent studies have indicated that environmental cues and molecular mediators can regulate macrophage polarization via protein–glycan interactions.1 The glycans decorating the Mφ membrane transduce a cascade of feedback signals from the cellular microenvironment into internal signaling pathways, shifting the functional Mφ phenotype that forms the molecular complex.2,3 Therefore, glycans and their derivatives might be attractive targets for controlling Mφ polarization at the supramolecular level. However, the influence of galactose supramolecular docking on cell behavior is currently unknown.
In this study, functional galactose probes were designed (see Supplementary Material) to target proteins on the macrophage membrane. As confirmed by fluorescence assay (Fig. 1a) and FACS data (Fig. 1b), Gal beads had significant binding activity with the macrophage membrane, while plain beads were not. Gal bead-binding affinity and sensitivity were verified by selecting a range of cell:bead ratios between 1:10 and 1:100 and using plain beads as a negative control (contr(−)). When the green fluorescence signal measured on single cells was compared among various cell:bead ratios, the number of anchored beads increased linearly with the cell:bead ratio (Fig. S3a). The fluorescence intensity correlated with the concentration of galactose, and the dissociation constant, KD, was 1.25 mM (Fig. S3b).3,4 Furthermore, with increasing Gal bead concentration, the percentage of the Gal-bead-coated Mφs increased almost linearly (Fig. S3c).
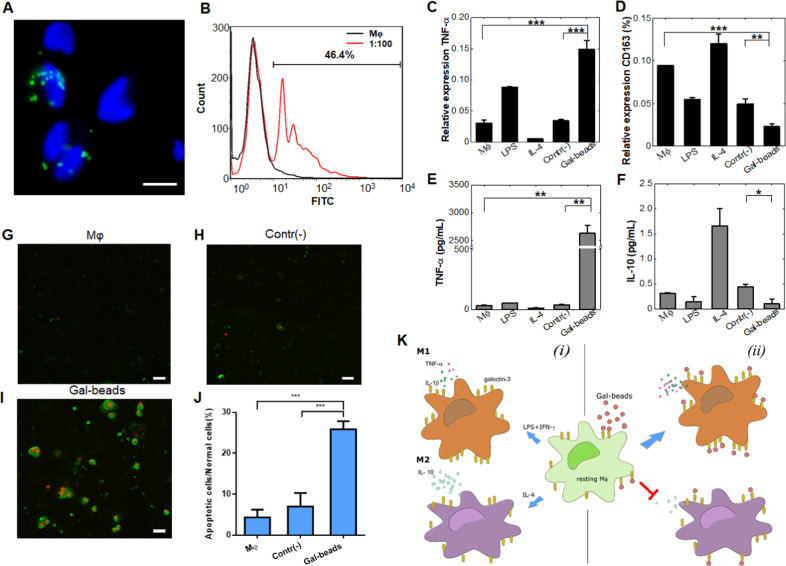
Fig. 1. Galactose docking educated macrophages such that they polarized into a proinflammatory phenotype.
a Analysis of the binding between Gal beads and Mφs by fluorescence image overlay. Gal beads were labeled with FITC, and the Mφs were stained with DAPI; scale bar = 20 μm. b FACS analysis results of the binding between naïve Mφs and Gal beads. The Gal beads coated with Mφ were gated. c–f Results from the quantitative analysis of M1 and M2 phenotype markers. The induced expression of transcript levels of IL-1β (c, an M1 phenotype marker) and CD163 (d, an M2 phenotype marker) as related to the internal GAPDH control. The induced secretion levels of proinflammatory e and anti-inflammatory f cytokines. The values represent the means ± SD of three individual experiments. g–i Combining Gal beads and Mφs leads to cancer cell apoptosis. Mφ itself g or with either bare beads h or Gal beads i were placed in the upper chamber, and the cancer cells were placed in the lower chamber. Cancer cells in the lower chamber were stained with Annexin V (green) and propidium iodide (red) to determine cell apoptosis. Scale bar = 50 μm for g–i. j Quantitative data indicating the number of apoptotic cells to normal cells. The cell counts in each experiment were obtained from 10 to 15 fields of view using a fluorescence microscope. The values represent the means ± SD of three individual experiments. k Schematic diagram illustrating Mφ polarization influenced by the cellular microenvironment in this study. Under standard conditions, resting Mφs can polarize to either the M1 or M2 phenotype upon exposure to the stimulating molecules LPS/IFN-γ or IL-4 (panel i). In a galactose-rich environment, the resting Mφs polarize to the M1 phenotype while the M2 type is inhibited (panel (ii)). The asterisks indicate significance, and the p values were calculated with Student’s t-test (*p < 0.05; **p < 0.01; and ***p < 0.001).
Binding activity represents a miniscule aspect of the interaction of cells with galactose moieties. To test our initial hypothesis that active moieties can induce macrophage polarization, we mimicked the in vivo interaction by incubating resting Mφs (M0 phenotype) with LPS/IFN-γ to induce the M1 phenotype, IL-4 to induce the M2 phenotype, Gal beads or control plain beads.5 The relative transcript levels of the cytokines TNF-α and IL-1β and the scavenger CD163 (which is commonly used to evaluate the M1 or M2 phenotypic status of Mφs6) were measured. As expected, M1 Mφs were found to highly express proinflammatory cytokines (TNF-α and IL-1β), while CD163 expression was suppressed, and the results for M2 Mφs were the opposite (Fig. 1c, d, S4a). Interestingly, incubation of Mφs with Gal beads resulted in obviously increased expression of both TNF-α (Fig. 1c) and IL-1β (Fig. S4a) compared to their levels in the untreated and negative controls. The CD163 transcript levels recorded in Mφ incubated with Gal beads were lower than those in naïve Mφs and negative control-treated Mφs (Fig. 1d). A more advanced step of determining protein production was used to quantify the levels of TNF-α, IL-1β, and IL-10 released from the Mφs when activated by the biochemical factors described above. Similar to the results from the genomic analyses, the data from the biochemical treatments showed that M1 Mφs released more proinflammatory cytokines (TNF-α, Fig. 1e; IL-1β, Fig. S4b), and that IL-4 exposure suppressed these cytokines. As expected, M2 Mφs released more IL-10 than LPS/IFN-γ-induced M1 Mφs (Fig. 1f). These crucial results indicate that, after interacting with galactose moieties, resting Mφs were educated to release a notably greater amount of proinflammatory cytokines, and this release was more profound than that caused by LPS/IFN-γ induction (Fig. 1e, S3b). In addition, IL-10 production was suppressed (Fig. 1f).
Docking by Gal beads induced resting Mφs to polarize and release proinflammatory cytokines, which possess potent immunoregulatory properties that could be exploited to facilitate host defenses or tumor surveillance. An in vitro Transwell system was used to evaluate cancer cell apoptosis by educated Mφs. As shown in Fig. 1g–j, after being cocultured with either Mφs only or Mφs together with plain beads, few cancer cells underwent apoptosis (green) or another type of death (red) (Fig. 1g, h). However, when Mφs were combined with Gal beads, the number of cancer cells undergoing apoptosis or emitting signals indicating death increased (Fig. 1i). The quantitative results, shown as the ratio of apoptotic cells to normal cells, indicated that the apoptosis rate of the cancer cells cocultured with Gal beads bound to Mφs was significantly higher than it was for the cancer cells cocultured with plain beads and Mφs or with only resting Mφs (Fig. 1j).
Recent biochemical studies have underscored the value of galactose as an attractive target influencing Mφ behavior,7,8 but the structural basis of the role of galactose moieties in Mφ polarization remains unresolved. Here, as shown in the scheme presented in Fig. 1k, resting Mφs can be polarized to acquire either the M1 or M2 phenotype according to the cellular microenvironment (panel i in Fig. 1k). Following Gal probe binding, the resting macrophages polarized into the proinflammatory phenotype, giving them the potential to induce cancer cell apoptosis, rather than the anti-inflammatory phenotype (panel ii in Fig. 1k). The results presented here provide a rational basis for designing small molecules to block the mechanisms induced by galectins (tumorigenesis, metastasis, and so on). We have highlighted a potential approach to rebalance inflammation, maintain immunological homeostasis, and possibly develop more effective vaccines.
Supplementary information
Acknowledgements
The authors want to thank Zhe Wang (School of Life Sciences, Northwestern Polytechnical University) for his support with the fluorescence microscope and Yan Feng (School of Materials Science and Engineering, Northwestern Polytechnical University; Shaanxi materials analysis and research center) for the help with scanning electron microscopy. In addition, the authors would like to thank Sufang Wang (School of Life Sciences, Northwestern Polytechnical University) for sharing comments about the manuscript. The authors are thankful for the support from the Foreigner Students Exchanging Program between the Northwestern Polytechnical University and Avans University. This work was supported by the National Natural Science Foundation of China (11722220, 11672246, and 11472224) and Fundamental Research Funds for the Central Universities (G2016KY0302 and G2017KY0320). N. Zhang and G. Simone contributed equally to this work.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Nu Zhang, Giuseppina Simone
Contributor Information
Hui Yang, Email: kittyyh@nwpu.edu.cn.
Giuseppina Simone, Email: giuseppina.simone@nwpu.edu.cn.
Supplementary information
The online version of this article (10.1038/s41423-019-0358-2) contains supplementary material.
References
- 1.Shang Q, et al. Delivery of adipose-derived stem cells attenuates adipose tissue inflammation and insulin resistance in obese mice through remodeling macrophage phenotypes. Stem Cells Dev. 2015;24:2052–2064. doi: 10.1089/scd.2014.0557. [DOI] [PubMed] [Google Scholar]
- 2.Su L, et al. Glycocalyx-mimicking nanoparticles for stimulation and polarization of macrophages via specific interactions. Small. 2015;11:4191–4200. doi: 10.1002/smll.201403838. [DOI] [PubMed] [Google Scholar]
- 3.Simone G, et al. Protein–carbohydrate complex reveals circulating metastatic cells in a microfluidic assay. Small. 2013;9:2152–2161. doi: 10.1002/smll.201202867. [DOI] [PubMed] [Google Scholar]
- 4.Zhang XM, Pastorino F, Simone G. Microliter chip for sensitive detection of the binding activity of glycans and cognate molecules. Sens. Actuators B-Chem. 2018;273:342–349. doi: 10.1016/j.snb.2018.05.096. [DOI] [Google Scholar]
- 5.Murray PJ, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuchs AK, et al. Carboxyl- and amino-functionalized polystyrene nanoparticles differentially affect the polarization profile of M1 and M2 macrophage subsets. Biomaterials. 2016;85:78–87. doi: 10.1016/j.biomaterials.2016.01.064. [DOI] [PubMed] [Google Scholar]
- 7.MacKinnon AC, et al. Regulation of alternative macrophage activation by galectin-3. J. Immunol. 2008;180:2650–2658. doi: 10.4049/jimmunol.180.4.2650. [DOI] [PubMed] [Google Scholar]
- 8.Novak R, Dabelic S, Dumic J. Galectin-1 and galectin-3 expression profiles in classically and alternatively activated human macrophages. BBA-Gen. Subj. 2012;1820:1383–1390. doi: 10.1016/j.bbagen.2011.11.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.