Genetic analysis reveals the dual function of an abscisic acid biosynthesis gene in violaxanthin isomerization into cis-neoxanthin and cis-violaxanthin, which are the last carotenoid precursors in abscisic acid biosynthesis.
Abstract
Abscisic acid (ABA), a plant hormone synthesized from carotenoids, functions in seed germination and abiotic stress responses. ABA is derived from the cleavage of 9-cis-isomers of violaxanthin and neoxanthin, which are oxygenated carotenoids, also called xanthophylls. Although genes encoding enzymes responsible for most steps of the ABA biosynthesis pathway have been identified, enzymatic reactions leading to the production of these cis-isomers from trans-violaxanthin remain poorly understood. Two mutants that lack trans- and cis-neoxanthin, tomato (Solanum lycopersicum) neoxanthin-deficient1 (nxd1) and Arabidopsis (Arabidopsis thaliana) ABA-deficient4 (aba4), were identified previously, but only aba4 exhibited ABA-deficient phenotypes. No enzymatic activity was detected for ABA4 and NXD1 proteins, and their exact function remained unknown. To further investigate ABA4 and NXD1 function in Arabidopsis, we compared phenotypes of single and double mutants, and analyzed the effect of ABA4 overexpression on ABA and carotenoid accumulation in wild-type and mutant backgrounds. We provide convergent evidence that ABA4 is not only required for the formation of trans- and 9′-cis-neoxanthin from trans-violaxanthin, but also controls 9-cis-violaxanthin accumulation. While nxd1 produces high amounts of 9-cis-violaxanthin and ABA, aba4 nxd1 exhibits reduced levels in both leaves and seeds. Furthermore, ABA4 constitutive expression in nxd1 increases both 9-cis-violaxanthin and ABA accumulation. Subcellular localization of NXD1 protein in transient expression assays suggests that production of the NXD1-derived factor required for neoxanthin synthesis takes place in the cytosol. Finally, we postulate that ABA4, with additional unknown cofactor(s), is required for, or contributes to, trans-to-cis violaxanthin isomerase activity, producing both cis-xanthophyll precursors of ABA.
Abscisic acid (ABA) accumulates in plants in response to abiotic stresses and contributes to the induction of many adaptive responses. In developing seeds, hormone levels peak during maturation and promote dormancy, reserve storage, and desiccation tolerance. In early studies, evidence of these different roles was notably provided by the phenotypes of ABA-deficient or insensitive mutants, with plants hypersensitive to water deficit and seeds being less dormant. Later studies revealed that ABA played other important roles throughout the plant life cycle (Mine, 2019; Yoshida et al., 2019).
ABA is a sesquiterpenoid that is synthesized in plants via the cleavage of carotenoid precursors. The early steps of ABA biosynthesis take place in plastids, and the direct precursors are oxygenated carotenoids, also called xanthophylls, produced from β-carotene (Fig. 1). The oxidative cleavage of cis-isomers of epoxycarotenoids, 9-cis-violaxanthin (c-viola) and 9′-cis-neoxanthin (c-neo), by a 9-cis-epoxycarotenoid dioxygenase (NCED) is generally considered the first specific step to ABA biosynthesis and has been widely described as the rate-limiting reaction of the pathway. The cleavage product, xanthoxin, is then transported to the cytosol by an unknown mechanism and converted to ABA via abscisic aldehyde. Reduction of active ABA levels is achieved by either oxidation or conjugation to form a glucosyl ester. The major oxidative pathway is through hydroxylation of ABA on C-8′ by members of the cytochrome P450 monooxygenase CYP707A family, followed by spontaneous isomerization to phaseic acid (PA), which is then converted to dihydrophaseic acid (DPA; Seo and Marion-Poll, 2019).
Figure 1.
The ABA biosynthesis pathway from zeaxanthin to xanthoxin. Synthesis of trans-violaxanthin (t-viola) is catalyzed by zeaxanthin epoxidase (ZEP). The formation of cis-isomers of c-viola and c-neo is thought to require two enzymes, NSY to produce trans-neoxanthin (t-neo) and an isomerase for trans-to-cis conversion of both neoxanthin and violaxanthin. Cleavage of cis-xanthophylls is catalyzed by a family of NCEDs and produces xanthoxin, the 15-carbon precursor of ABA.
Most of the genes involved in the ABA biosynthesis and catabolism pathways have been identified. Nevertheless, the enzymatic reactions involved in the production of cis-isomers of violaxanthin and neoxanthin remain poorly understood (Fig. 1). Zeaxanthin is produced from β-carotene as a trans-isomer and is converted by ZEP to t-viola via the intermediate antheraxanthin. t-viola is then converted to t-neo, and two genes in this pathway have been identified, namely, ABA-DEFICIENT4 (ABA4) and NEOXANTHIN-DEFICIENT1 (NXD1), whose loss of function prevents neoxanthin synthesis but whose exact function has not been established yet (North et al., 2007; Neuman et al., 2014). In wild-type plants, cis-isomers of violaxanthin and neoxanthin are produced from t-viola and t-neo, respectively, by an unknown cis-isomerase. c-neo is more abundant than c-viola and contributes to photosystem protection (Dall’Osto et al., 2007). The presence of a 9-cis double bond has been reported as necessary for precursor cleavage by recombinant NCED, but it remains uncertain whether both cis-isomers are used for ABA biosynthesis (Schwartz et al., 1997, 2003). The Arabidopsis (Arabidopsis thaliana) aba4 mutant was isolated in a screen for germination resistance to paclobutrazol, which is an inhibitor of GA synthesis (North et al., 2007). The absence of both t- and c-neo in this mutant was associated with a substantial reduction in ABA levels in both seeds and water-stressed rosettes, suggesting that ABA was likely produced from c-viola, but at a limiting rate. In accordance, ABA-related phenotypes, plant stature, stress-induced water loss, and seed dormancy were less affected in aba4 than in the ZEP defective mutant aba1. The other neoxanthin-deficient mutant nxd1 was identified in tomato (Solanum lycopersicum) based on its paler yellow flower color (Neuman et al., 2014). Despite nxd1 mutation completely preventing neoxanthin synthesis, nxd1 mutants exhibited no alterations in ABA level. Furthermore, tomato nxd1 plants showed a better tolerance to progressive water stress than did the wild type. In tomato nxd1, unlike Arabidopsis aba4, the formation of c-viola appeared to provide sufficient amounts of ABA precursors. North et al. (2007) could not establish ABA4 enzymatic function, since no similarity to known proteins was found and no in vitro activity was detected for recombinant protein produced in Escherichia coli. Furthermore, attempts to synthesize neoxanthin through expression of recombinant NXD1 alone or together with ABA4 in an E. coli strain producing violaxanthin were also unsuccessful (Neuman et al., 2014). In contrast to ZEP and ABA4, the NXD1 protein has not been found in chloroplast proteomes, and accordingly, the NXD1 sequence does not contain a predicted transit peptide; this led Neuman et al. (2014) to postulate that NXD1 might be involved in an auxiliary process required for neoxanthin synthesis.
To investigate further the function of ABA4 and NXD1 in the biosynthesis of ABA in Arabidopsis, we compared the phenotypes of single and double mutants and analyzed the effect of ABA4 overexpression on ABA and carotenoid accumulation in wild-type and mutant plants. Our study provides evidence that ABA4 not only functions in t-neo synthesis but is also involved in ABA production from t-viola via the alternative c-viola pathway.
RESULTS
Arabidopsis NXD1 Loss of Function Increases ABA Levels and Water Stress Tolerance
In Arabidopsis, At1g28100 has been identified as the single putative ortholog of tomato NXD1. In accordance, a transfer DNA (T-DNA) insertion mutant called Atnxd1 was shown to lack neoxanthin isomers, similar to the tomato knockout mutant nxd1-2 (Neuman et al., 2014). However, as mentioned above, ABA levels were not affected in tomato nxd1. This observation was unexpected since, in an earlier study, we reported that all three Arabidopsis aba4 mutant alleles in both Columbia (Col-0; aba4-3) and Wassilewskija (Ws; aba4-1 and aba4-2) accessions, which also lack neoxanthin isomers, had ABA-deficient phenotypes (North et al., 2007). Since only leaf carotenoid composition has been assessed in Arabidopsis nxd1 (Neuman et al., 2014), we decided to perform a detailed comparative analysis of this mutant with aba4-3, both of which are in the Col-0 background. In nxd1, renamed here nxd1-1, the T-DNA insertion is located 475 nucleotides upstream of the start codon of the NXD1 gene, in an intron that belongs to the 5′ UTR region (Supplemental Fig. S1). To assess nxd1 phenotypes, we isolated additional alleles using a CRISPR-Cas9 strategy. The allele nxd1-2 carries a 13-nucleotide deletion in exon 4, which is expected to result in the production of a truncated 89-amino acid protein that would contain seven amino acid changes in the C-terminal sequence before the generated stop codon. The allele nxd1-3 carries a single nucleotide deletion in exon 1 and a single nucleotide insertion in exon 4, and the predicted protein of 29 amino acids would contain 12 amino acid changes in the C-terminal region before the generated stop codon. The allele nxd1-4 carries a single nucleotide insertion in exon 1, and the predicted protein of 46 amino acids would contain 31 amino acid changes in the C-terminal region before the generated stop codon (Supplemental Fig. S1).
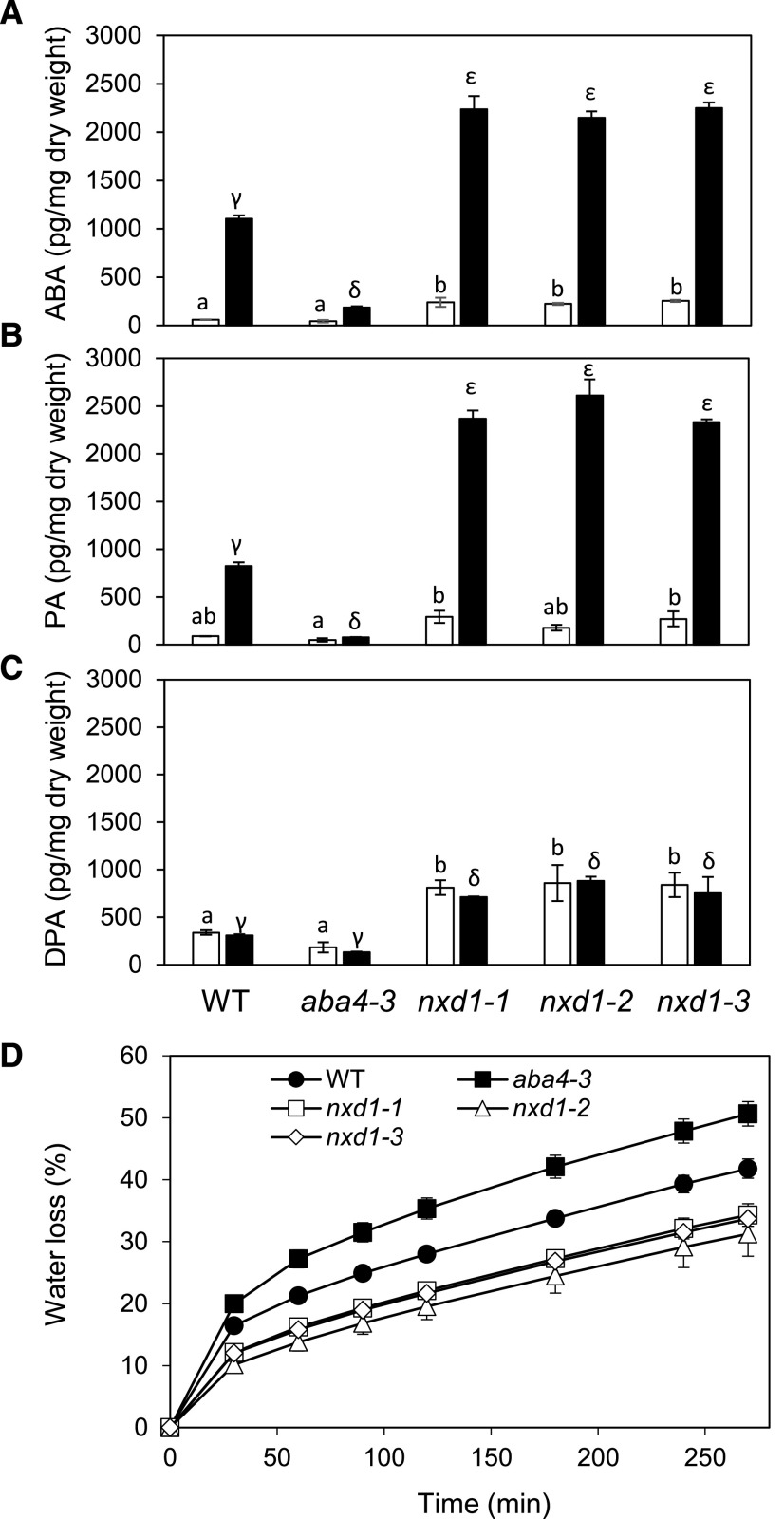
As previously described for Arabidopsis nxd1-1 (Neuman et al., 2014), neoxanthin isomers t-neo and c-neo were absent in nxd1-2, nxd1-3, and nxd1-4 whereas t-viola and c-viola levels were higher than in the wild type (Supplemental Table S1). The mutant response to rapid dehydration was analyzed by measurement of ABA and catabolite levels as well as water loss from detached rosettes of three mutant alleles, nxd1-1, nxd1-2, and nxd1-3. ABA levels in turgid rosettes were about 4-fold higher than in the wild type and 6-fold higher than in aba4-3 (Fig. 2A). After detachment and water loss to 15% of the initial fresh weight, ABA levels in aba4-3 rosettes were strongly reduced compared to wild-type levels, confirming ABA deficiency in the mutant (Fig. 2A; North et al., 2007). In contrast, ABA content in desiccated rosettes of all three nxd1 alleles was 2-fold higher than in the wild type and about 12-fold higher than in aba4-3. In all genotypes, the content of major ABA catabolites corresponded well with that of ABA, with PA being more abundant than the downstream inactive catabolite DPA under rapid dehydration conditions (Fig. 2, B and C). Taken together, these data suggest that in contrast to aba4-3, c-viola can be efficiently used as an ABA precursor in water-stressed Arabidopsis nxd1 mutants. Furthermore, increased ABA accumulation in nxd1 did not result from decreased ABA catabolism through the CYP707A pathway. Finally, in good agreement with the inverse effects of their mutation on ABA levels, water loss of detached rosettes was increased in aba4-3 and reduced in nxd1 alleles (Fig. 2D).
Figure 2.
Effect of dehydration on accumulation of ABA metabolites and water loss of detached rosettes of the nxd1-1, nxd1-2, and nxd1-3 mutants as compared to the wild type (WT) and aba4-3. A to C, ABA (A), phaseic acid (PA; B) and dihydrophaseic acid (DPA; C) levels were measured in detached rosettes prior to desiccation (white bars) and after 15% water loss (black bars). D, Water loss of detached rosettes as a percentage of initial fresh weight. Means of three (A–C) or four (D) biological replicates are shown as the mean ± se. Lowercase Latin and Greek letters, for control and desiccation treatments, respectively, indicate statistically significant differences as determined by one-way ANOVA followed Tukey’s HSD mean-separation test (P < 0.05).
nxd1 Dry Seeds Exhibit High ABA Levels and Deep Dormancy
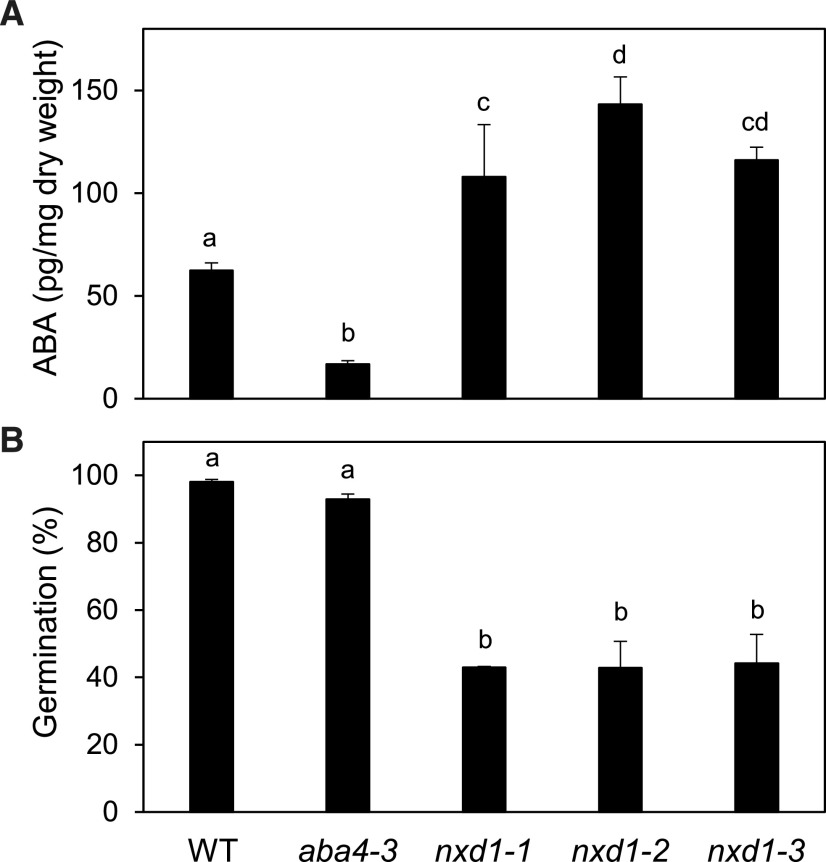
In aba4 alleles, lack of neoxanthin isomers has been shown to result in reduced seed dormancy and lower ABA levels in dry seeds (North et al., 2007). As in vegetative tissues, the opposite phenotypes were observed in nxd1 seeds compared to aba4-3 (Fig. 3). In contrast to the strong reduction observed in aba4-3 seeds, ABA levels in nxd1 mature dry seeds were increased by up to 2-fold compared to wild-type levels (Fig. 3A). Dormancy is progressively released upon dry storage of freshly harvested seeds (Graeber et al., 2012). Dormancy depth was assessed in nxd1 mutant seeds compared to aba4-3 by measuring the maximal percentage of germination of the different genotypes after 2 weeks of dry storage (Fig. 3B). In accordance with their higher ABA content, germination percentages for nxd1 seeds were lower than in the wild type and aba4-3, indicating that nxd1 seeds are more dormant. To determine whether NXD1 loss of function affected other germination characteristics, we assessed the sensitivity of seeds to germination inhibitors after stratification treatment using paclobutrazol, which reduces GA biosynthesis, ABA, and NaCl (Supplemental Fig. S2). As previously described (North et al., 2007), aba4-3 seeds were more resistant to paclobutrazol inhibition of germination (Supplemental Fig. S2A). In contrast, nxd1-1 seeds were more sensitive than wild-type seeds, in good agreement with their increased ABA content. Thus, the two mutations had opposing effects on germination, likely due to an altered ABA/GA balance. More surprisingly, the nxd1-1 mutation also affected ABA sensitivity (Supplemental Fig. S2B), suggesting that nxd1 phenotypes may result from a combination of increased ABA synthesis and sensitivity. Finally, a mild salt-hypersensitive response was also observed (Supplemental Fig. S2C).
Figure 3.
ABA content in and germination of nxd1-1, nxd1-2, and nxd1-3 seeds, as compared to wild-type (WT) and aba4-3 seeds. A and B, ABA content was measured in freshly harvested mature seeds (A), and germination (as a percentage of the total number of seeds) was scored 7 d after imbibition of seeds that had been stored for 2 weeks (B). The means ± se of three biological replicates are shown. Lowercase letters indicate statistically significant differences as determined by one-way ANOVA followed by Tukey’s HSD mean-separation test (P < 0.05).
NXD1 Is Not Localized in Plastids
Carotenoid biosynthesis takes place in plastids, and a number of enzymes have been reported to be associated with chloroplast envelope membranes (Gloaguen et al., 2017). In accordance with its function in neoxanthin synthesis, ABA4 contains a predicted transit peptide and has been exclusively found in the envelope fraction. Moreover, it is likely an integral protein in the plastid envelope, since the protein sequence is predicted to contain four transmembrane domains (North et al., 2007). In contrast to ABA4, the NXD1 sequence does not contain a predicted transit peptide and the protein has not been detected in plastid proteomes. Therefore, NXD1 has been postulated to be involved in an auxiliary process required for neoxanthin synthase (NSY) activity (Neuman et al., 2014). To determine more precisely the localization of the NXD1 protein, Arabidopsis wild-type and nxd1 mutant plants were transformed with p35S:NXD1-GFP and p35S:GFP-NXD1 constructs. Neoxanthin accumulation was restored in complemented mutants, confirming that both N- and C-terminal fusions to NXD1 were active. However, the GFP signal detected by confocal microscopy in these transgenic plants was very low. Therefore, both constructs, together with p35S:NXD1-mCherry and p35S:mCherry-NXD1, were transiently expressed in Nicotiana benthamiana leaves. NXD1-GFP and NXD1-mCherry protein fusions colocalized in the same subcellular compartments, suggesting that both reflected similar NXD1 protein targeting (Fig. 4, A and B; Supplemental Fig. S3). Both C- and N-terminal NXD1 fusions had a diffuse fluorescent pattern typical of cytosolic accumulation, but only N-terminal fusions were also detected in small organelles. Neither GFP nor mCherry signals colocalized with the chloroplast autofluorescence signal (Fig. 4). Subcellular compartment markers with GFP or YFP tags were used in coexpression experiments to further investigate the localization of the mCherry-NXD1 fusion. Colocalization was found with markers for the cytosolic Ran-binding protein (RANBP1A; Fig. 4E) and the peroxisomal enzymes citrate synthase and hydroxypyruvate reductase1 (Fig. 4, C and D). These two enzymes each contain one of the two different peroxisome-targeting signals, which are located either near the amino terminus or at the carboxy terminus, respectively (Mano et al., 2002). No colocalization was found with chloroplast (CD3-997), mitochondria (CD3-990), Golgi (memb11), or endoplasmic reticulum (PAS1) markers (Supplemental Fig. S4; Supplemental Table S2).
Figure 4.

Subcellular colocalization of NXD1 protein fusions with peroxisome and cytosol markers in N. benthamiana leaf epidermal cells. A and B, Colocalization of NXD1-mCherry and NXD1-GFP (A) and mCherry-NXD1 and GFP-NXD1 (B). C and D, Colocalization of mCherry-NXD1 and GFP protein fusions containing the targeting peptides of peroxisomal citrate synthase (CS-GFP; C) or peroxisomal hydroxypyruvate reductase1 (GFP-HPR1; D). E, Colocalization of mCherry-NXD1 and the GFP protein fusion RANBP1A-GFP containing the cytosolic Ran-binding protein. GFP signals are shown in green, mCherry in magenta, and chlorophyll autofluorescence in cyan. Scale bars = 10 µm.
These data strongly suggested that NXD1 has a cytosolic and/or peroxisomal localization, and they confirmed the absence of targeting to plastids. They also implied that ABA4 and NXD1 proteins are not located in the same subcellular compartment and therefore are not expected to interact in an enzyme complex. While the convergent evidence indicated that NXD1 was not likely to be part of the plastidial, possibly multimeric, NSY enzyme, ABA4 still remained a potential candidate and its function was investigated further.
More t-neo and Less t-viola Are Accumulated in p35S:ABA4 Transgenic Roots
Previous attempts to detect recombinant protein activity have been unsuccessful (North et al., 2007; Neuman et al., 2014), probably due to the absence of an unknown component whose production requires NXD1. Therefore, for a better understanding of ABA4 function in carotenoid interconversion, we decided to perform a more detailed analysis of transgenic plants carrying the ABA4 gene under the control of the 35S promoter. We previously showed that ABA4 overexpression in leaves of Agrobacterium tumefaciens-transformed wild-type (Col-0) plants resulted in limited modification of carotenoid composition, presumably due to the tight regulation of the accumulation of these photosystem protective components (North et al., 2007). Nevertheless, up to 2-fold increases in t-neo and c-viola contents were detected, in good accordance with the putative ABA4 function in the conversion of t-viola to t-neo; however, t-viola levels were similar to wild-type levels. Here, we analyzed the carotenoid contents of the nongreen tissues of transgenic plants, which had been previously obtained by transformation of Col-0 and Ws wild types (North et al., 2007). Progeny from four independent transgenic lines in each accession were grown hydroponically for one month, and the carotenoid composition of their roots was compared to that of their respective wild types. Roots of all eight lines exhibited up to a 2-fold increase in t-neo content compared to the wild type, with corresponding reductions in t-viola content (Fig. 5). Content of c-neo was also increased, although to a smaller extent. Very low levels of c-viola were detected and no increase was observed in transgenic roots, suggesting a possible preferential production of t-neo at the expense of c-viola.
Figure 5.
Carotenoid content in roots of transgenic plants carrying a p35S:ABA4 construct, compared to wild-type and aba4 roots. Four transformants in Arabidopsis Col-0 (A) and Ws (B) backgrounds were grown hydroponically together with controls. Results for three biological replicates are shown as the mean ± sd. Different lowercase letters for a given carotenoid indicate statistically significant differences as determined by one-way ANOVA followed by Tukey’s HSD mean-separation test (P < 0.05).
The inverse relationship between increased levels of t-neo and decreased t-viola levels in roots of ABA4 transgenics strongly corroborated that the ABA4 protein plays a key role in the conversion of t-viola to t-neo, possibly acting as a component of the NSY enzyme.
Dehydration Tolerance and ABA Levels Are Increased in Transgenic Plants
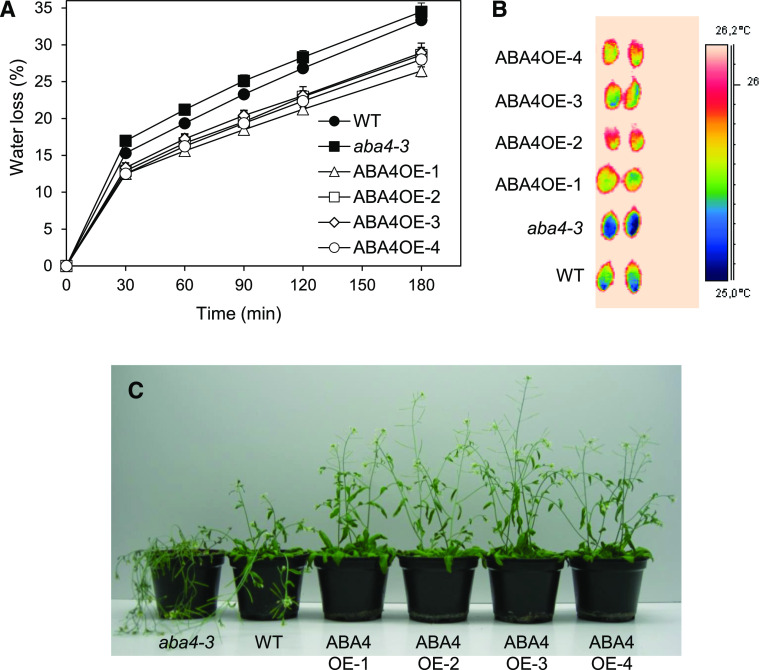
To investigate the impact of alterations in xanthophyll content, the phenotypes of all four ABA4 transgenic lines in the Col-0 background were assessed under different types of dehydration treatment. Water loss of detached rosettes was reduced compared to that of wild-type rosettes (Fig. 6A). Water loss can also be assessed by thermal imaging because of the cooling effect of transpiration on leaf temperature. When measured a few minutes after detachment, leaf temperature in aba4-3 was lower than in the wild type (Fig. 6B). In contrast, leaves of all four Col-0 transgenic lines exhibited an increased temperature compared to wild-type leaves, confirming their decreased transpiration rate. Tolerance of long-term water deficit was also investigated by withholding water from flowering plants for 14 d, and transgenic lines were less affected than mutant and wild-type plants (Fig. 6C). These results indicated that ABA4 constitutive expression in transgenic plants increased tolerance to both rapid and progressive water deficit, even when relatively modest alterations in carotenoid composition were observed, as in ABA4OE-3 and ABA4OE-4.
Figure 6.
Water-stress tolerance of transgenic plants carrying a p35S:ABA4 construct, as compared to wild-type (WT; Col-0) and aba4-3 plants. A, Water loss of detached rosettes expressed as the percentage of initial fresh weight. Results for four biological replicates are shown as the mean ± se. B, Temperature of detached leaves visualized by thermal imaging a few minutes after detachment, with the corresponding false color temperature scale. C, Tolerance to water deficit. Three plants were grown in each pot and photographed 14 d after withholding water.
ABA levels were also measured in detached rosettes and seeds of the two Col-0 transgenic lines that exhibited the highest t-neo accumulation. In accordance with the increased precursor abundance, the ABA content of detached rosettes after 15% water loss was higher in ABAOE-1 and ABAOE-2 compared to the wild type (Fig. 7A). A similar observation was made in dry seeds, where ABA content was increased in both transgenic lines compared to the wild type (Fig. 7B). The increase in ABA abundance in transgenic plants relative to wild-type plants was higher in seeds (47% to 63%) than in rosette leaves (27% to 28%). Altogether these results provided convergent evidence for the contribution of ABA4 to the modulation of ABA levels in seeds and water-stressed plants.
Figure 7.
ABA content in detached rosettes and dry seeds of transgenic wild-type plants carrying a p35S:ABA4 construct, as compared to wild-type (WT) and aba4-3 plants. A, Detached rosettes prior to dehydration (white bars) and after 15% water loss (black bars). Lowercase Latin and Greek letters were used for control and desiccation treatments, respectively. B, Dry seeds. The mean ± se of three biological replicates is shown. Different lowercase letters indicate statistically significant differences as determined by one-way ANOVA followed by Tukey’s HSD mean-separation test (P < 0.05).
Constitutive Expression of ABA4 in nxd1 Mutants Promotes c-viola and ABA Accumulation
The phenotypes of aba4 mutants and ABA4OE transgenic plants in the wild-type background provided strong evidence for a role of ABA4 in neoxanthin synthesis and also showed that ABA production occurs preferentially via c-neo rather than c-viola. However, c-viola has been shown in tomato nxd1 (Neuman et al., 2014), and confirmed here in Arabidopsis mutants, to be a potent ABA precursor.
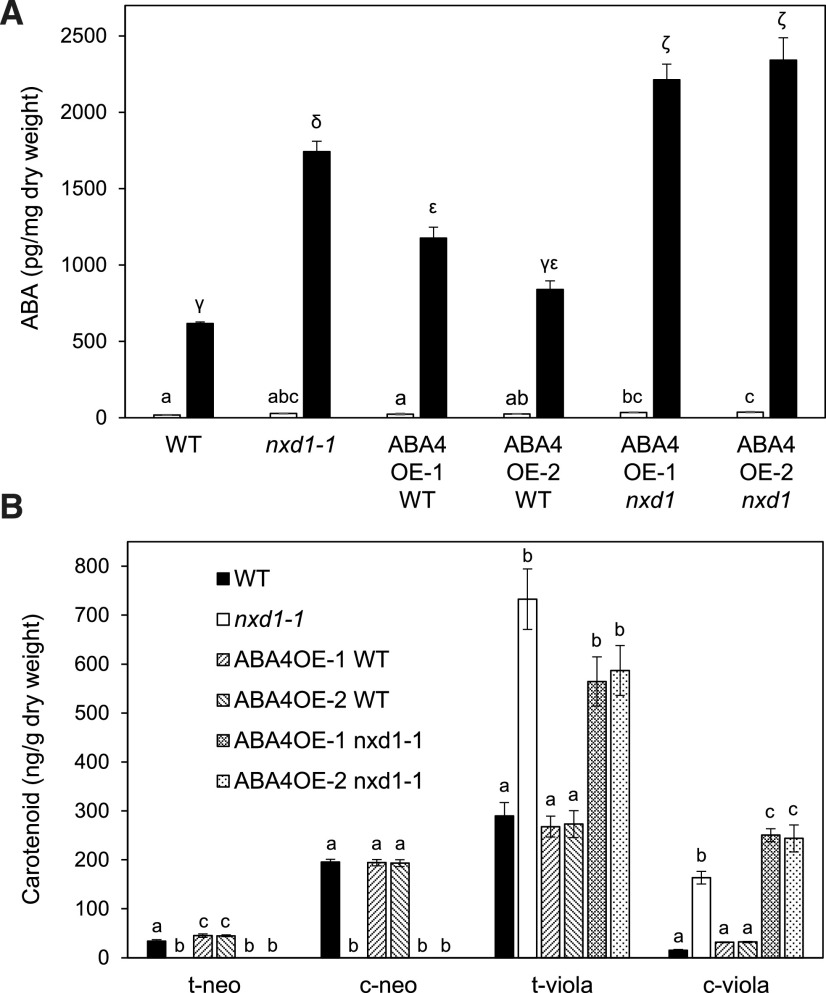
To explore further ABA4 function in the xanthophyll biosynthesis pathway, the ABA4OE-1 and ABA4OE-2 transgenic lines were crossed to the nxd1-1 mutant, and homozygous nxd1-1 F2 progeny containing the p35S:ABA4 construct were selected. The ABA content of detached rosettes after 15% water loss was higher in ABAOE-1 and ABAOE-2 in the nxd1-1 background, compared to the nxd1-1 untransformed control, suggesting that ABA4 could increase ABA production through the alternative c-viola pathway (Figs. 1 and 8A). Furthermore, analysis of leaf carotenoid composition in both nxd1-1 transgenic lines showed an increase in c-viola content, associated with a decrease in t-viola, compared to nxd1-1 (Fig. 8B). Thus, in the absence of NXD1, and with the prevention of neoxanthin isomer synthesis, an additional function of ABA4 was revealed in c-viola synthesis. This also implied that the aba4 mutant defect blocks ABA biosynthesis from both c-neo and c-viola routes, thus explaining its ABA-deficient phenotypes, in contrast to nxd1, which is still able to produce ABA from c-viola.
Figure 8.
ABA and carotenoid content in transgenic lines carrying a p35S:ABA4 construct in the wild-type (WT) and nxd1-1 mutant background. A, ABA levels in detached rosettes prior to dehydration (white bars) and after 15% water loss (black bars). The mean ± se of three biological replicates is shown. Lowercase Latin and Greek letters were used for control and desiccation treatments, respectively. B, Carotenoid content in cauline leaves. The mean ± se of five biological replicates is shown. Lowercase letters indicate statistically significant differences as determined by one-way ANOVA followed by Tukey’s HSD mean-separation test (P < 0.05). Different letters indicate significant differences for a given carotenoid.
Loss of Function of ABA4 Reduces c-viola and ABA Accumulation in the nxd1 Background
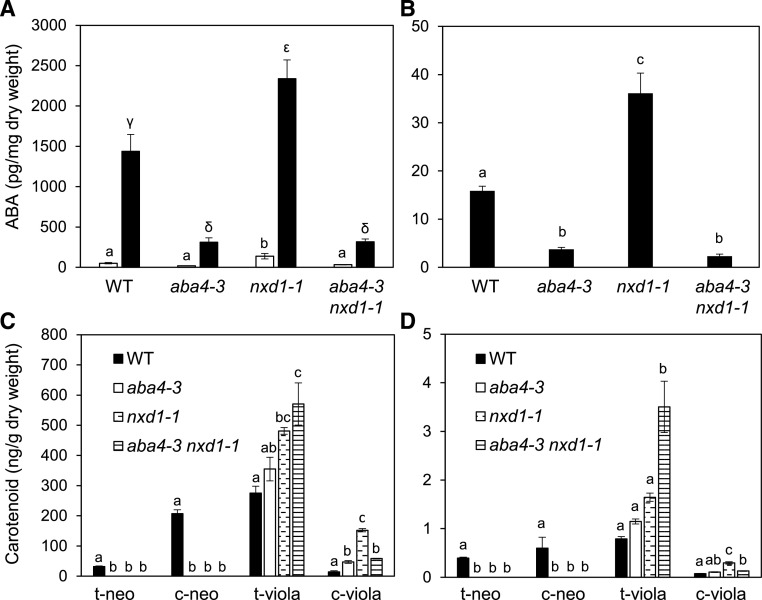
To further determine the respective functions of ABA4 and NXD1, we analyzed the levels of ABA in the aba4-3 nxd1-1 double mutant compared to each single mutant. In detached rosettes, ABA levels in aba4-3 nxd1-1 were similar to those in aba4-3 when measured either before or after water deficit (Fig. 9A). In dry seeds also, both aba4-3 and aba4-3 nxd1-1 exhibited similarly reduced ABA levels (Fig. 9B). Thus, as compared to nxd1-1, ABA levels were strongly decreased in the double mutant seeds and desiccated rosettes.
Figure 9.
ABA and carotenoid contents in the aba4-3 nxd1-1 double mutant compared to the wild type (WT), aba4-3, and nxd1-1. A, ABA levels in detached rosettes prior to desiccation (white bars) and after 15% water loss (black bars). Lowercase Latin and Greek letters were used for control and desiccation treatments, respectively. B, ABA levels in dry seeds. C and D, Carotenoid content in cauline leaves (C) and dry seeds (D). The mean ± se of three biological replicates is shown. Different lowercase letters indicate statistically significant differences for a given carotenoid, as determined by one-way ANOVA, followed by Tukey’s HSD mean-separation test (P < 0.05).
We then determined whether the combination of aba4-3 and nxd1-1 mutations affected carotenoid composition (Fig. 9, C and D). Similar c-viola levels were observed in aba4-3 nxd1-1 and aba4-3 in both leaves and seeds, indicating that ABA4 loss of function in the nxd1-1 background reduced c-viola production relative to that of t-viola. Moreover, in both seeds and leaves, t-viola levels in the double mutant were higher than those in nxd1-1.
The concomitant decrease of ABA and c-viola levels by introducing the aba4-3 mutation in the nxd1 background indicated that loss of function of ABA4 likely reduces ABA synthesis by limiting c-viola accumulation. Taken together, results obtained from the analysis of mutant and ABA4OE transgenic lines lead to convergent conclusions regarding ABA4 involvement in the c-viola alternative pathway for ABA production.
DISCUSSION
In this study, we aimed to gain a better understanding of the function of two genes, ABA4 and NXD1, that have been implicated in neoxanthin synthesis but were identified and characterized in Arabidopsis and tomato, respectively. In contrast to ABA4 in Arabidopsis, NXD1 loss of function in tomato was reported to have no detectable effect on ABA accumulation. Here, a more detailed analysis has been performed in Arabidopsis using single and double mutants, ABA4 overexpression, and NXD1 protein localization. Taken together, these data provide insights into the function of ABA4 in carotenoid and ABA synthesis and its possible interaction with NXD1.
NXD1 Subcellular Localization Makes Unlikely a Direct Interaction with ABA4 in Plastids
As pointed out by Neuman et al. (2014), the NXD1 protein sequence does not contain a predicted transit peptide for targeting to plastids and is not found in plastid proteomes. In contrast, Arabidopsis ABA4 has been reported to contain a transit peptide (North et al., 2007) and be located in the inner envelope membrane of plastids, in which zeaxanthin epoxidase and other enzymes upstream of the carotenoid pathway were also detected (Bouchnak et al., 2019). In accordance, protein fusion of the rice (Oryza sativa) ABA4 homolog MAO HUZI4 with GFP has been shown to colocalize with plastid chlorophyll fluorescence in transient expression assays (Ma et al., 2014). To obtain further evidence that NXD1 is not located in plastids and determine its subcellular localization, we performed here transient expression assays in N. benthamiana using NXD1 C- or N-terminal fusion with GFP or mCherry. In accordance with proteome data, no fluorescence overlap of NXD1 fusion proteins was detected with either plastid marker or chlorophyll autofluorescence. Moreover, for all four NXD1 fusion proteins, fluorescence was detected in the cytosol, in agreement with its predicted location in SUBA4, the subcellular localization database for Arabidopsis proteins (Hooper et al., 2017; http://suba.live). However, when GFP and mCherry were fused at the N-terminal end of NXD1, an intense fluorescence signal was also detected in small organelles and was shown to colocalize with peroxisome markers.
Plant peroxisomes house a number of metabolic pathways, such as fatty acid degradation, the glyoxylate cycle, photorespiration, and detoxification of reactive oxygen species. They notably contribute to the synthesis of phytohormones including jasmonic acid, auxin, and salicylic acid (Kao et al., 2018). Chloroplasts and peroxisomes are physically tethered through the formation of peroxules by peroxisome extension (Oikawa et al., 2015). Therefore, it could be hypothesized that the production of cis-neo in plastids, which contributes to photosystem protection, might be promoted by the provision of a metabolite whose production would require NXD1 in peroxisomes.
Most peroxisomal matrix proteins contain a peroxisome targeting signal, either a C-terminal tripeptide or a nonapeptide near the N terminus (Pan et al., 2020). Despite the fact that mCherry-NXD1 fusions are targeted to peroxisomes, NXD1 does not contain the SKL tripeptide most frequently found in peroxisomal proteins (Wang et al., 2017). The existence of a noncanonical targeting peptide remains possible, but NXD1 is not listed in peroxisome proteomes (http://suba.live; http://ppdb.tc.cornell.edu).
Both C- and N-terminal NXD1 protein fusions were shown to complement neoxanthin accumulation in nxd1 mutants, and they share a common localization in the cytosol. Thus, despite both mCherry-NXD1 and GFP-NXD1 signals overlapping with those of peroxisome markers, it seems probable that NXD1 targeting to peroxisomes may be artifactual. Current evidence therefore suggests that NXD1 is a cytosolic protein that cannot directly interact with ABA4, which is located in the inner plastid envelope. However, localization of NXD1 in the cytosol does not give any clues to its function in neoxanthin synthesis.
ABA4 Has a Dual Function in c-neoxanthin and c-violaxanthin Synthesis
In contrast to NXD1, ABA4 is likely to have a direct function in neoxanthin synthesis. First, it is located in the same subcompartment as upstream carotenoid enzymes, which might favor carotenoid metabolic channeling by putative membrane-associated multienzyme complexes, as suggested by Cunningham and Gantt (1998) and supported by recent evidence reviewed by Wurtzel (2019). Second, its constitutive expression in Arabidopsis wild-type roots has been clearly shown here to concomitantly decrease t-viola and increase t-neo accumulation, in accordance with previous observations of transgenic leaves by North et al. (2007). After introduction of the p35S:ABA4 construct into nxd1, a similar decrease in t-viola was observed in transgenic mutant leaves; moreover, in the absence of t-neo synthesis, the decrease in t-viola was concomitant with an increase in c-viola, clearly indicating a positive effect of ABA4 on violaxanthin isomerization. We did not detect here higher c-viola levels in roots of wild-type transformants, in contrast to leaf levels (North et al., 2007). Since c-neo levels also varied between roots and leaves, this may suggest a possible tissue specificity of carotenoid pathway regulation. Alternatively, it may simply be due to the very low levels of c-viola in roots. The hypothesis of a direct role of ABA4 in c-viola synthesis is further supported by the observed decrease in c-viola accumulation, and a contrasting t-viola increase, in the aba4 nxd1 double mutant compared to nxd1. Differences in t-viola accumulation in the double mutant compared to single mutants suggest a possible additivity of the two mutations, which remains to be explained. Taken together, these data strongly indicate that in addition to a function in neoxanthin synthesis, ABA4 has violaxanthin trans-to-cis isomerase activity, or contributes to such activity.
A number of carotenoid isomerases have been identified. The 15-cis-ζ-carotene isomerase (ZISO) catalyzes the cis-to-trans conversion of the 9,15,9′-tri-cis-ζ-carotene into the 9,9′-di-cis-ζ-carotene, whereas carotenoid isomerase (CRTISO) transforms 7,9,7′,9′-tetra-cis-lycopene (prolycopene) into all-trans-lycopene (Park et al., 2002; Li et al., 2007; Beltrán et al., 2015). The β-carotene isomerase DWARF27 (D27) is a trans-to-cis isomerase that converts all-trans-β-carotene into 9-cis-β-carotene, the precursor of strigolactones (Lin et al., 2009; Alder et al., 2012). CRTISO and D27 proteins were described as being associated with inner envelope membranes, and ZISO as an integral membrane protein (Shumskaya and Wurtzel, 2013; Bouchnak et al., 2019). The presence of transmembrane domains is a common structural characteristic of ABA4 and ZISO, suggesting that the putative integration of ABA4 into the plastid inner envelope would be compatible with isomerase activity. However, recombinant ABA4 activity in the conversion of t-viola to c-viola has yet to be demonstrated (North et al., 2007; Neuman et al., 2014). Nevertheless, an unknown cofactor may be missing, as observed for ZISO, which requires a redox-regulated heme cofactor for its activity (Beltrán et al., 2015). However, if ABA4 was indeed a violaxanthin isomerase that needed an unknown cofactor, the production of this cofactor by NXD1 would not be necessary for the isomerization of t-viola to c-viola, since high amounts of c-viola are accumulated in nxd1 mutants.
Both ZISO and CRTISO isomerization activity can be compensated in green tissues by photoisomerization (Park et al., 2002; Beltrán et al., 2015). The existence of such spontaneous isomerization would explain the presence of c-viola in aba4 mutants, which would be sufficient to produce ABA in normal conditions but not in water-stress conditions. Furthermore, other cis-trans isomerases, such as D27 or D27-like proteins, may be able to act with lower affinity on alternative substrates (i.e. t-viola when accumulated in high amounts in the aba4 background) and reduce phenotype severity. Such a compensation mechanism has been described for the Arabidopsis mutant Arabidopsis aldehyde oxidase3 (aao3), in which related aldehyde oxidases would be able to act on abscisic aldehyde in the absence of AAO3 (Seo et al., 2004). In accordance with this hypothesis, d27 mutants are affected in ABA biosynthesis, with lower ABA levels detected in both rice and Arabidopsis mutants (Bruno and Al-Babili, 2016; Abuauf et al., 2018). However, although purified D27 protein exhibits 9-cis isomerase activity on β-carotene, neither rice nor Arabidopsis D27 recombinant proteins acted on t-viola.
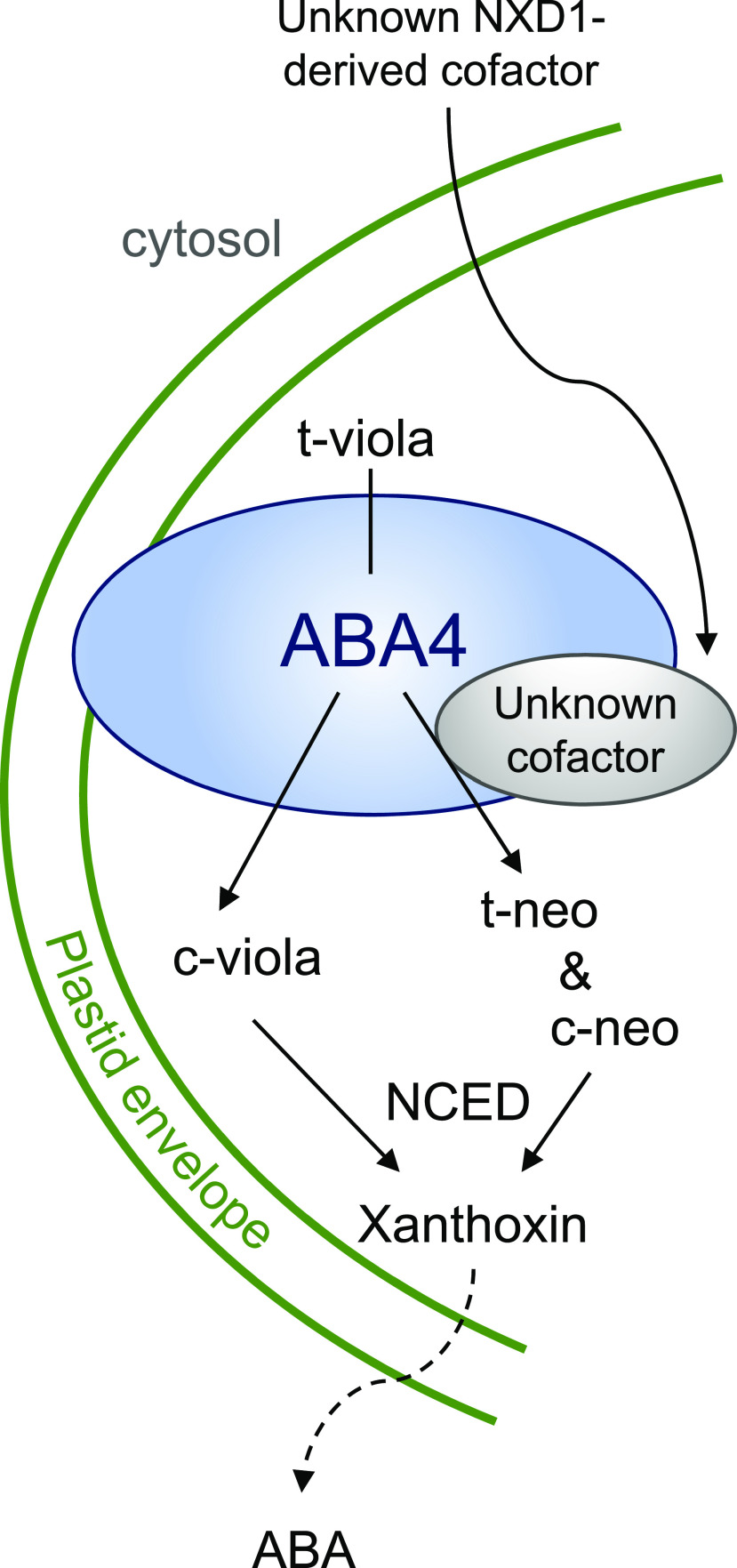
Our results provide convergent evidence that ABA4 is essential for the synthesis of both t-neo/c-neo and c-viola from t-viola and thus would be involved in different types of catalytic activity. Since the aba4 mutation is epistatic to nxd1 for both c-viola and ABA production, ABA4 may act upstream of NXD1, with the latter being required only for conversion of t-viola to t-neo. We propose a simple explanatory model (Fig. 10). After binding to ABA4, t-viola could be converted to t-neo and then c-neo if the NXD1-derived factor is present, or to c-viola independent of the presence of this unknown factor. If ABA4 is involved in trans-to-cis isomerization of t-viola, it can be supposed to have the same function in t-neo isomerization. Nevertheless, in the absence of biochemical evidence, this model remains hypothetical, and ABA4 may contribute to NSY activity in a bigger complex, as observed for carotenoid desaturase enzymes, which require several cofactors to be active (Ruiz-Sola and Rodríguez-Concepción, 2012). No protein interactions with ABA4 have been reported in the Bio-Analytic Resource for Plant Biology Web site (http://bar.utoronto.ca). Thus, in future studies it will be important to determine which proteins or cofactors interact with ABA4.
Figure 10.
Hypothetical model for the synthesis of c-viola, t-neo, and c-neo from t-viola. The trans-to-cis isomerization of t-viola would be catalyzed by ABA4, giving rise to either c-viola, t-neo and c-neo if the unknown cofactor whose synthesis requires NXD1 is present, or only c-viola if this cofactor is absent.
ABA4 and NXD1 Contribute to Control of ABA Accumulation
Our previous study on the Arabidopsis aba4 mutant (North et al., 2007) led to the conclusion that ABA was produced from c-neo in wild-type plants upon water stress, because ABA deficiency and reduced tolerance to water deficit in this mutant were associated with the lack of c-neo, whereas c-viola was still detected. This assumption was in good accordance with previous studies. Indeed, c-viola and c-neo had both been shown to be cleaved in vitro by NCED to produce xanthoxin, but the higher abundance of c-neo (20- to 100-fold higher than c-viola or ABA) suggested that in vivo it could likely be the main ABA precursor (Parry and Horgan, 1991; Schwartz et al., 2003). Nevertheless, subsequent studies have reported that both the parasitic plant Cuscuta reflexa and the tomato mutant nxd1 were able to produce ABA, despite being neoxanthin deficient (Qin et al., 2008; Neuman et al., 2014). C. reflexa plants were shown to accumulate high amounts of c-viola, which replaced c-neo in light-harvesting complexes (Snyder et al., 2004). Furthermore, upon water stress, increased ABA levels were observed in parasitic stems, likely resulting from c-viola cleavage. In accordance with these findings, the inability of the aba4 mutant to produce ABA from c-viola was postulated to result from limited availability of c-viola due to its integration in photosystems (Qin et al., 2008).
As discussed above, aba4 mutation itself likely restricts c-viola availability for ABA biosynthesis, and possible photoisomerization of c-viola or compensatory activity of other isomerases would be insufficient to produce sufficient ABA amounts for water-stress tolerance. As previously shown in tomato and C. reflexa (Qin et al., 2008; Neuman et al., 2014), analysis of nxd1 mutants in Arabidopsis further proved that c-viola is an adequate ABA precursor. Interestingly, ABA levels in Arabidopsis nxd1 were higher than in the wild type, suggesting that c-viola might be more efficiently used as NCED substrate than c-neo. If this is the case, it can be further hypothesized that NXD1 has a regulatory role in channelling t-viola metabolism toward c-neo production, thus limiting c-viola availability for ABA synthesis. Moreover, a dual function for ABA4 in both c-viola and c-neo synthesis would negatively impact ABA synthesis, in agreement with the higher ABA levels accumulated when neoxanthin isomers are not produced in nxd1, suggesting increased availability of ABA4 for c-viola synthesis and further cleavage. In accordance, the constitutive expression of ABA4 in nxd1 was also shown to promote ABA accumulation.
The parallel variation in c-viola and ABA levels in Arabidopsis nxd1 is not sufficient to prove that wild-type plants preferentially use c-viola rather than c-neo as an in vivo precursor for ABA synthesis. In fact, in aba4 also, c-viola levels are reproducibly higher than in the wild type, although to a small extent compared to nxd1. This finding indicates, first, that a substantial amount of c-viola is still produced in the absence of ABA4, possibly resulting from the availability of larger t-viola pools for alternative isomerization. Second, the finding that ABA levels in aba4 seeds or well-watered plants are reduced suggests that the c-viola pool produced in the absence of ABA4 is less efficiently used for ABA biosynthesis than in the wild type or nxd1. If c-viola is a suitable substrate for NCED, further hypotheses can be envisaged, such as a role of ABA4 in c-viola channeling to NCED.
In an early review on ABA biosynthesis, Taylor et al. (2000) proposed several possible routes for the formation of xanthoxin from xanthophylls. One of these was described as follows: “all-trans-violaxanthin is simultaneously subjected to two alternative isomerizations, leading to the formation of 9-cis-violaxanthin and all-trans-neoxanthin. Only the latter would be further isomerized to form 9-cis-neoxanthin. Either of these two 9-cis-xanthophylls could be used indiscriminately as a substrate for oxidative cleavage” (p. 1564). In accordance with current knowledge and further evidence provided here, we can now postulate that ABA4 could be responsible for both of these alternative isomerizations without the need for any other isomerase. However, identification of the putative NXD1-derived factor, as well as possible additional partners (either proteins or cofactors), and the exact function of NXD1 will be necessary to provide evidence for in vitro isomerase activity and to validate this model pathway.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis (Arabidopsis thaliana) wild-type and mutant seeds were surface sterilized, sown in petri dishes containing Arabidopsis Gamborg B5 medium (Duchefa; http://www.duchefa.com) supplemented with 30 mm Suc, and stratified at 4°C in the dark for 3 d. Petri dishes were then placed for 4 d in a growth chamber (16-h photoperiod, 50 μmol m−2 s−1 light intensity, 18°C, and 60% relative humidity). Germinated seedlings were transferred to soil (Tref Substrates; http://www.trefgroup.com) and, unless otherwise stated, grown in a glasshouse with a minimum photoperiod of 13 h, assured by supplementary lighting. In each experiment, all genotypes were grown together.
As previously described (North et al., 2007; Neuman et al., 2014), the aba4-3 (SALK_137455) and nxd1-1 (SALK_020928) mutants in the Arabidopsis Col-0 background were obtained from the Salk database (Alonso et al., 2003; http://signal.salk.edu/cgi-bin/tdnaexpress) and aba4-1 in the Arabidopsis Ws background was selected by screening the Versailles T-DNA insertion lines for paclobutrazol resistance. Production of the stable transformants of wild-type plants from the Col-0 and Ws accessions was described in North et al. (2007). A genomic fragment from 9 bp before the ATG to 183 bp after the STOP codon that contains the ABA4 gene was cloned downstream of the 35S promoter and the construct was introduced by Agrobacterium tumefaciens transformation.
For hydroponics, seeds were sterilized and stratified in water at 4°C in the dark for 4 d. Seeds were then sown on agar-filled tubes placed in solution containing 3 mm KNO3, 1.5 mm Ca(NO3)2, 2 mm MgSO4, 2 mm KH2PO4, 1 mm K2SO4, 0.7 mm CaCl2, 10 mg L−1 Fe-EDTA, 10 μm MnSO4, 24 μm H3BO3, 3 μm ZnSO4, 0.9 μm CuSO4, and 0.04 μm (NH4)6Mo7O24. Plants were grown in short-day growth cabinets as described by Orsel et al. (2004). After 4 weeks, they were subjected to nitrogen starvation for 9 d in order to enhance root growth. Roots were harvested and frozen in liquid nitrogen.
Germination Experiments
For dormancy assays, freshly harvested seeds stored at room temperature for 2 weeks were sown in triplicate in petri dishes containing 0.5% (w/v) agarose and then placed in a growth chamber (continuous light, 25°C, and 70% relative humidity). After 2 weeks of dry seed storage at room temperature, dormancy was analyzed by germination scoring. Germination was scored 7 d after sowing based on radicle protrusion. For inhibitor resistance tests, surface-sterilized seeds were sown on 0.5% (w/v) agarose supplemented with paclobutrazol (Syngenta; http://www.syngenta-agro.fr), ABA (JunDa Pharm Chem Plant Co.; http://jundapharmchem.en.ecplaza.net/), or NaCl, stratified at 4°C for 3 d, then incubated for 4 d in the same conditions used for the dormancy assays. Seedlings were scored as resistant if they developed green cotyledons. All mother plants were grown together in a glasshouse, and three independent seed lots were harvested for each genotype; each seed lot was obtained by pooling seeds from three to four plants. Similar results were obtained from at least two independent cultures.
Dehydration Assays
Rapid dehydration assays were carried out using 3-week-old plants grown in the glasshouse. Four rosettes per genotype were cut from the root system and water loss was measured as previously described (North et al., 2007). Leaf thermal imaging was carried out on detached leaves of 5-week-old plants grown in the same conditions within a few minutes after cutting using a FLIR A320 infrared camera (FLIR Systems; http://www.flir.com) equipped with a 45° lens (Plessis et al., 2011).
Long-term dehydration assays were carried out in a growth chamber (21°C day and 17°C night temperatures, 150 µmol m−2 s−1 light intensity, 16-h photoperiod, and 65% relative humidity; Frey et al., 2012). All pots contained the same amount of compost, and each contained three 5-week-old plants, which were watered to saturation before draining the excess water. Watering was continued for six plants per genotype and stopped for six others, and plants were examined daily for wilting. These experiments were repeated at least twice using plants from independent cultures.
Determination of Carotenoid, ABA, and Catabolite Contents
Hormone analysis was performed on detached rosettes prior to desiccation and after 15% water loss, as well as on mature dry seeds. Samples were freeze-dried and ground before extraction in acetone:water:acetic acid (80:19:1 [v/v/v]) with addition of 1 ng d4-ABA, 2 ng d3-PA, 8 ng d3-DPA, 10 ng d5-ABA-glucosyl ester, and 5 ng d4-7′OH-ABA isotopically labeled standards (NRC Plant Biotechnology Institute). ABA and catabolites were analyzed as previously described (Chauffour et al., 2019) using liquid chromatography-tandem mass spectrometry (I-Class UPLC system coupled with Xevo TQ-S; Waters; http://www.waters.com). Carotenoid analysis was performed on cauline leaves, whose carotenoid composition is similar to that of rosette leaves, roots, and mature dry seeds. Samples were ground and subsequently extracted with acetone containing 0.01% (v/v) ammoniac, and carotenoid content was measured using LC-UV DAD, as previously described (North et al., 2005). Three or more biological replicates were analyzed for each genotype, and experiments were repeated at least twice using samples from independent plant cultures. Absorbance spectra and retention times of the peaks of neoxanthin and violaxanthin isomers detected in HPLC chromatograms of Arabidopsis leaf extracts are shown in Supplemental Figure S5.
Statistical analyses of carotenoid, ABA, and catabolite content were performed using icalcu.com. Statistical differences among treatments were evaluated by one-way ANOVA, followed by Tukey’s honestly significant difference (HSD) mean-separation test. Lowercase Latin or Greek letters represent significant differences between genotypes within each treatment. P < 0.05 indicates significant difference.
Selection of nxd1 Mutant Alleles by CRISPR-Cas9 Strategy
Two different guide RNAs were designed using the CRISPOR Web site (http://crispor.tefor.net), and specificity for the NXD1 gene was checked by a BLAST search against the Arabidopsis genome. Guide RNAs 1 (5′-GCTGGTTATGCCAAGCCTCCA-3′) and 2 (5′-ACATACGCGCATGATGTAGG-3′) were synthesized with Arabidopsis U6 and U3 promoter sequences, respectively, and flanked by Gateway (Thermofisher Lifetech) attB1 and attB2 recombination sites. As described by Morineau et al. (2017), both cassettes were synthesized, cloned individually into pDONR207 (Life Technologies) by Gateway recombination and then recombined into pDE-Cas9 using the Gateway LR clonase. The pDE-Cas9 vector (kind gift from Holger Puchta, Karlsruhe Institute of Technology) was modified by exchanging the Basta resistance cassette with a DsRed cassette.
Arabidopsis Col-0 wild-type plants were transformed by the floral-dip method (Clough and Bent, 1998) and transgenic T1 lines were selected by detection of the DsRed marker fluorescence in seeds and the presence of mutations in PCR-amplified fragments containing NXD1 guide sequences. Homozygous nxd1 mutants were selected in the T2 progeny by screening for both neoxanthin deficiency and absence of DsRed fluorescence. Sequence analysis was used to identify nxd1 mutations and check the segregation of Cas9 sequence in the selected lines. The absence of neoxanthin and presence of homozygous nxd1 mutations were confirmed in their T3 progeny.
Reporter Gene Constructs
The NXD1 coding sequence (AT1G28100) was amplified from wild-type Col-0 genomic DNA by PCR, using Taq Phusion high-fidelity DNA polymerase (www.thermofisher.com), from ATG to STOP (TAA) codons and from ATG to the last amino acid (AAG) codons using NXD1-For (GGGGACAAGTTTGTACAAAAAAGCAGGCTTCATGGACGTTGAAGAAAAGAG) and NXD1-Rev1 (GGGGACCACTTTGTACAAGAAAGCTGGGTCTTACTTTGAAGGGTAAATT) or NXD1-Rev2 primers (GGGGACCACTTTGTACAAGAAAGCTGGGTCCTTTGAAGGGTAAATTACAA), respectively.
Amplified fragments were cloned by recombination with BP clonase (www.invitrogen.com) into the pDONR207 vector (www.arabidopsis.org/servlet/TairObject?id=501000106&type=vector) and transformed into Escherichia coli strain DH10B. Sequence analysis was carried out to confirm the absence of PCR-induced errors in the sequence of the cloned fragments before introduction by recombination into the binary Gateway destination vectors. The pMDC43 (www.arabidopsis.org/servlet/TairObject?id=501100109&type=vector) and pMDC83 (www.arabidopsis.org/servlet/TairObject?id=501100110&type=vector) binary vectors were used to obtain constructs encoding NXD1 protein fusion with GFP at the N-terminal (p35S:GFP-NXD1) or C-terminal (p35S:NXD1-GFP) end, respectively, under the control of a dual 35S promoter. Similarly, the pMDC32-mCherry and pMDC140-mCherry (www.arabidopsis.org/servlets/TairObject?type=vector&id=501100120) vectors were used to clone NXD1 downstream (p35S:mCherry-NXD1) or upstream (p35S:NXD1-mCherry) of the reporter gene. These latter binary vectors were constructed by cloning the mCherry gene into pMDC32 and replacing the GUS reporter with the mCherry gene in the pMDC140 vector, respectively, as previously described (Curtis and Grossniklaus, 2003; Deruyffelaere et al., 2018). The fragment amplified using the NXD1-For and NXD1-Rev1 primers was cloned into pMDC43 and pMDC32-mCherry vectors to produce the p35S:GFP-NXD1 and p35S:mCherry-NXD1 constructs, respectively. In parallel, the fragment amplified using the NXD1-For and NXD1-Rev2 primers was cloned into pMDC83 and pMDC140-mCherry vectors to produce p35S:NXD1-GFP and p35S:NXD1-mCherry constructs, respectively. The resulting binary vectors were transformed into A. tumefaciens C58C1pMP90 by electroporation prior to either stable or transient transformation.
A. tumefaciens Infiltration and Confocal Imaging
Agrobacteria carrying each of the four binary vectors described above were cultured overnight at 28°C in Luria-Bertani medium containing antibiotics (50 μg mL−1 rifampicin, 50 μg mL−1 gentamycin, and 50 μg mL−1 kanamycin), centrifuged, and resuspended in infiltration buffer (10 mm MgCl2 and 10 mm MES [pH 5.7]) at OD600 = 0.1. Coinfiltration of each strain alone, or of pairs of GFP and mCherry strains, was performed with an Agrobacterium strain containing the RNA-silencing inhibitor P19 (Voinnet et al., 2015), which was cultured in parallel and added at OD600 = 0.1 to the infiltration buffer to prevent gene silencing. For colocalization experiments, the p35S:mCherry-NXD1 strain was coinfiltrated with A. tumefaciens strains transformed with binary vectors containing GFP or YFP markers (Supplemental Table S2) for different subcellular compartments (cytosol, Golgi, endoplasmic reticulum, mitochondria, plastids, and peroxisomes). At 3 d before imaging, Agrobacteria cultures were mixed into 5 mL infiltration buffer and infiltration was performed on the abaxial face of leaves from 4-week-old plants using a syringe. For each construct or combination, leaf samples from four infiltrations of two independent plants were analyzed. Colocalization experiments using GFP, YFP, and mCherry constructs were imaged using a TCS-SP8-AOBS spectral confocal laser scanning microscope (Leica Microsystems). GFP/YFP and mCherry were excited with the 488-nm argon laser line or the 561-nm diode laser line, respectively, with an emission band of 495 to 530 nm for GFP/YFP, 585 to 625 nm for mCherry, and 665 to 705 nm for chlorophyll autofluorescence and bright-field images. Bright-field imaging was performed using the transmission photomultiplier tube.
ACCESSION NUMBERS
Sequence data from this article can be found in the Arabidopsis Genome Initiative data library under accession numbers At1g28100 (NXD1) and At1g67080 (ABA4).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Schematic diagram of NEOXANTHIN DEFICIENT1 (NXD1) and ABA DEFICIENT4 (ABA4) genes.
Supplemental Figure S2. Germination of nxd1-1 seeds compared to aba4-3 and wild-type seeds in the presence of increasing amounts of paclobutrazol, ABA, and NaCl.
Supplemental Figure S3. Subcellular colocalization of GFP or mCherry protein fusions with NXD1 at the N or C terminus in N. benthamiana leaf epidermal cells.
Supplemental Figure S4. Subcellular localization of mCherry-NXD1 fusion protein.
Supplemental Figure S5. Absorbance spectra of carotenoid peaks of Arabidopsis leaf extracts detected in HPLC chromatograms.
Supplemental Table S1. Carotenoid content in cauline leaves of nxd1 mutant alleles obtained by CrispR-Cas9, compared to wild-type and aba4-3 cauline leaves.
Supplemental Table S2. Details of the GFP and YFP markers used for colocalization experiments with mCherry-NXD1.
Acknowledgments
We are grateful to Institut Jean-Pierre Bourgin Plant Observatory technicians for plant culture, to Cécile Collonnier for her help in CRISPR-Cas9 technology, Ayuko Kuwahara and Clément Dionisio for contributions to the experimental work, and Helen North for critical reading of and helpful suggestions on the manuscript.
Footnotes
This work was supported by the Agence Nationale de la Recherche-Génoplante (grant no. GNP05075) and Saclay Plant Sciences, also under the auspices of the Agence Nationale de la Recherche (grant no. ANR–17–EUR–0007).
References
- Abuauf H, Haider I, Jia KP, Ablazov A, Mi J, Blilou I, Al-Babili S(2018) The Arabidopsis DWARF27 gene encodes an all-trans-/9-cis-β-carotene isomerase and is induced by auxin, abscisic acid and phosphate deficiency. Plant Sci 277: 33–42 [DOI] [PubMed] [Google Scholar]
- Alder A, Jamil M, Marzorati M, Bruno M, Vermathen M, Bigler P, Ghisla S, Bouwmeester H, Beyer P, Al-Babili S(2012) The path from β-carotene to carlactone, a strigolactone-like plant hormone. Science 335: 1348–1351 [DOI] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Beltrán J, Kloss B, Hosler JP, Geng J, Liu A, Modi A, Dawson JH, Sono M, Shumskaya M, Ampomah-Dwamena C, et al. (2015) Control of carotenoid biosynthesis through a heme-based cis-trans isomerase. Nat Chem Biol 11: 598–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchnak I, Brugière S, Moyet L, Le Gall S, Salvi D, Kuntz M, Tardif M, Rolland N(2019) Unraveling hidden components of the chloroplast envelope proteome: Opportunities and limits of better MS sensitivity. Mol Cell Proteomics 18: 1285–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno M, Al-Babili S(2016) On the substrate specificity of the rice strigolactone biosynthesis enzyme DWARF27. Planta 243: 1429–1440 [DOI] [PubMed] [Google Scholar]
- Chauffour F, Bailly M, Perreau F, Cueff G, Suzuki H, Collet B, Frey A, Clément G, Soubigou-Taconnat L, Balliau T, et al. (2019) Multi-omics analysis reveals sequential roles for ABA during seed maturation. Plant Physiol 180: 1198–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF(1998) Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Cunningham FX, Gantt E(1998) Genes and enzymes of carotenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol 49: 557–583 [DOI] [PubMed] [Google Scholar]
- Curtis MD, Grossniklaus U(2003) A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol 133: 462–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall’Osto L, Cazzaniga S, North H, Marion-Poll A, Bassi R(2007) The Arabidopsis aba4-1 mutant reveals a specific function for neoxanthin in protection against photooxidative stress. Plant Cell 19: 1048–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deruyffelaere C, Purkrtova Z, Bouchez I, Collet B, Cacas JL, Chardot T, Gallois JL, D’Andrea S(2018) PUX10 is a CDC48A adaptor protein that regulates the extraction of ubiquitinated oleosins from seed lipid droplets in Arabidopsis. Plant Cell 30: 2116–2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey A, Effroy D, Lefebvre V, Seo M, Perreau F, Berger A, Sechet J, To A, North HM, Marion-Poll A(2012) Epoxycarotenoid cleavage by NCED5 fine-tunes ABA accumulation and affects seed dormancy and drought tolerance with other NCED family members. Plant J 70: 501–512 [DOI] [PubMed] [Google Scholar]
- Gloaguen P, Bournais S, Alban C, Ravanel S, Seigneurin-Berny D, Matringe M, Tardif M, Kuntz M, Ferro M, Bruley C, et al. (2017) ChloroKB: A web application for the integration of knowledge related to chloroplast metabolic network. Plant Physiol 174: 922–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeber K, Nakabayashi K, Miatton E, Leubner-Metzger G, Soppe WJJ(2012) Molecular mechanisms of seed dormancy. Plant Cell Environ 35: 1769–1786 [DOI] [PubMed] [Google Scholar]
- Hooper CM, Castleden IR, Tanz SK, Aryamanesh N, Millar AH(2017) SUBA4: The interactive data analysis centre for Arabidopsis subcellular protein locations. Nucleic Acids Res 45(D1): D1064–D1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao YT, Gonzalez KL, Bartel B(2018) Peroxisome function, biogenesis, and dynamics in plants. Plant Physiol 176: 162–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Murillo C, Wurtzel ET(2007) Maize Y9 encodes a product essential for 15-cis-zeta-carotene isomerization. Plant Physiol 144: 1181–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Wang R, Qian Q, Yan M, Meng X, Fu Z, Yan C, Jiang B, Su Z, Li J, et al. (2009) DWARF27, an iron-containing protein required for the biosynthesis of strigolactones, regulates rice tiller bud outgrowth. Plant Cell 21: 1512–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B, Yin CC, He SJ, Lu X, Zhang WK, Lu TG, Chen SY, Zhang JS(2014) Ethylene-induced inhibition of root growth requires abscisic acid function in rice (Oryza sativa L.) seedlings. PLoS Genet 10: e1004701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano S, Nakamori C, Hayashi M, Kato A, Kondo M, Nishimura M(2002) Distribution and characterization of peroxisomes in Arabidopsis by visualization with GFP: Dynamic morphology and actin-dependent movement. Plant Cell Physiol 43: 331–341 [DOI] [PubMed] [Google Scholar]
- Mine A. (2019) Multifaceted involvement of abscisic acid in plant interactions with pathogenic and mutualistic microbes. In Seo M, Marion-Poll A, eds, Abscisic Acid in Plants, Advances in Botanical Research, volume 92, Elsevier, Amsterdam, pp 219–253 [Google Scholar]
- Morineau C, Bellec Y, Tellier F, Gissot L, Kelemen Z, Nogué F, Faure JD(2017) Selective gene dosage by CRISPR-Cas9 genome editing in hexaploid Camelina sativa. Plant Biotechnol J 15: 729–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman H, Galpaz N, Cunningham FX Jr., Zamir D, Hirschberg J(2014) The tomato mutation nxd1 reveals a gene necessary for neoxanthin biosynthesis and demonstrates that violaxanthin is a sufficient precursor for abscisic acid biosynthesis. Plant J 78: 80–93 [DOI] [PubMed] [Google Scholar]
- North HM, De Almeida A, Boutin JP, Frey A, To A, Botran L, Sotta B, Marion-Poll A(2007) The Arabidopsis ABA-deficient mutant aba4 demonstrates that the major route for stress-induced ABA accumulation is via neoxanthin isomers. Plant J 50: 810–824 [DOI] [PubMed] [Google Scholar]
- North HN, Frey A, Boutin JP, Sotta B, Marion-Poll A(2005) Analysis of xanthophyll cycle gene expression during the adaptation of Arabidopsis to excess light and drought stress: Changes in RNA steady-state levels do not contribute to short-term responses. Plant Sci 169: 115–164 [Google Scholar]
- Oikawa K, Matsunaga S, Mano S, Kondo M, Yamada K, Hayashi M, Kagawa T, Kadota A, Sakamoto W, Higashi S, et al. (2015) Physical interaction between peroxisomes and chloroplasts elucidated by in situ laser analysis. Nat Plants 1: 15035. [DOI] [PubMed] [Google Scholar]
- Orsel M, Eulenburg K, Krapp A, Daniel-Vedele F(2004) Disruption of the nitrate transporter genes AtNRT2.1 and AtNRT2.2 restricts growth at low external nitrate concentration. Planta 219: 714–721 [DOI] [PubMed] [Google Scholar]
- Pan R, Liu J, Wang S, Hu J(2020) Peroxisomes: Versatile organelles with diverse roles in plants. New Phytol 225: 1410–1427 [DOI] [PubMed] [Google Scholar]
- Park H, Kreunen SS, Cuttriss AJ, DellaPenna D, Pogson BJ(2002) Identification of the carotenoid isomerase provides insight into carotenoid biosynthesis, prolamellar body formation, and photomorphogenesis. Plant Cell 14: 321–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry AD, Horgan R(1991) Carotenoids and abscisic acid (ABA) biosynthesis in higher plants. Physiol Plant 82: 320–326 [Google Scholar]
- Plessis A, Cournol R, Effroy D, Silva Pérez V, Botran L, Kraepiel Y, Frey A, Sotta B, Cornic G, Leung J, et al. (2011) New ABA-hypersensitive Arabidopsis mutants are affected in loci mediating responses to water deficit and Dickeya dadantii infection. PLoS One 6: e20243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Yang SH, Kepsel AC, Schwartz SH, Zeevaart JA(2008) Evidence for abscisic acid biosynthesis in Cuscuta reflexa, a parasitic plant lacking neoxanthin. Plant Physiol 147: 816–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Sola MA, Rodríguez-Concepción M(2012) Carotenoid biosynthesis in Arabidopsis: A colorful pathway. The Arabidopsis Book 10: e0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz SH, Tan BC, Gage DA, Zeevaart JA, McCarty DR(1997) Specific oxidative cleavage of carotenoids by VP14 of maize. Science 276: 1872–1874 [DOI] [PubMed] [Google Scholar]
- Schwartz SH, Tan BC, McCarty DR, Welch W, Zeevaart JAD(2003) Substrate specificity and kinetics for VP14, a carotenoid cleavage dioxygenase in the ABA biosynthetic pathway. Biochim Biophys Acta 1619: 9–14 [DOI] [PubMed] [Google Scholar]
- Seo M, Aoki H, Koiwai H, Kamiya Y, Nambara E, Koshiba T(2004) Comparative studies on the Arabidopsis aldehyde oxidase (AAO) gene family revealed a major role of AAO3 in ABA biosynthesis in seeds. Plant Cell Physiol 45: 1694–1703 [DOI] [PubMed] [Google Scholar]
- Seo M, Marion-Poll A (2019) Abscisic acid metabolism and transport. In Seo M, Marion-Poll A, eds, Abscisic Acid in Plants, Advances in Botanical Research, volume 92, Elsevier, Amsterdam, pp 1–49 [Google Scholar]
- Shumskaya M, Wurtzel ET(2013) The carotenoid biosynthetic pathway: Thinking in all dimensions. Plant Sci 208: 58–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder AM, Clark BM, Robert B, Ruban AV, Bungard RA(2004) Carotenoid specificity of light-harvesting complex II binding sites. Occurrence of 9-cis-violaxanthin in the neoxanthin-binding site in the parasitic angiosperm Cuscuta reflexa. J Biol Chem 279: 5162–5168 [DOI] [PubMed] [Google Scholar]
- Taylor IB, Burbidge A, Thompson AJ(2000) Control of abscisic acid synthesis. J Exp Bot 51: 1563–1574 [DOI] [PubMed] [Google Scholar]
- Voinnet O, Rivas S, Mestre P, Baulcombe D(2015) Retraction: An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. Plant J 84: 846. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang Y, Gao C, Jiang L, Guo D(2017) PPero, a computational model for plant PTS1 type peroxisomal protein prediction. PLoS One 12: e0168912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzel ET.(2019) Changing form and function through carotenoids and synthetic biology. Plant Physiol 179: 830–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Christmann A, Yamaguchi-Shinozaki K, Grill E, Fernie AR(2019) Revisiting the basal role of ABA—Roles outside of stress. Trends Plant Sci 24: 625–635 [DOI] [PubMed] [Google Scholar]