Phytochrome B contributes to a wave of reactive oxygen species as well as local and systemic stomatal aperture closure in response to local excess light stress.
Abstract
Perception of a change in light intensity leads to the activation of multiple physiological, metabolic, and molecular responses in plants. These responses allow acclimation to fluctuating light conditions, e.g. sunflecks in field grown plants, preventing cellular damage associated with excess light stress. Perception of light stress by a single Arabidopsis (Arabidopsis thaliana) leaf was recently shown to activate different local and systemic responses that include rapid changes in stomatal aperture size; these were found to be coordinated by a systemic process of reactive oxygen species (ROS)-derived ROS production (i.e. the ROS wave). How light intensity is perceived, and how long the ROS wave stays “on” during this process are, however, unknown. Here we show that triggering of the ROS wave by a local excess light stress treatment results in the induction and maintenance of high levels of systemic ROS for up to 6 h. Despite these high systemic ROS levels, stomatal aperture size returns to control size within 3 h, and the systemic stomatal response can be retriggered within 6 h. These findings suggest that the ROS wave triggers a systemic stress memory mechanism that lasts for 3 to 6 h, but that within 3 h of its activation, stomata become insensitive to ROS and open. We further show that the excess light stress-triggered ROS wave, as well as the excess light stress-triggered local and systemic stomatal aperture closure responses, are dependent on phytochrome B function. Our findings reveal a delicate interplay between excess light stress, phytochrome B, ROS production, and rapid systemic stomatal responses.
The absolute dependency of photosynthetic organisms on sunlight as an overall energy source has led to the evolution of multiple photoreceptors that integrate light quality and intensity cues to direct many physiological, molecular, and developmental responses that optimize growth, acclimation, and adaptation to different environments (Chen et al., 2012; Ballard et al., 2019; Matthews et al., 2019; Liscum et al., 2020). Among the different photoreceptors of vascular plants, phytochromes (Phys) were found to play a key role in the regulation of many plant-environment interactions (Boccalandro et al., 2009; Wang et al., 2010; Casson and Hetherington, 2014; Jung et al., 2016; Legris et al., 2016, 2019; Ballaré, 2017; Klose et al., 2020). Phys are synthesized in their inactive, red light (R)-absorbing (Pr) form and convert upon absorption of light to their physiologically active far-red light (FR)-absorbing (Pfr) form. Light-activated Phys then translocate into the nucleus, interact with many different transcriptional regulators, and control a variety of different processes. Of the different members belonging to the Phy family, PhyB was found to play a key role in regulating many different plant responses to their environment. PhyB regulates responses to changes in light intensity and quality, temperature, hormone levels, cold and drought stresses, light- and heat-driven reactive oxygen species (ROS) scavenging mechanisms, stomatal opening, stomatal aperture oscillations, and various plant developmental responses (Boccalandro et al., 2009; Wang et al., 2010, 2016; González et al., 2012; Jung et al., 2016; Legris et al., 2016; Han et al., 2019; Kostaki et al., 2020).
In addition to regulating different responses to changes in environmental conditions at the particular tissue impacted by stress, PhyB was found to be involved in regulating different systemic whole-plant responses to a localized application of stress or pathogen infection. For example, PhyB expression in mesophyll and phloem cells was shown to regulate light-stimulated systemic stomatal development in new and emerging leaves (Casson and Hetherington, 2014). PhyB was also found to be involved in regulating light-induced root growth during shoot-to-root signaling in Arabidopsis (Arabidopsis thaliana; Lee et al., 2016), as well as shoot-induced abscisic acid (ABA) synthesis that regulates ROS production in roots (Ha et al., 2018). The light dependency of systemic acquired resistance (SAR) to pathogen attack was also found to be dependent on Phys (PhyA and PhyB; Griebel and Zeier, 2008). Of particular importance to systemic signaling in response to light stress are studies in tomato (Solanum lycopersicum) that demonstrated a role for PhyB and auxin in regulating photosynthesis and other physiological responses in lower canopy leaves in response to light changes occurring at the upper leaves (Guo et al., 2016; Wang et al., 2018). Although these studies did not address systemic stomatal aperture responses, they nevertheless highlighted a potential role for PhyB in regulating systemic ROS levels that control different physiological and molecular acclimation mechanisms.
Changes in systemic ROS/redox levels, as well as ROS-mediated systemic signaling, were shown to play a canonical role in the systemic acclimation response of plants to abiotic stress, a process termed systemic acquired acclimation (SAA; Karpinski et al., 1999; Suzuki et al., 2013; Kollist et al., 2019). Several recent studies revealed that an active process, or state, of ROS-induced ROS production can propagate through the plant, originating at the treated, stimulated, or stressed local tissue, and spreading within minutes to the entire plant (systemic tissues). This process was termed the “ROS wave” and was shown to regulate and coordinate systemic metabolic, molecular, and physiological responses among the different parts of the plant (Choudhury et al., 2018; Devireddy et al., 2018; Zandalinas et al., 2019, 2020a, 2020b; Fichman et al., 2019; Fichman and Mittler, 2020a), as well as to be required for SAA (Suzuki et al., 2013; Devireddy et al., 2018; Zandalinas et al., 2019, 2020a). Interestingly, in Arabidopsis, an excess light stress treatment with broad-wavelength white light was found to cause a local and systemic stomatal aperture closure response that was mediated by the ROS wave and required for plant acclimation (Devireddy et al., 2018, 2020; Kollist et al., 2019; Zandalinas et al., 2020a). Whether this stomatal aperture closure response and its systemic coordination depend on PhyB, and whether the initiation of the ROS wave itself involves PhyB are, however, unknown at present.
To address the role of PhyB in the regulation of systemic stomatal aperture closure responses and ROS wave initiation, we studied these processes in Arabidopsis plants and Phy-deficient mutants (phyA, phyB, and phyAphyB). Our findings reveal that PhyB is essential for ROS wave initiation and the stomatal aperture closure response at the local leaf, and that in its absence, systemic ROS signaling and stomatal aperture closure responses are suppressed.
RESULTS
Characterization of the Systemic Stomatal Aperture Closure Response of Arabidopsis
The systemic stomatal aperture response of Arabidopsis to excess light stress occurs within minutes and depends on the ROS wave for its propagation (Devireddy et al., 2018, 2020; Kollist et al., 2019; Zandalinas et al., 2020a). Although the maximum extent of this response was recorded within 10 min of excess white light stress application (Devireddy et al., 2018, 2020), it is not known for how long stomata would remain closed following recovery from excess light stress. To address this question we applied a 10-min excess white light stress treatment to a single Arabidopsis leaf and measured stomatal aperture and ROS levels in local and systemic leaves of plants at 10, 30, 60, 90, 120, 150, 180, and 360 min following initiation of the treatment. As shown in Figure 1, stomatal aperture returned to control levels (0-min time point) in local and systemic leaves within 3 h of initiation of the treatment. In contrast, local and systemic ROS levels peaked at 90 min, but remained higher than control levels (0-min time point) even at 360 min after initiation of treatment. As shown in Supplemental Figures S1 and S2, similar changes in stomatal aperture and ROS levels were not found in untreated control plants sampled in parallel at the time points shown in Figure 1. These findings reveal that although the levels of ROS remained high in local and systemic leaves, stomatal aperture was able to return to control levels, suggesting that the sensitivity of the stomatal aperture response to ROS levels could subside over time. Forward-looking infrared camera measurements of local and systemic leaf temperature after application of light stress to the local leaf (Devireddy et al., 2018; Zandalinas et al., 2020a) further confirmed the measurements of stomatal aperture closure (Fig. 1A; Supplemental Fig. S1) and demonstrated that this process was accompanied by a transient increase in leaf temperature that lasted for at least 1 h (Supplemental Fig. S3). Interestingly, in the cytosolic ascorbate peroxidase1 (apx1) mutant, which is unable to scavenge hydrogen peroxide (H2O2) at the cytosol and accumulates high levels of H2O2 in response to light stress (Davletova et al., 2005), stomata of local and systemic leaves closed and remain closed for the entire duration of the experiment (Fig. 1C). These findings suggest that the higher levels of ROS produced during light stress in the apx1 mutant (Davletova et al., 2005) may cause stomata to close for a much longer period of time in the apx1 mutant compared to wild-type plants.
Figure 1.
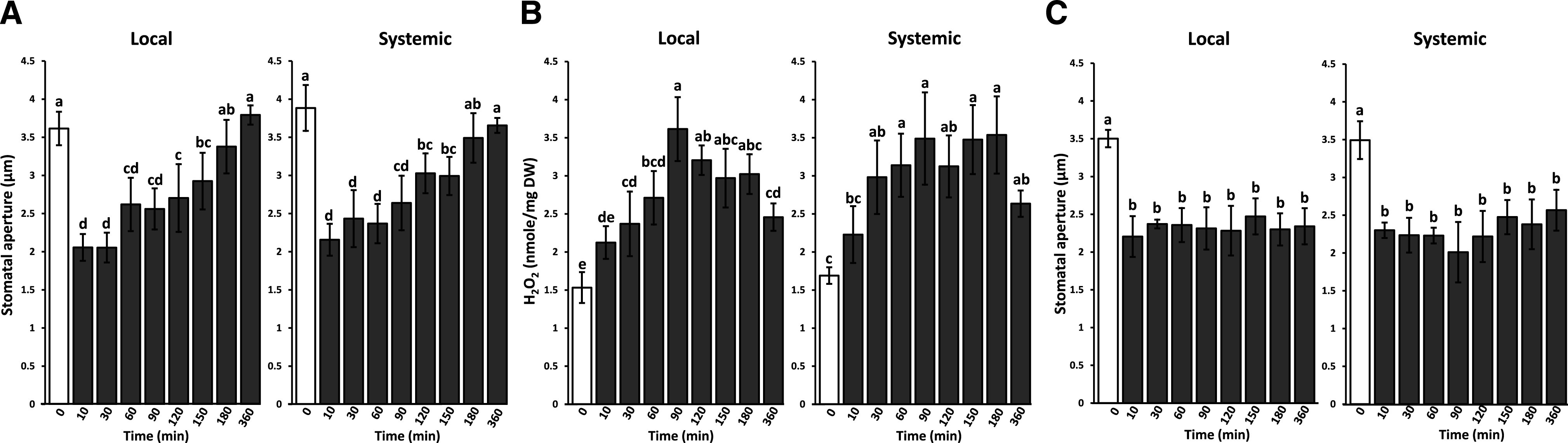
Characterization of the stomatal aperture closure response to a local treatment of excess light stress in Arabidopsis. A, Time course of local and systemic changes in stomatal aperture size in wild-type plants in response to a 10-min local treatment of excess white light stress. B, Time course of local and systemic changes in hydrogen peroxide levels in wild type plants in response to a 10-min local treatment of excess white light stress. C, Time course of local and systemic changes in stomatal aperture size in the cytosolic ascorbate peroxidase1 (apx1) mutant in response to a 10-min local treatment of excess white light stress. White bars indicate local or systemic stomatal aperture or H2O2 accumulation responses at 0 min. Black bars indicate local or systemic stomatal aperture or H2O2 accumulation responses at different time points in the experiment. Statistical analysis was conducted using ANOVA followed by Tukey’s honestly significant difference (HSD) mean-separation test and indicated by lowercase letters, n = 500 stomata from 10 different plants for each group. Error bars indicate the mean ± se; P < 0.05. DW, Dry weight.
To determine whether the reopening of stomatal aperture observed in wild-type plants at 3 and 6 h following the local 10-min excess white light stress treatment in the presence of higher-than-control levels of ROS (Fig. 1) resulted from stomatal insensitivity to ROS or ABA, and whether a subsequent excess light treatment would cause a stomatal aperture closure in stomata that had opened at 3 and 6 h, we applied a second 10-min excess white light stress treatment or sprayed plants with H2O2 (250 μm; Supplemental Fig. S4) or ABA (20 μm) at 3 or 6 h post the initial 10-min excess light stress treatment. As shown in Figure 2A, a second stomatal aperture closure response to a subsequent 10-min excess white light stress did not occur at 3 h, but occurred at 6 h after the initial local 10-min excess white light stress treatment. A similar trend was observed with H2O2 application (i.e. limited, but not significant, stomatal aperture closure response to H2O2 at 3 h, with a recovery of the response at 6 h; Fig. 2B). In contrast, ABA treatment at 3 or 6 h after the initial local 10-min excess white light stress treatment caused a stomatal aperture closure response (Fig. 2C). The findings presented in Figures 1 and 2 and Supplemental Figures S1 to S4 reveal that the systemic stomatal aperture closure response of Arabidopsis in response to a this treatment lasts for at least 3 h post initiation, that ROS levels remain high in local and systemic leaves for at least 6 h, and that stomata in local and systemic leaves of wild-type plants might become insensitive to H2O2 treatment or a second round of local 10-min excess white light stress treatment at 3 h (though this insensitivity is abolished by 6 h). In contrast, stomata in local and systemic leaves of the apx1 mutant close and remain closed for up to 6 h.
Figure 2.
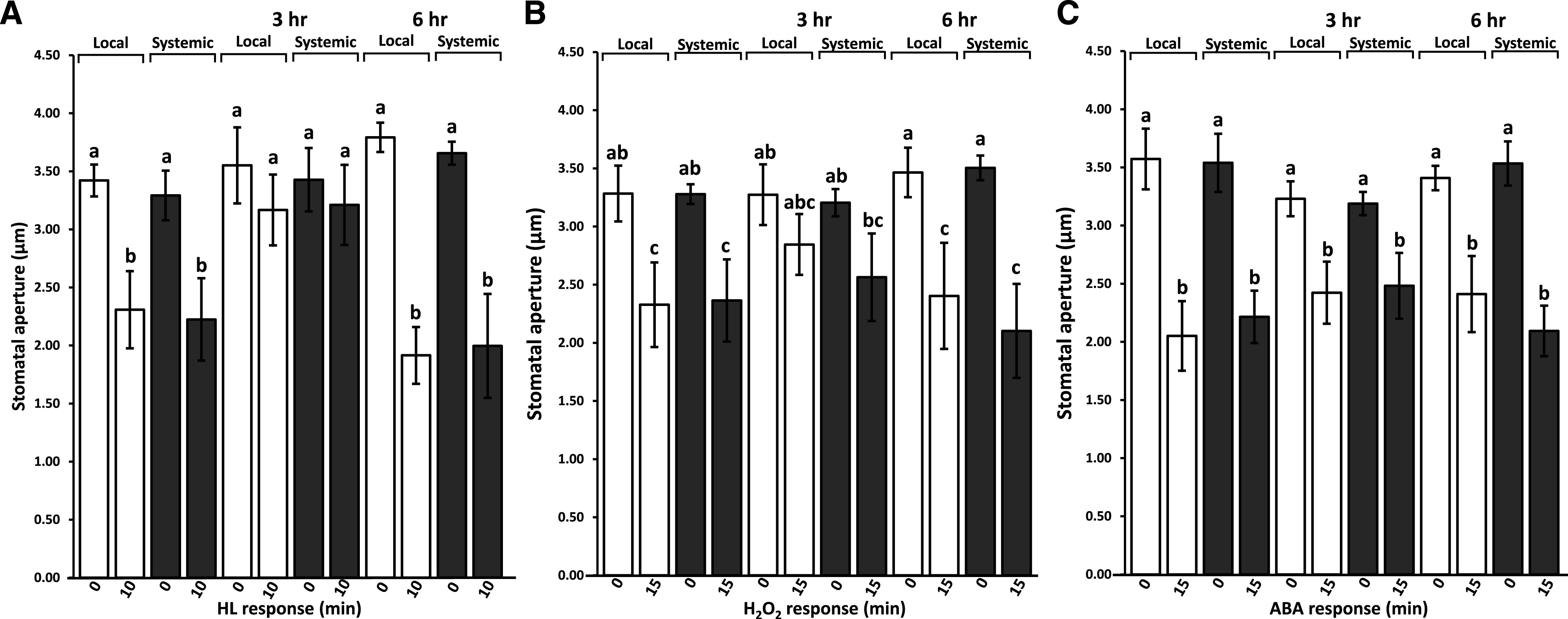
Recovery of stomatal responses to light stress at 3 and 6 h after an initial 10-min white light stress treatment. A, Local and systemic stomatal aperture responses to a second 10-min white light stress treatment applied to a local or systemic leaf 3 or 6 h after the initial 10-min white light stress treatment. B and C, Local and systemic stomatal aperture responses to a 15-min treatment of H2O2 (250 μm; B) or ABA (20 μm; C) applied to the entire plant at 3 or 6 h after the initial 10-min white light stress treatment. White and black bars indicate the stomatal response of local and systemic leaves to 0-, 10-, or 15-min treatments, respectively. Statistical analysis was conducted using ANOVA followed by Tukey’s honestly significant difference (HSD) mean-separation test and indicated by lowercase letters; n = 500 stomata from 10 different plants. Error bars indicate the mean ± se, P < 0.05. HL, High light.
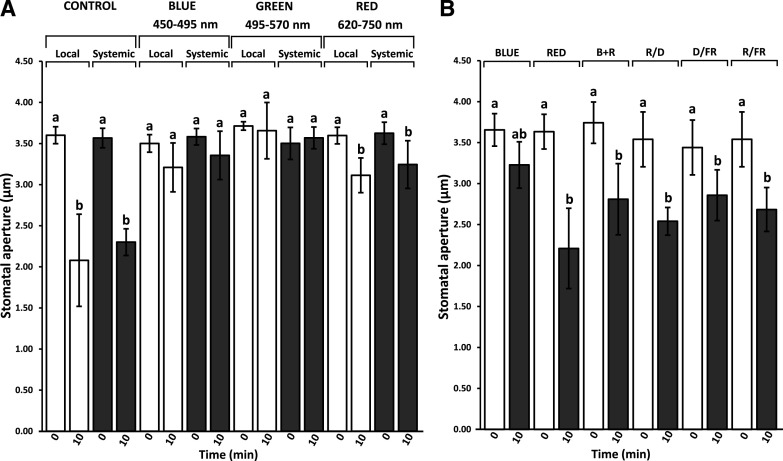
Wavelength Dependency of the Local and Systemic Stomatal Aperture Closure Responses
The findings that systemic photosynthetic and/or ROS responses to changes in light intensity are dependent on PhyB in Arabidopsis and tomato (Guo et al., 2016; Ha et al., 2018; Wang et al., 2018) could suggest that the systemic responses observed in stomatal aperture closure (Figs. 1 and 2; Supplemental Figs. S1 and S3; Devireddy et al., 2018, 2020) are mediated via a similar light perception system. We therefore examined the wavelength dependency of local and systemic stomatal aperture closure responses. As shown in Figure 3A, a 10-min treatment of excess white light stress, or filtered red light (applied through the same light source; Table 1; Supplemental Fig. S5A), applied to a local Arabidopsis leaf caused a local and systemic stomatal closure response. In contrast, filtered blue or green light (B or G, respectively) did not (Fig. 3A; Table 1; Supplemental Fig. S5A). As shown in Figure 3B, a similar stomatal aperture closure response was obtained when R was applied to the entire plant using a light-emitting diode (LED) array (Table 1; Supplemental Fig. S5B). We further used the LED array to test the effect of other light qualities on stomatal aperture changes, as well as the potential FR photoreversibility of the R response. Interestingly, B had no significant effect on stomatal aperture closure, nor did it impact the response of stomatal aperture to R when the two light wavelengths were combined (B+R). The lack of a B-induced stomatal closure response is not particularly surprising, as B is known to induce stomatal opening via the action of phototropin photoreceptors (Kinoshita et al., 2001; Inoue and Kinoshita, 2017).
Figure 3.
Local and systemic stomatal aperture responses to light at different wavelength spectrums applied to a local leaf. A, Local and systemic stomatal aperture responses to a 10-min white light treatment or blue (B), green (G), or red (R) filtered light treatment applied to a local leaf (Table 1; Supplemental Fig.S3A, spectra). B, Local and systemic stomatal aperture responses to 10-min LED B, R, B+R, or oscillating cycles (alternating 30-s periods for a total of 10 min) of R and D (R/D), D and FR (D/FR), or R and FR (R/FR) treatments applied to the entire plant (Table 1; Supplemental Fig. S3B). White and black bars indicate stomatal response of local and systemic leaves to 0- or 10-min light treatments, respectively. Statistical analysis was conducted using ANOVA followed by Tukey’s honestly significant difference (HSD) mean-separation test and indicated by lowercase letters; n = 500 stomata from 10 different plants. Error bars indicate the mean ± se; P < 0.05.
Table 1. Wavelength spectra and intensities of light.
| Light Sources | Wavelength | Light Intensity |
|---|---|---|
| nm | μmol m−2 s−1 | |
| Excess white light | 420–1050 | 1,700 |
| Blue filter | 450–550 | 145 |
| Red filter | 595–800 | 330 |
| Far red filter | 700–800 | 53 |
| Green filter | 495–600 | 175 |
| LED blue | 430–500 | 60 |
| LED red | 600–700 | 230 |
| LED far red | 550–780 | 180 |
| LED blue + red | 430–500 | 151 |
| 600–700 | ||
| LED red + far red | 600–780 | 270 |
The finding that high light-induced stomatal aperture closure is mediated by the R portion of the electromagnetic spectrum (Fig. 3) suggests that a Phy molecule may be mediating this response. We therefore examined the systemic stomatal aperture closure response to light stress under R and FR LEDs to examine its potential Phy dependency. As shown in Figure 3B, exposure of plants to alternating cycles (alternating 30-s periods for a total of 10 min) of R and darkness (D; R/D), D and FR (D/FR), or R and FR (R/FR) resulted in similar stomatal aperture closure responses. While the observation that R/D conditions induce stomatal closure is consistent with the function of a light-stable Phy, such as PhyB, the findings that D/FR conditions can also induce stomatal closure suggests a role for light-labile PhyA as well (Ballaré, 2017; Legris et al., 2019; Klose et al., 2020). The inability of FR to counteract the influence of R (R/FR condition) is consistent with independent cooperative interactions between R and FR signaling, dependent upon both PhyA and a light-stable Phy.
To test whether the intensity of R impacted stomatal responses and performance of the photosynthetic apparatus, we conducted dose-response curves for R generated through the filter or LED source (Supplemental Fig. S6) and measured the impact of R on the quantum yield of PSII and the maximum photochemical efficiency of PSII in the dark-adapted state (Fv/Fm) at local and systemic leaves (Supplemental Fig. S7). As shown in Supplemental Fig. S6, filtered- and LED-generated R triggered the stomatal aperture closure response at about the same intensity (200–230 μmol m−2 s−1). In contrast, white light triggered stomatal closure at a light intensity of 1,000 μmol m−2 s−1 (Supplemental Fig. S6). In addition, while white light impacted the quantum yield of PSII and Fv/Fm at the local leaf, R only impacted the quantum yield of PSII. Moreover, the impact of white light or R was only apparent for ∼30 min (Supplemental Fig. S7), in contrast to the local and systemic stomatal aperture closure responses, which lasted for about 1 to 3 h (Fig. 1; Supplemental Figs. S1 and S3). No impact on thequantum yield of PSII or Fv/Fm was observed at the systemic leaves of white light- or R-treated plants, and B had no effect on these parameters at the local or systemic leaves (Supplemental Fig. S7). The results presented in Supplemental Figs. S6 and S7 suggest, therefore, that the local and systemic responses observed in wild-type plants were not the result of light stress impacting photosynthetic performance and thereby indirectly causing long-term stomatal closure due to metabolic- or damage-induced responses.
PhyA and PhyB Regulate the Stomatal Aperture Closure Response
The findings presented in Figure 3 and Supplemental Figure S6 suggest that Phys are involved in the stomatal aperture closure response to excess light stress. To test whether this response is mediated by PhyA, PhyB, or both, we compared the local and systemic stomatal aperture closure responses of wild-type and Phy-deficient mutants (phyA, phyB, and phyAphyB) to a local application of a 10-min excess white light stress treatment. As shown in Figure 4A, in contrast to the wild type, phyB and phyAphyB plants did not display a local or systemic stomatal aperture closure response to the treatment. As further shown in Figure 4A, in contrast to phyB and phyAphyB, phyA plants displayed a local, but not a systemic, stomatal aperture closure response to the treatment. The results presented in Figure 4A suggest that PhyB is required for local, as well as systemic, stomatal aperture closure responses under broad-wavelength light conditions, while PhyA might be required only for systemic stomatal responses. To confirm that all phy mutants used in this study are capable of closing their stomatal aperture, we tested whether a dark treatment for 24 h resulted in stomatal aperture closure in these mutants. As shown in Supplemental Figure S8, phyA, phyB, phyAphyB, and the wild type all displayed a stomatal aperture closure response to the 24-h dark incubation, confirming that all genotypes can indeed close their stomates.
Figure 4.
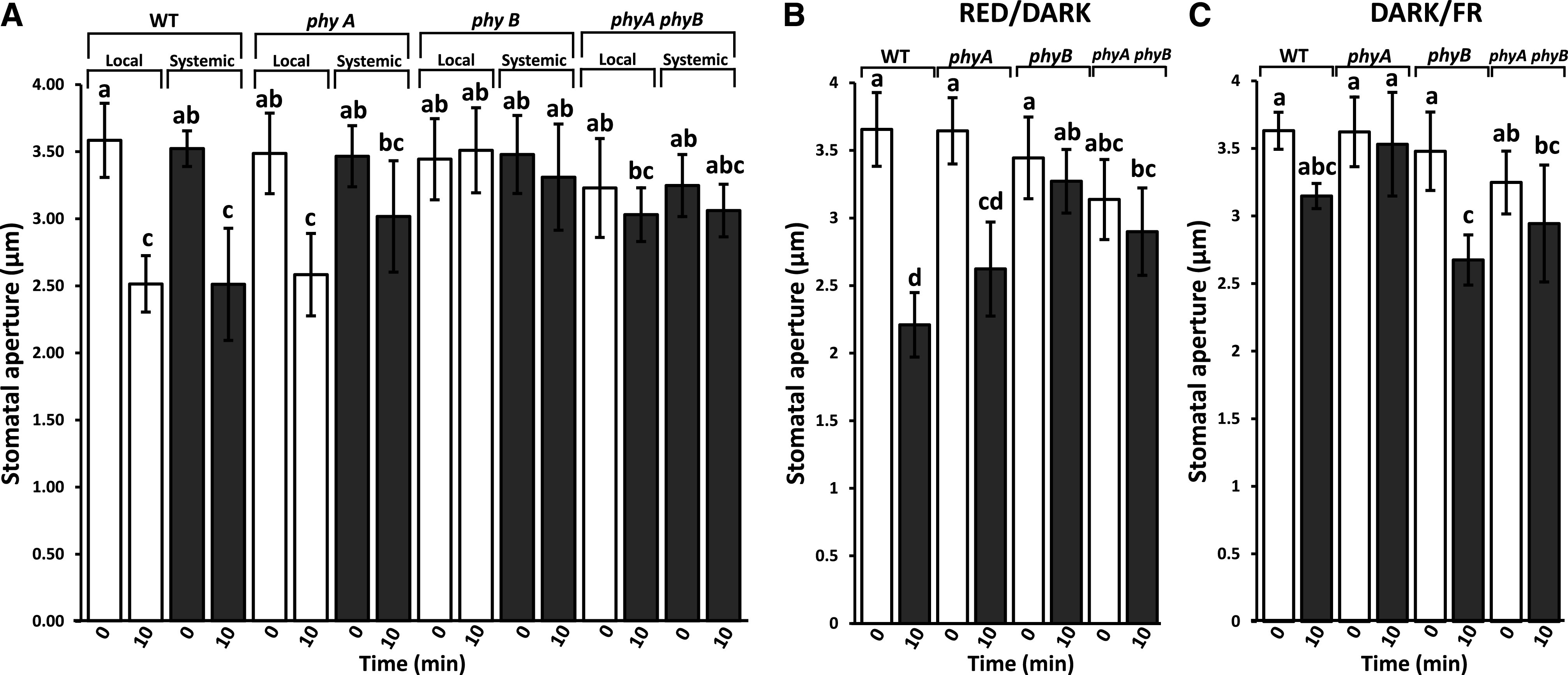
Local and systemic stomatal aperture responses of different phy mutants to a local light stress treatment. A, Local and systemic stomatal aperture responses to a 10-min excess white light stress treatment applied to a local leaf of wild-type (WT), phyA, phyB, phyAphyB plants. B, Stomatal aperture responses to a 10-min oscillating cycle (alternating 30-s periods for a total of 10 min) of LED R and D (R/D) applied to entire wild-type or phy mutant plants. C, Stomatal aperture responses to a 10-min oscillating cycle (alternating 30-s periods for a total of 10 min) of D and LED FR (D/FR) applied to entire wild-type or phy mutant plants. White and black bars indicate stomatal responses of local and systemic leaves, respectively, to 0- or 10-min light treatments in wild-type and phy mutants. Statistical analysis was conducted using ANOVA followed by Tukey’s honestly significant difference (HSD) mean-separation test and indicated by lowercase letters; n = 500 stomata from 10 different plants. Error bars indicate the mean ± se; P < 0.05.
To test whether the stomatal aperture closure response of the different phy mutants is wavelength dependent, we used the LED array to apply alternating cycles (alternating 30-s periods for a total of 10 min) of R and D (R/D; Fig. 4B), or D and FR (D/FR; Fig. 4C; Supplemental Fig. S5B) to entire phyA, phyB, phyAphyB, and wild-type plants. As shown in Figure 4B, R/D cycles resulted in stomatal aperture closure in wild-type and phyA plants, but not in phyB and phyAphyB plants. In contrast, as shown in Figure 4C, D/FR cycles resulted in stomatal aperture closure in wild-type and phyB plants. Taken together with the results shown in Figure 3, our findings suggest that PhyB is primarily involved in regulating stomatal aperture closure in local and systemic leaves when R is prominent, and that PhyA also contributes to stomatal aperture closure in systemic leaves when FR light is abundant.
PhyB Is Required for ROS Wave Initiation in Local Leaves
The suppression of local and systemic stomatal aperture closure responses in the phyB mutant (Fig. 4) could suggest that PhyB itself is required for the stomatal response, the accumulation of ROS levels at the local leaf, and/or activation of the ROS wave signal required for systemic stomatal aperture responses (Devireddy et al., 2018, 2020). To determine whether PhyB is required for ROS accumulation at the local leaf, as well as for activation of the systemic ROS wave response, we subjected phyA, phyB, phyAphyB, and wild-type plants to a local 10-min excess white light stress treatment and imaged ROS accumulation in whole plants using our newly developed imaging platform (Fichman et al., 2019; Zandalinas et al., 2020a). As shown in Figure 5A, local and systemic whole-plant ROS accumulation occurred in response to a local 10-min treatment of R or excess white light stress in wild-type plants. In contrast, in phyA plants, R and excess white light stress caused a local ROS accumulation response, but only white light caused a systemic ROS accumulation response (Fig. 5B), while in phyB and phyAphyB mutants, neither excess white light stress nor R caused a local or systemic ROS accumulation response (Fig. 5, C and D). These findings suggest that PhyB is required for light sensing during local excess white light stress treatment, and that light sensing through PhyB at the local leaf regulates local and systemic aperture closure responses (Figs. 3 and 4) as well as activation of the systemic ROS wave (Fig. 5; Supplemental Fig. S9) required for systemic stomatal aperture closure responses; Devireddy et al., 2018, 2020; Kollist et al., 2019; Zandalinas et al., 2020a).
Figure 5.
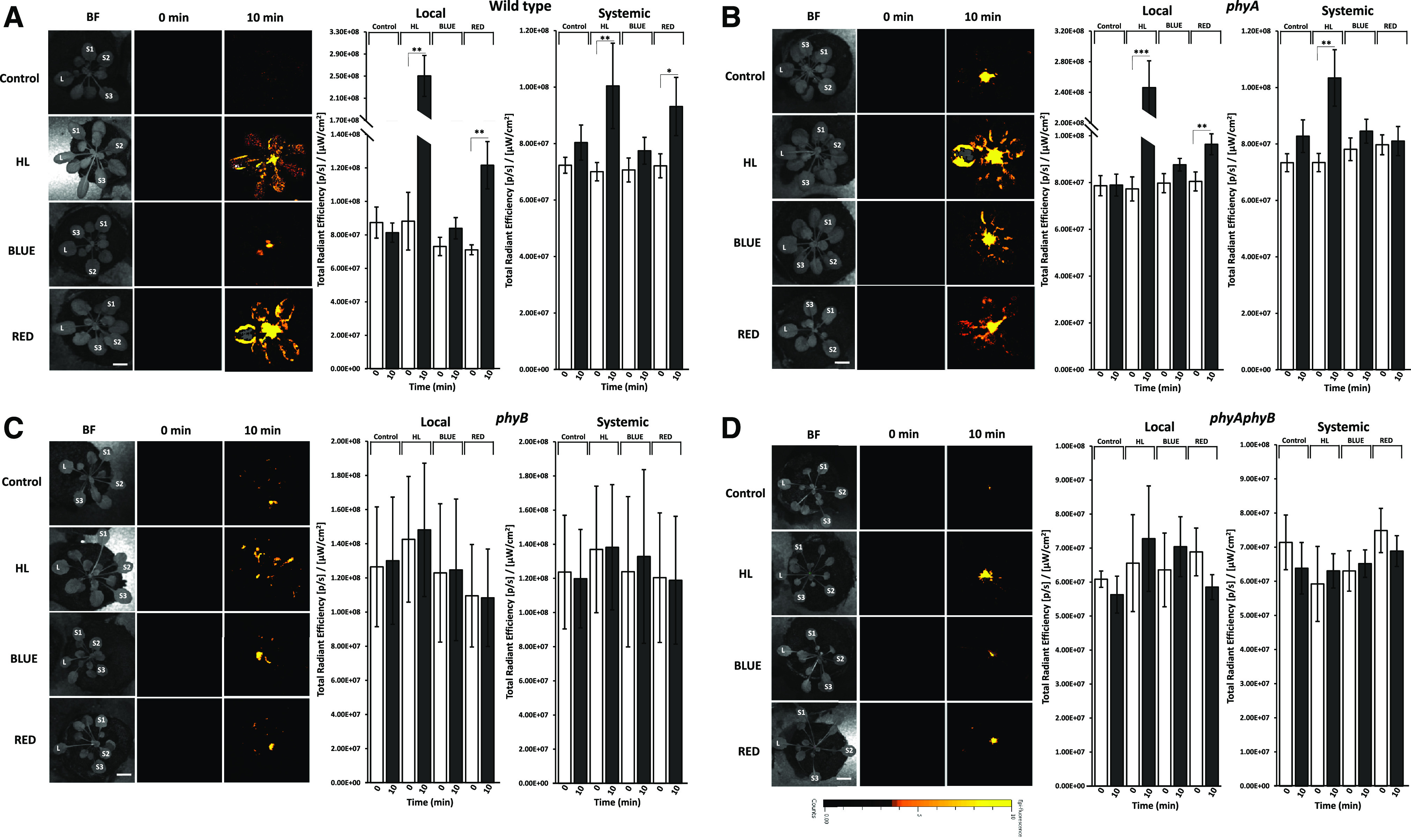
Whole-plant imaging of ROS accumulation in response to a local 10-min light stress treatment in the wild type and the different phy mutants. Time-lapse imaging of whole-plant ROS accumulation in plants subjected to a 10-min local white high light (HL) or B- or R-filtered light treatments (applied to the local leaf [L] only; Table 1; Supplemental Fig. S3A) is shown at left and statistical analysis of ROS accumulation in local and systemic (average of S1, S2, and S3) leaves at 0 and 10 min at right for the wild type (A) and phyA (B), phyB (C), and phyAphyB (D), is shown at right. All experiments were repeated at least three times with 10 plants per biological repeat. White and black bars indicate ROS accumulation in response to 0-min and 10-min light treatments, respectively. Error bars indicate the mean ± se of n = 10 plants. Asterisks indicate statistica significance determined by Student’s t test (*P < 0.05, **P < 0.01, and ***P < 0.001). Scale bars = 1 cm. HL, High light; BF, bright field; L, local; S, systemic.
DISCUSSION
Systemic stomatal aperture responses are thought to play an important role in plant acclimation to different abiotic and/or biotic conditions (Devireddy et al., 2018, 2020; Yoshida and Fernie, 2018; Kollist et al., 2019; McLachlan, 2020; Zandalinas et al., 2020a, 2020b). In Arabidopsis, systemic stomatal closure occurs in response to excess light stress and injury, potentially to prevent catastrophic xylem failure, rapid desiccation, and further injury to the plant (Devireddy et al., 2018, 2020; Zandalinas et al., 2020a), and to heat stress, potentially to enable the rapid cooling of leaves (Devireddy et al., 2020; Zandalinas et al., 2020a). In soybean (Glycine max), systemic stomatal opening was recently reported to occur in response to a rapid increase in light intensity, but this was not accompanied by systemic changes in photosynthetic activity, suggesting that systemic stomatal aperture responses could play a distinct role that is not directly related to changes in photosynthetic activity (Zandalinas et al., 2020b). Here we show that the excess white light stress-induced systemic stomatal aperture closure response can last for up to 3 h, suggesting that a stress memory mechanism may exist for this stress-induced systemic response. Interestingly, although ROS levels remained high in systemic leaves for up to 6 h, the stomatal aperture size returned to control levels within 3 h, and the potential for a systemic stomatal aperture response to a subsequent excess white light stress treatment was regained within 6 h (Fig. 1). These findings suggest that the systemic response of plants to abiotic stress and its memory may occur in two different phases: In the first phase (0–3 h), the ROS wave triggered at the local leaf causes stomatal aperture responses and activation of acclimation mechanisms (Figs. 1 and 2; Devireddy et al., 2018, 2020; Fichman et al., 2019; Zandalinas et al., 2019, 2020a, 2020b), while at the second phase (3–6 h), ROS levels remain high to keep acclimation mechanisms upregulated (stress memory), but stomatal responses return to normal (Figs. 1 and 2). One possible mechanism that could explain this result is that stomata become insensitive to high ROS levels. Indeed, as shown in Figure 2, while the stomatal aperture returned to its open state at 3 h, it was tolerant to H2O2 treatment or a subsequent application of excess white light stress (but not to ABA treatment). By 6 h, although ROS levels remained higher than control levels, this insensitivity was removed and stomata could respond to a subsequent excess light stress treatment or H2O2. An alternative explanation could be that a certain threshold of ROS level might be needed to maintain stomatal aperture closure, and the decrease in ROS levels occurring between 3 and 6 h, although not statistically significant compared to time 0 (Fig. 1B), coupled with activation of ROS scavenging mechanisms in guard cells may be sufficient to allow a return of stomatal responses. Further studies are needed to address this possibility (e.g. measurements of ROS levels in guard cells of intact leaves), as well as to determine the potential role of ROS in systemic stress memory in plants. The finding that in the apx1 mutant, which is unable to scavenge H2O2 at the cytosol, stomata of local and systemic leaves close and remain closed for the entire duration of the experiment (Fig. 1C) further supports the role of H2O2 in controlling and maintaining systemic stomatal responses.
Phys have primarily been studied for their role in stomatal aperture opening in response to light and other stimuli (Wang et al., 2010; Chen et al., 2012; Jung et al., 2016; Legris et al., 2016; Ballard et al., 2019; Matthews et al., 2019; Kostaki et al., 2020). In contrast, very little is known about the role of Phys in stomatal aperture closure. At least one study has shown that PhyB is involved in enhancing ABA sensitivity, increasing drought tolerance, and impacting stomatal closure during drought (González et al., 2012). Although it is not clear whether this response is similar to the stomatal aperture closure response of plants to light stress (which is also ABA dependent; Devireddy et al., 2018), taken together, the study of González et al. (2012) and our present study (Figs. 3 and 4) suggest a broader role for PhyB in regulating stomatal closure responses to light stress. PhyB in particular may therefore be involved in regulating light- or drought-induced stomatal closure responses.
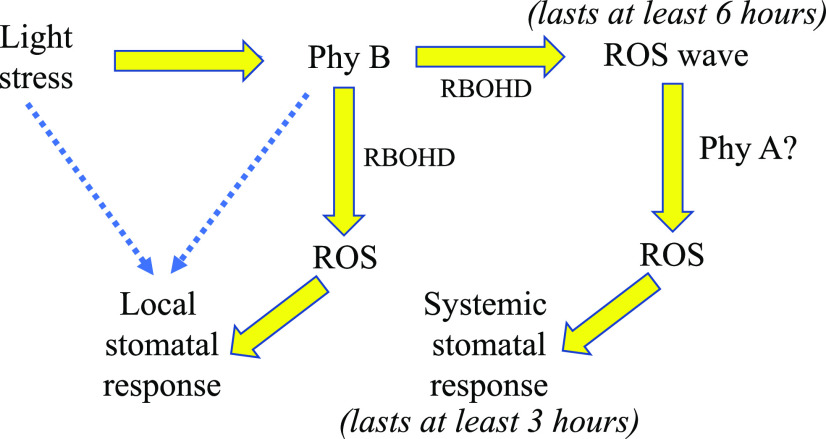
Our findings suggest that PhyB could serve as a potential signaling conduit between light, ROS, and stomatal aperture responses (Fig. 6), highlighting an interesting relationship between light intensity, photosynthesis, and ROS production in plants. It was traditionally thought that ROS produced by the photosynthetic apparatus in chloroplasts or peroxisomes during photorespiration is used as a signaling molecule to trigger stomatal responses and acclimation at the local leaf (Mittler, 2002, 2017; Kollist et al., 2019). In contrast, the findings presented in this study suggest that PhyB, a photoreceptor that is not directly involved in photosynthesis or photorespiration, is required for excess white light- or R-driven stomatal responses (Figs. 3 and 4). This finding could mean that excess light-driven production of ROS at the chloroplast or peroxisomes is primarily used for other regulatory functions during excess white light stress, and that sensing of R by PhyB is the primary signaling route for regulating the stomatal aperture closure response during light stress.
Figure 6.
A hypothetical model for the role of PhyB in regulating systemic stomatal aperture closure responses and ROS wave initiation during light stress. Excess white light stress is shown to be sensed by PhyB, leading to local accumulation of ROS, closure of stomatal aperture in local leaves, and triggering of the ROS wave. The ROS wave is shown to induce a state of high ROS accumulation in systemic leaves for up to 6 h and to trigger stomatal aperture closure in systemic leaves. Stomata in systemic leaves are shown to remain closed for at least 3 h. PhyB could be playing a paramount role in triggering local and systemic responses to light stress in Arabidopsis, and these responses are maintained in systemic tissues for up to 3 to 6 h, potentially serving as a systemic stress memory mechanism. Yellow arrows indicate pathways proposed by this study, and blue dashed arrows represent additional possible pathways.
Another process previously thought to be triggered by excess light stress-driven ROS production at the chloroplast or peroxisome is the initiation of the ROS wave during light stress (Mittler et al., 2011; Kollist et al., 2019; Fichman and Mittler, 2020a). Our findings that this process is also dependent on PhyB (Fig. 5) suggest that R sensing by PhyB, and not ROS produced in the chloroplast and/or peroxisomes, is involved in triggering the ROS wave. We previously showed that the initiation and propagation of the ROS wave is dependent on the function of the respiratory burst oxidase homolog D (RBOHD) protein (Miller et al., 2009; Mittler et al., 2011). The rapidity of this process further suggested that it is triggered posttranscriptionally via changes in calcium levels and/or phosphorylation of RBOHD (Fichman and Mittler, 2020a). Because RBOHD is localized to the plasma membrane, and the site of activated PhyB function is thought to be primarily nuclear through direct interaction with transcriptional regulators (Klose et al., 2020), it is not immediately obvious how PhyB might posttranscriptionally activate RBOHD. However, two possible scenarios may provide clues: (1) Rapid transcriptional changes may be involved (Suzuki et al., 2015); and (2) PhyB may activate a kinase/phosphatase relay that results in rapid activation of ROS production by RBOHD. In support of the second possibility, PhyB was found to interact with, or affect the function of, different kinases via its C-terminal domain (Qiu et al., 2017; Paik et al., 2019). The possible interaction between PhyB and respiratory burst oxidase homologs such as RBOHD should be further studied to reveal possible links between light sensing and different local and systemic signaling pathways in plants.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis (Arabidopsis thaliana) ecotype Columbia-0, apx1 mutant (Davletova et al., 2005), and phytochrome mutant phyA-211 (Reed et al., 1994), phyB,(SALK_069700C; Seo et al., 2006), and phyAphyB plants (Reed et al., 1993, 1994) were grown in peat pellets (Jiffy-7, Jiffy; http://www.jiffygroup.com) at 23°C under short-day growth conditions (8 h light/16 h dark, 50 μmol m−2 s−1) for 4 to 5 weeks.
Light Stress, ABA, and H2O2 Treatments
Local leaves of 4- to 5-week-old plants grown under short-day growth conditions were exposed to light stress (1,700 μmol m−2 s−1) for 10 min using a ColdVision fiber optic LED light source (A20980, Schott), as described in Devireddy et al., (2018). B (450–495 nm), R (620–700 nm), or FR (700–780 nm) filters (Roberts et al., 2011) were also used in conjunction with the ColdVision fiber optic LED light source to apply different filtered light wavelengths to a single leaf (Table 1). Light filters were placed immediately below the fiber optic light source output and the distance between the filters and the leaf surface was ∼2.3 cm. To apply specific light wavelengths to the entire plant, an LED light diode array (DuoStrip I033–Light engine, LEDdynamics) was used (Table 1). The LED light source was placed ∼10 cm from plants. Dose-response curves were conducted for the stomatal aperture closure response for each light source, as shown in Supplemental Figure S6. The wavelength and intensity of the different light sources was measured using a PS-200 Spectroradiometer, or an LI-250 Quantum Photometer (LI-COR Biosciences; Table 1; Supplemental Fig. S5). ABA and H2O2 treatments were performed by spraying ABA (20 μm) or H2O2 (250 μm; Supplemental Fig. S4) on the entire plant, as described in Devireddy et al. (2018), and leaving plants to incubate for 15 min before taking stomatal aperture measurements. Leaf temperature was determined using a forward-looking infrared camera (FLIR Systems), as described in Devireddy et al. (2018) and Zandalinas et al. (2020a). Quantum yield of PSII and Fv/Fm were determined using a portable fluorometer (model no. 110/S FluorPen, Photon Systems Instruments) as described in Balfagón et al. (2019). Briefly, plants were treated with the different light sources for 10 min and quantum yield of PSII of local and systemic leaves was measured at different time points up to 30 min. For Fv/Fm experiments, plants were treated with the different light sources for 10 min and incubated in the dark for 30 min before Fv/Fm measurements were conducted for local and systemic leaves (Balfagón et al., 2019).
Stomatal Aperture Measurements
Stomatal aperture analyses were performed as described in Morillon and Chrispeels (2001) and Devireddy et al., (2018). In brief, a local or a systemic leaf from each plant was cut and the lower surface was immediately stuck to a microspore slide with a medical adhesive (Hollister). After 1 to 2 min, the leaf was peeled away under distilled water. The lower epidermis imprint stuck to the glass was then visualized under the microscope, and stomatal images were recorded. Measurements of stomatal aperture were performed using ImageJ software, version 6. At least 500 different stomata were measured from 10 different plants for each time point, treatment, or genetic background.
H2O2 Measurements
The accumulation of H2O2 in local or systemic leaves was measured using Amplex Red (Molecular Probes, Invitrogen), as described in Suzuki et al. (2015). Briefly, 500 μL of 50 mm sodium phosphate buffer (pH 7.4) containing 50 mm Amplex Red and 0.05 U mL−1 horseradish peroxidase was added to ground tissues and samples were centrifuged at 12,000g for 12 min at 4°C. Following the centrifugation, 450 μL of supernatant was transferred into fresh tubes and incubated in the dark for 30 min at room temperature. Absorbance at 560 nm was then measured using Qubit 4 Fluorometer and the concentration of H2O2 in each sample was determined from a standard curve consisting of 0 and 25 μm H2O2. Following the measurement of absorbance, tissue samples were completely dried using a speed vacuum concentrator at 30°C for 120 min and H2O2 accumulation per milligram dry weight was calculated.
Whole-Plant ROS Imaging
ROS accumulation in local and systemic leaves of wild-type and phy mutants was performed using the IVIS Lumina S5 platform in acquisition mode (PerkinElmer), as described previously (Fichman et al., 2019; Fichman and Mittler, 2020b). Briefly, 4-week-old plants were placed in a glass container at a relative humidity of 70% and fumigated with H2DCFDA solution for 30 min using nebulizers (Fichman et al., 2019; Fichman and Mittler, 2020b; Zandalinas et al., 2020a). Light stress was then applied to the local leaf for 10 min. Plants were immediately placed in the IVIS Lumina S5 fluorescence imager (PerkinElmer) and images were captured using a filter with excitation/emission wavelengths 480 nm/520 nm and small binning setting). Visible-light images and fluorescent images were captured every 30 s for 10 min. Image analysis was conducted using the region of interest and image math tools of Living Image 4.7.2 software (Fichman et al., 2019; Fichman and Mittler, 2020b; Zandalinas et al., 2020a). Total counts of fluorescence were used for all calculations with the Living Image 4.7.2 image math tool. The first image (at 0 min) was subtracted from the last image (at 10 min) to evaluate signal intensity, so the image at 0 min appears black or holds low signal intensity. Radiant efficiency of selected regions of interest was calculated by Living Image 4.7.2.
Statistical Analysis
Statistical analysis was performed by a two-way ANOVA followed by Tukey’s honestly significant difference (HSD) mean-separation test or Student’s t test (***P < 0.001, **P < 0.01, and *P < 0.05).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Stomatal aperture changes in control Arabidopsis plants.
Supplemental Figure S2. Hydrogen peroxide levels in control Arabidopsis plants.
Supplemental Figure S3. Local and systemic leaf temperature in control plants and plants locally treated with excess light stress.
Supplemental Figure S4. Stomatal closure response of local and systemic leaves sprayed with different concentrations of hydrogen peroxide.
Supplemental Figure S5. Wavelength spectrums of filtered and light-emitting diode light sources used in this study.
Supplemental Figure S6. Stomatal closure response of local and systemic leaves treated with different intensities of white or red light.
Supplemental Figure S7. Quantum yield of PSII and Fv/Fm of local and systemic leaves treated with white or red light.
Supplemental Figure S8. Changes in stomatal aperture size in response to a 24-h dark treatment of wild type and different Phy mutants.
Supplemental Figure S9. Whole-plant ROS imaging of the wild type and the different phy mutants under controlled growth conditions.
Acknowledgments
We acknowledge all authors of articles not mentioned in this manuscript due to space limitations.
Footnotes
This work was supported by the National Science Foundation, Directorate of Biological Sciences, Divisions of Integerative Organismal Systems (grant nos. IOS–1932639 and IOS–1353886) and National Science Foundation, Directorate of Biological Sciences, Molecular and Cellular Bioscience (grant no. MCB–1936590) and the University of Missouri.
Articles can be viewed without a subscription.
References
- Balfagón D, Sengupta S, Gómez-Cadenas A, Fritschi FB, Azad RK, Mittler R, Zandalinas SI(2019) Jasmonic acid is required for plant acclimation to a combination of high light and heat stress. Plant Physiol 181: 1668–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard T, Peak D, Mott K(2019) Blue and red light effects on stomatal oscillations. Funct Plant Biol 46: 146–151 [DOI] [PubMed] [Google Scholar]
- Ballaré CL.(2017) Phytochrome responses: Think globally, act locally. Trends Plant Sci 22: 909–911 [DOI] [PubMed] [Google Scholar]
- Boccalandro HE, Rugnone ML, Moreno JE, Ploschuk EL, Serna L, Yanovsky MJ, Casal JJ(2009) Phytochrome B enhances photosynthesis at the expense of water-use efficiency in Arabidopsis. Plant Physiol 150: 1083–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casson SA, Hetherington AM(2014) phytochrome B is required for light-mediated systemic control of stomatal development. Curr Biol 24: 1216–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Xiao Y-GG, Li X, Ni M(2012) Light-regulated stomatal aperture in Arabidopsis. Mol Plant 5: 566–572 [DOI] [PubMed] [Google Scholar]
- Choudhury FK, Devireddy AR, Azad RK, Shulaev V, Mittler R(2018) Local and systemic metabolic responses during light-induced rapid systemic signaling. Plant Physiol 178: 1461–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davletova S, Rizhsky L, Liang H, Shengqiang Z, Oliver DJ, Coutu J, Shulaev V, Schlauch K, Mittler R(2005) Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 17: 268–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devireddy AR, Arbogast J, Mittler R(2020) Coordinated and rapid whole-plant systemic stomatal responses. New Phytol 225: 21–25 [DOI] [PubMed] [Google Scholar]
- Devireddy AR, Zandalinas SI, Gómez-Cadenas A, Blumwald E, Mittler R(2018) Coordinating the overall stomatal response of plants: Rapid leaf-to-leaf communication during light stress. Sci Signal 11: eaam9514. [DOI] [PubMed] [Google Scholar]
- Fichman Y, Miller G, Mittler R(2019) Whole-plant live imaging of reactive oxygen species. Mol Plant 12: 1203–1210 [DOI] [PubMed] [Google Scholar]
- Fichman Y, Mittler R(2020a) Rapid systemic signaling during abiotic and biotic stresses: Is the ROS wave master of all trades? Plant J 102: 887–896 [DOI] [PubMed] [Google Scholar]
- Fichman Y, Mittler R(2020b) Noninvasive live ROS imaging of whole plants grown in soil. Trends Plant Sci 25: 1052–1053 [DOI] [PubMed] [Google Scholar]
- González CV, Ibarra SE, Piccoli PN, Botto JF, Boccalandro HE(2012) Phytochrome B increases drought tolerance by enhancing ABA sensitivity in Arabidopsis thaliana. Plant Cell Environ 35: 1958–1968 [DOI] [PubMed] [Google Scholar]
- Griebel T, Zeier J(2008) Light regulation and daytime dependency of inducible plant defenses in Arabidopsis: Phytochrome signaling controls systemic acquired resistance rather than local defense. Plant Physiol 147: 790–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Wang F, Xiang X, Ahammed GJ, Wang M, Onac E, Zhou J, Xia X, Shi K, Yin X, et al. (2016) Systemic induction of photosynthesis via illumination of the shoot apex is mediated sequentially by phytochrome B, auxin and hydrogen peroxide in tomato. Plant Physiol 172: 1259–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha JH, Kim JH, Kim SG, Sim HJ, Lee G, Halitschke R, Baldwin IT, Kim JIJH, Park CM(2018) Shoot phytochrome B modulates reactive oxygen species homeostasis in roots via abscisic acid signaling in Arabidopsis. Plant J 94: 790–798 [DOI] [PubMed] [Google Scholar]
- Han S-HH, Park Y-JJ, Park C-MM(2019) Light primes the thermally induced detoxification of reactive oxygen species during development of thermotolerance in Arabidopsis. Plant Cell Physiol 60: 230–241 [DOI] [PubMed] [Google Scholar]
- Inoue SI, Kinoshita T(2017) Blue light regulation of stomatal opening and the plasma membrane H+-ATPase. Plant Physiol 174: 531–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JH, Domijan M, Klose C, Biswas S, Ezer D, Gao M, Khattak AK, Box MS, Charoensawan V, Cortijo S, et al. (2016) Phytochromes function as thermosensors in Arabidopsis. Science 354: 886–889 [DOI] [PubMed] [Google Scholar]
- Karpinski S, Reynolds H, Karpinska B, Wingsle G, Creissen G, Mullineaux P(1999) Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science 284: 654–657 [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Doi M, Suetsugu N, Kagawa T, Wada M, Shimazaki K(2001) phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 414: 656–660 [DOI] [PubMed] [Google Scholar]
- Klose C, Nagy F, Schäfer E(2020) Thermal reversion of plant phytochromes. Mol Plant 13: 386–397 [DOI] [PubMed] [Google Scholar]
- Kollist H, Zandalinas SI, Sengupta S, Nuhkat M, Kangasjärvi J, Mittler R(2019) Rapid responses to abiotic stress: Priming the landscape for the signal transduction network. Trends Plant Sci 24: 25–37 [DOI] [PubMed] [Google Scholar]
- Kostaki KI, Coupel-Ledru A, Bonnell VC, Gustavsson M, Sun P, McLaughlin FJ, Fraser DP, McLachlan DH, Hetherington AM, Dodd AN, et al. (2020) Guard cells integrate light and temperature signals to control stomatal aperture. Plant Physiol 182: 1404–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H-J, Ha J-H, Kim S-G, Choi H-K, Kim ZH, Han Y-J, Kim J-I, Oh Y, Fragoso V, Shin K, et al. (2016) Stem-piped light activates phytochrome B to trigger light responses in Arabidopsis thaliana roots. Sci Signal 9: ra106. [DOI] [PubMed] [Google Scholar]
- Legris M, Ince YÇ, Fankhauser C(2019) Molecular mechanisms underlying phytochrome-controlled morphogenesis in plants. Nat Commun 10: 5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legris M, Klose C, Burgie ES, Rojas CC, Neme M, Hiltbrunner A, Wigge PA, Schäfer E, Vierstra RD, Casal JJ(2016) Phytochrome B integrates light and temperature signals in Arabidopsis. Science 354: 897–900 [DOI] [PubMed] [Google Scholar]
- Liscum E, Nittler P, Koskie K(2020) The continuing arc toward phototropic enlightenment. J Exp Bot 71: 1652–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews JSA, Vialet-Chabrand S, Lawson T(2019) Role of blue and red light in stomatal dynamic behaviour. J Exp Bot 71: 2253–2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan DH.(2020) Systemic signalling, and the synchronization of stomatal response. New Phytol 225: 5–6 [DOI] [PubMed] [Google Scholar]
- Miller G, Schlauch K, Tam R, Cortes D, Torres MA, Shulaev V, Dangl JL, Mittler R(2009) The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci Signal 2: ra45. [DOI] [PubMed] [Google Scholar]
- Mittler R.(2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7: 405–410 [DOI] [PubMed] [Google Scholar]
- Mittler R.(2017) ROS are good. Trends Plant Sci 22: 11–19 [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F(2011) ROS signaling: The new wave? Trends Plant Sci 16: 300–309 [DOI] [PubMed] [Google Scholar]
- Morillon R, Chrispeels MJ(2001) The role of ABA and the transpiration stream in the regulation of the osmotic water permeability of leaf cells. Proc Natl Acad Sci USA 98: 14138–14143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik I, Chen F, Ngoc Pham V, Zhu L, Kim J-I, Huq E(2019) A phyB-PIF1-SPA1 kinase regulatory complex promotes photomorphogenesis in Arabidopsis. Nat Commun 10: 4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Pasoreck EK, Reddy AK, Nagatani A, Ma W, Chory J, Chen M(2017) Mechanism of early light signaling by the carboxy-terminal output module of Arabidopsis phytochrome B. Nat Commun 8: 1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Nagatani A, Elich TD, Fagan M, Chory J(1994) Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiol 104: 1139–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Nagpal P, Poole DS, Furuya M, Chory J(1993) Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell 5: 147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D, Pedmale UV, Morrow J, Sachdev S, Lechner E, Tang X, Zheng N, Hannink M, Genschik P, Liscum E(2011) Modulation of phototropic responsiveness in Arabidopsis through ubiquitination of phototropin 1 by the CUL3-Ring E3 ubiquitin ligase CRL3(NPH3). Plant Cell 23: 3627–3640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo M, Hanada A, Kuwahara A, Endo A, Okamoto M, Yamauchi Y, North H, Marion-Poll A, Sun TP, Koshiba T, et al. (2006) Regulation of hormone metabolism in Arabidopsis seeds: Phytochrome regulation of abscisic acid metabolism and abscisic acid regulation of gibberellin metabolism. Plant J 48: 354–366 [DOI] [PubMed] [Google Scholar]
- Suzuki N, Devireddy AR, Inupakutika MA, Baxter A, Miller G, Song L, Shulaev E, Azad RK, Shulaev V, Mittler R(2015) Ultra-fast alterations in mRNA levels uncover multiple players in light stress acclimation in plants. Plant J 84: 760–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Miller G, Salazar C, Mondal HA, Shulaev E, Cortes DF, Shuman JL, Luo X, Shah J, Schlauch K, et al. (2013) Temporal-spatial interaction between reactive oxygen species and abscisic acid regulates rapid systemic acclimation in plants. Plant Cell 25: 3553–3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Guo Z, Li H, Wang M, Onac E, Zhou J, Xia X, Shi K, Yu J, Zhou Y(2016) Phytochrome a and b function antagonistically to regulate cold tolerance via abscisic acid-dependent jasmonate signaling. Plant Physiol 170: 459–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Wu N, Zhang L, Ahammed GJ, Chen X, Xiang X, Zhou J, Xia X, Shi K, Yu J, et al. (2018) Light signaling-dependent regulation of photoinhibition and photoprotection in tomato. Plant Physiol 176: 1311–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F-F, Lian H-L, Kang C-Y, Yang H-Q(2010) Phytochrome B is involved in mediating red light-induced stomatal opening in Arabidopsis thaliana. Mol Plant 3: 246–259 [DOI] [PubMed] [Google Scholar]
- Yoshida T, Fernie AR(2018) Remote control of transpiration via ABA. Trends Plant Sci 23: 755–758 [DOI] [PubMed] [Google Scholar]
- Zandalinas SI, Cohen IH, Fritschi FB, Mittler R(2020b) Coordinated systemic stomatal responses in soybean. Plant Physiol 183: 1428–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandalinas SI, Fichman Y, Devireddy AR, Sengupta S, Azad RK, Mittler R(2020a) Systemic signaling during abiotic stress combination in plants. Proc Natl Acad Sci USA 117: 13810–13820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandalinas SI, Sengupta S, Burks D, Azad RK, Mittler R(2019) Identification and characterization of a core set of ROS wave-associated transcripts involved in the systemic acquired acclimation response of Arabidopsis to excess light. Plant J 98: 126–141 [DOI] [PMC free article] [PubMed] [Google Scholar]