A heat shock factor mediates flavonoid synthesis and abscisic acid signaling to regulate drought tolerance in apple.
Abstract
Drought is an important environmental factor affecting the growth and production of agricultural crops and fruits worldwide, including apple (Malus domestica). Heat shock factors (HSFs) have well-documented functions in stress responses, but their roles in flavonoid synthesis and the flavonoid-mediated drought response mechanism remain elusive. In this study, we demonstrated that a drought-responsive HSF, designated MdHSFA8a, promotes the accumulation of flavonoids, scavenging of reactive oxygen species, and plant survival under drought conditions. A chaperone, HEAT SHOCK PROTEIN90 (HSP90), interacted with MdHSFA8a to inhibit its binding activity and transcriptional activation. However, under drought stress, the MdHSP90-MdHSFA8a complex dissociated and the released MdHSFA8a further interacted with the APETALA2/ETHYLENE RESPONSIVE FACTOR family transcription factor RELATED TO AP2.12 to activate downstream gene activity. In addition, we demonstrated that MdHSFA8a participates in abscisic acid-induced stomatal closure and promotes the expression of abscisic acid signaling-related genes. Collectively, these findings provide insight into the mechanism by which stress-inducible MdHSFA8a modulates flavonoid synthesis to regulate drought tolerance.
With global climate change, plant growth and development are often subject to diverse environmental stresses, such as drought, heat, cold, and high salinity (Zhu, 2016). Drought stress, in particular, has become a critical problem worldwide as temperatures rise and arid lands expand, seriously affecting the growth and productivity of the most important crops (Fedoroff et al., 2010; Gilbert, 2012). Therefore, it is of considerable importance for the sustainable development of global agriculture to breed drought-tolerant cultivars and elucidate the mechanisms of abiotic stress responses.
In plants, drought stress results in a network of physiological and biochemical reactions, such as limitation of photosynthesis, enhanced stomatal closure, changes in cellular components, stimulation of osmolyte production, and accumulation of reactive oxygen species (ROS; Miao et al., 2006; Cutler et al., 2010; Krasensky and Jonak, 2012). These reactions occur in a disciplined spatiotemporal order and are interrelated, forming a systematic drought response mechanism. The primary stress signal caused by drought is hyperosmotic stress (or osmotic stress for short), which can result in rapid accumulation of abscisic acid (ABA) in plants (Zhu, 2002, 2016). The ABA signaling pathway plays a central role in drought responses in plants (Cutler et al., 2010). When drought stress induces ABA accumulation, the PYRABACTIN RESISTANCE (PYR)/PYR-LIKE/REGULATORY COMPONENTS OF ABA RECEPTOR combines with ABA and interacts with 2C-type protein phosphatases (PP2Cs; Fujii et al., 2009), thereby releasing Sucrose nonfermenting1-related protein kinases (SnRKs) previously inhibited by PP2Cs (Soon et al., 2012). The released SnRKs phosphorylate downstream ABA-responsive element-binding factors (ABFs; also termed AREBs), such as ABF2 (AREB1), ABF3, ABF4 (AREB2), and ABI5, which can bind to ABA-responsive element cis-acting elements (ACGTGG/TC) and activate the expression of downstream genes (Furihata et al., 2006; Yoshida et al., 2010). Generally, ABA accumulation and signal transduction occur rapidly, which enables plants to close stomata quickly and reduce water loss under drought stress (Watkins et al., 2017). However, when plants continue to experience drought stress, ROS will accumulate excessively in plant cells, leading to oxidative stress, which causes irreversible oxidative modifications of nucleic acids, proteins, and membrane lipids (Van Breusegem and Dat, 2006; Gill and Tuteja, 2010; Choudhury et al., 2017). To prevent oxidative stress, plant cells have evolved a network of enzymatic and nonenzymatic antioxidant mechanisms to maintain ROS homeostasis (Wrzaczek et al., 2013). Among these mechanisms, flavonoids have been extensively reported to participate in plant-environment interactions owing to their strong antioxidant and scavenging capacity for ROS (Winkel-Shirley, 2002; Agati and Tattini, 2010; Singh et al., 2016).
Flavonoids are an ancient, specialized group of secondary metabolites in plants. The accumulation of flavonoids in plant tissues has been considered to be a hallmark of plant stress (Winkel-Shirley, 2002). The primary structure of flavonoids is composed of three carbon-linked two aromatic rings, among which the structure that exhibits the highest antioxidant activity is a 3′,4′-o-dihydroxyl group in the B ring because of its strong capacity to donate electrons or hydrogen atoms (Williams and Grayer, 2004; Hernández et al., 2009; Supplemental Fig. S1). Under many environmental stresses, such as ultraviolet radiation, drought, high salinity, and cold, the synthesis of flavonoids increases, which provides an important mechanism for controlling the accumulation of stress-induced ROS (Li et al., 1993; Tattini et al., 2004; Walia et al., 2005; Korn et al., 2008). In addition, the flavonoid synthesis pathway is hormonally regulated, including by ABA, auxin, jasmonic acid, ethylene, and other hormone signals (Loreti et al., 2008; Lewis et al., 2011; An et al., 2015; Zhang et al., 2018). It has been observed that drought can substantially promote the expression of genes associated with flavonoid synthesis pathways (Castellarin et al., 2007; André et al., 2009; Ma et al., 2014). However, studies of flavonoid-mediated drought stress tolerance remain rare. In Arabidopsis (Arabidopsis thaliana), overexpression of MYB12/Production of Flavonol Glycosides1 or MYB75/Production of Anthocyanin Pigment1 alone, or coexpression of MYB12 and MYB75, demonstrated that overaccumulation of flavonoids mitigated the accumulation of ROS in vivo under drought stress (Nakabayashi et al., 2014). Overexpression of UGT79B2/B3 significantly increases anthocyanin accumulation and enhances antioxidant activity in response to low temperature, drought, and salt stresses (Li et al., 2017b). Interestingly, in addition to the elimination of ROS to confer drought tolerance, some studies have shown that flavonoids can participate in stomatal switches (Watkins et al., 2014, 2017). Although attempted by numerous studies, the elucidation of the molecular mechanism of the flavonoid-mediated response to drought stress remains elusive.
Heat shock factors (HSFs) are important components of the signal transduction chain that mediate the activation of genes responsive to diverse stresses and are common in bacteria, algae, animals, and plants (Nover et al., 2001; Scharf et al., 2012). Compared with other eukaryotes, which contain one to three HSFs, the plant HSF family shows more striking diversity in quantity, structure, and regulatory mechanisms, which may be due to the sessile lifestyle of plants and the continuous role of HSFs in stress tolerance throughout plant evolution (von Koskull-Döring et al., 2007; Ohama et al., 2016). The N-terminal region of the HSF protein sequence contains a highly conserved DNA-binding domain, which can accurately identify and bind the heat shock cis-elements (HSEs; nGAAnnTTCn or nTTCnnGAAn) of the downstream target gene promoter (Littlefield and Nelson, 1999). An oligomerization domain (or HR-A/B region) is linked to the DNA-binding domain by 15 to 80 amino acid residues. On the basis of the structural characteristics of the oligomerization domain region, HSFs in plants are classified into A, B, and C classes (Nover et al., 2001). Consistent with its nomenclature, the crucial role of HSFs in plants is the earliest and most widely studied function in heat tolerance. Initially in tomato (Solanum lycopersicum), three HSFs induced by heat stress were cloned and identified (Scharf et al., 1990). Subsequently, in Arabidopsis, group A1 (AtHSFA1a, AtHSFA1b, AtHSFA1d, and HSFA1e) and A2 (AtHSFA2) HSFs were shown to play important roles in heat shock response (Busch et al., 2005; Liu and Charng, 2013; Ohama et al., 2016). HSFA4 is considered to be a potent activator of heat stress-induced gene expression, whereas HSFA5 acts as a specific repressor of HSFA4 activity (Baniwal et al., 2007). In contrast, class B HSFs (AtHSFB1 and AtHSFB2b) are considered to be transcriptional inhibitors, which negatively regulate heat-induced HSFs (Ikeda et al., 2011). In addition to heat stress, HSFs also participate in salt (Xiang et al., 2013; Pérez-Salamó et al., 2014), drought (Sakuma et al., 2006; Hwang et al., 2014), strong light (Jung et al., 2013), and even cold (Zeng et al., 2016) stress responses. In the drought tolerance mechanism, AtHSFA3 can be transcriptionally induced by the Dehydration-Responsive Element Binding Protein2A (DREB2A) under drought and heat stress (Sakuma et al., 2006). HSFA6a, HSFA4, and HSFA9 have also been reported to play important roles in drought responses (Hwang et al., 2014; Personat et al., 2014). Although the role of HSFs in stress responses is well established, how HSFs participate in stress tolerance remains obscure, and many functions of HSFs require further investigation.
Apple (Malus domestica), as a widely cultivated and economically important fruit crop in temperate regions, has frequently suffered drought, hail, and other adverse climatic factors due to global climate anomalies in recent years (Richey and Barik, 2016). In contrast to annual and vegetable crops, fruit crops cannot be readily translocated owing to their perenniality and are vulnerable to multiple abiotic stresses over much of the production process, which may result in a decline in quality and yield. In this investigation, a previously unstudied drought-responsive HSF, designated MdHSFA8a, was cloned and identified from apple fruit. MdHSFA8a promoted the accumulation of flavonoids and the expression of flavonoid synthesis-related genes and also promoted ROS scavenging and plant survival under drought. In addition, the chaperone MdHSP90 (HEAT SHOCK PROTEIN90) was shown to interact with MdHSFA8a to inhibit its binding activity and transcriptional activation. However, under drought stress, the MdHSP90-MdHSFA8a complex dissociated and the released MdHSFA8a interacted with the APETALA2/ETHYLENE RESPONSIVE FACTOR (AP2/ERF) family transcription factor (TF) RELATED TO AP2.12 (MdRAP2.12) to activate downstream gene activity. Furthermore, we demonstrated that MdHSFA8a participated in ABA-induced stomatal closure and promoted the expression of ABA signaling-related genes. These findings elucidate a mechanism by which the stress-inducible TF MdHSFA8a modulates flavonoid synthesis and ABA signaling to regulate drought tolerance.
RESULTS
Drought Induces Flavonoid and Anthocyanin Accumulation in Apple
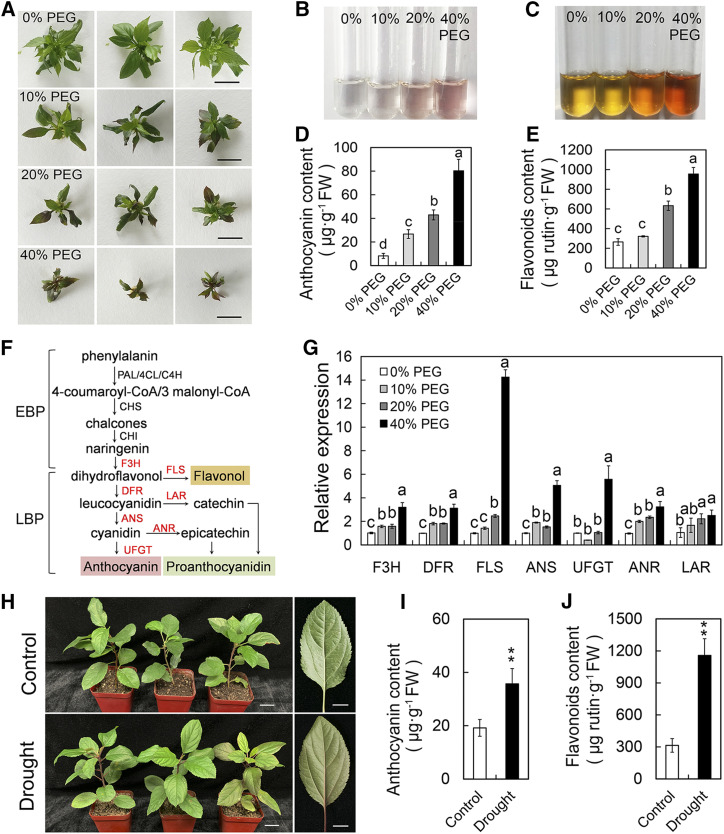
Previous studies indicated that drought stress can induce the accumulation of flavonoids and anthocyanins in tomato, Arabidopsis, rice (Oryza sativa), and wheat (Triticum aestivum; André et al., 2009; Lenka et al., 2011; Ma et al., 2014; Nakabayashi et al., 2014). In this study, we first examined if drought induced flavonoids and anthocyanin accumulation in apple plants. Solid Murashige and Skoog (MS) medium supplemented with different concentrations of polyethylene glycol 8000 (PEG) was used to simulate drought stress (Fig. 1A; Gopal and Iwama, 2007). The contents of anthocyanins and flavonoids in apple plants increased significantly after 15 d of simulated drought treatment, but the growth of the plants was significantly inhibited (Fig. 1, B–E). Reverse transcription quantitative PCR (RT-qPCR) analysis indicated that drought notably promoted the transcript levels of late biosynthetic genes of the flavonoid biosynthesis pathway, especially FLAVANOL SYNTHASE (MdFLS), ANTHOCYANIDIN SYNTHASE (MdANS), and FLAVONOID 3-O-GLUCOSYLTRANSFERASE (MdUFGT; Fig. 1, F and G). In addition, the contents of anthocyanins and flavonoids in apple plants also increased significantly after natural drought treatment, which was consistent with the results of simulated drought treatment (Fig. 1, H–J). These results confirmed that drought stress induced anthocyanin biosynthesis in apple and were consistent with previous findings.
Figure 1.
Drought induces flavonoid and anthocyanin accumulation in apple. A, Growth status of apple plants after simulated drought treatment. Different concentrations of PEG were used to simulate drought stress. Bars = 1 cm. B, Anthocyanins extracted in 1% (v/v) HCl-methanol. C, Flavonoids extracted in 1% (v/v) HCl-methanol and colored by NaNO2, Al(NO3)3, and NaOH. D and E, Anthocyanin (D) and flavonoid (E) contents in apple plants under simulated drought treatment. Values are means ± sd of three independent biological replicates. Different lowercase letters indicate significant differences by Tukey’s test using DPS software (P < 0.05). FW, Fresh weight. F, Simplified representation of the flavonoid biosynthetic pathway leading to the three major classes of end products: flavonols, proanthocyanidins, and anthocyanins. EBP, Early biosynthesis pathway; LBP, late biosynthesis pathway. G, Transcript levels of late biosynthesis pathway genes in apple plants under simulated drought treatments. MdActin was used as an internal control gene. Samples treated with 0% PEG were used as an internal standard. Values are means ± sd of three independent biological replicates. Different lowercase letters indicate significant differences by Tukey’s test using DPS software (P < 0.05). H, Wild-type apple ‘GL-3’ plants cultured in pots were subjected to natural drought treatment. Bars = 2 cm for the plants and 1 cm for the leaves. I and J, Anthocyanin (I) and flavonoid (J) contents in apple ‘GL-3’ plants under natural drought treatment. Values are means ± sd of three independent biological replicates. Asterisks indicate statistical significance by Tukey’s test using DPS software (**P < 0.01).
MdHSFA8a Is Involved in Drought-Induced Accumulation of Flavonoids and Anthocyanins
HSFs play crucial roles in plant drought tolerance (Sakuma et al., 2006; Hwang et al., 2014), but their function in flavonoid synthesis has not been explored previously. To accurately characterize the function of HSFs in flavonoid and anthocyanin accumulation in response to drought, we analyzed the expression levels of all members of the apple HSF family under drought stress. On the basis of previous studies and additional comparative analyses between the Arabidopsis HSF family and the apple genome (Giorno et al., 2012; Daccord et al., 2017), we screened 30 HSF family genes in apple (Supplemental Fig. S2). The RT-qPCR results showed that HSFA3a/b, HSFA4a/b/c, HSFA8a, HSFA9a, HSFB1a/b, and HSFC1a/b may be induced by drought to varying degrees, among which HSFA3a/b, HSFA4a, HSFA8a, and HSFC1a/b were significantly affected; their expression levels under 40% (w/v) PEG treatment were 4.13, 2.69, 13.02, and 5.92 times higher, respectively, than those of the control (Supplemental Fig. S3). The highest expression level of MdHSFA8a was detected by the unified analysis of these HSFs.
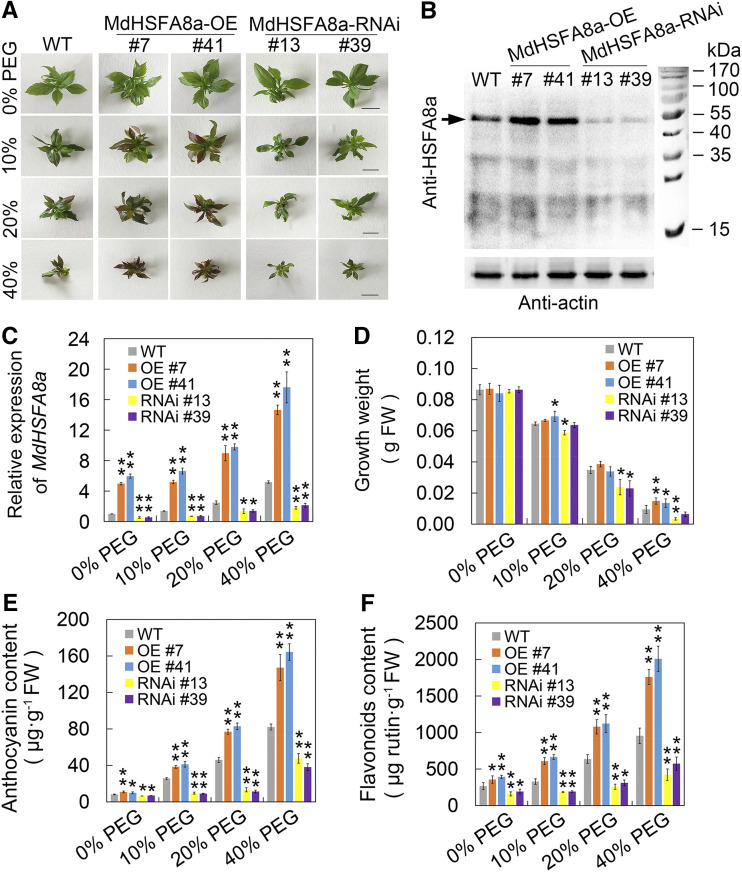
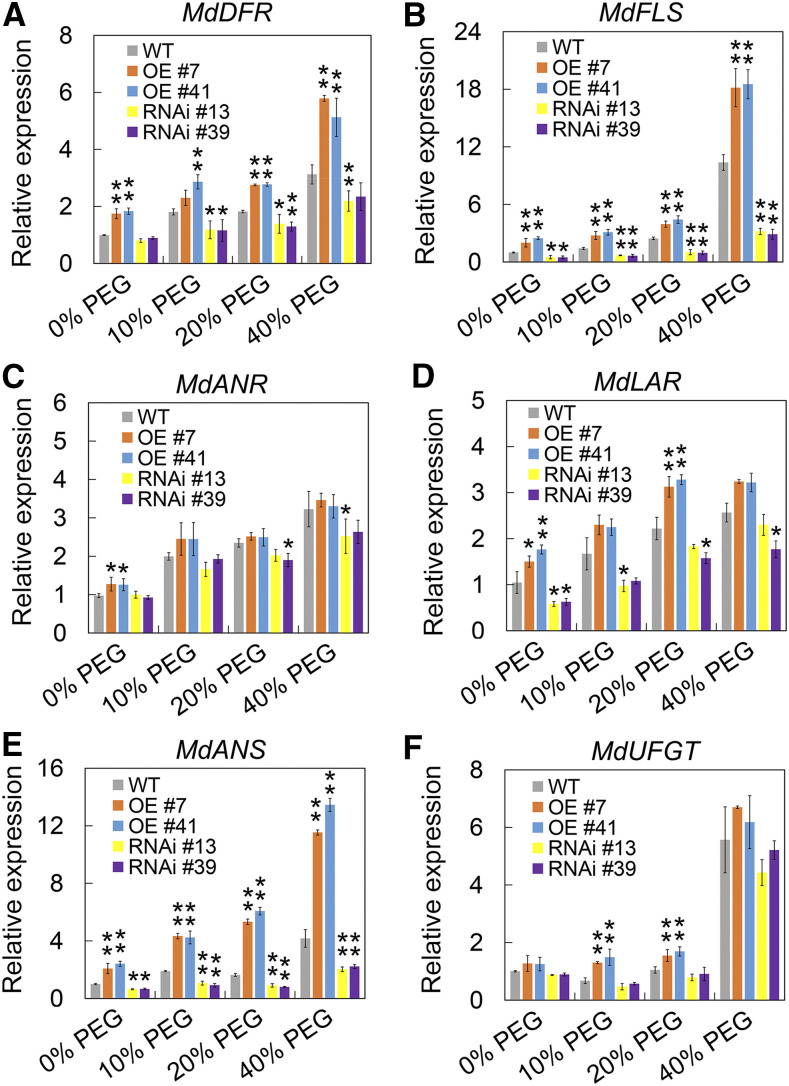
Unlike the majority of HSFs, HSFA8 is more sensitive to oxidative stress than heat stress and is considered to be a ROS and/or redox sensor in plants (Miller and Mittler, 2006). To evaluate the function of MdHSFA8a in drought-induced flavonoid synthesis, we generated transgenic lines that overexpressed MdHSFA8a (MdHSFA8a-OE) in the wild-type ‘GL-3’ apple plants. In addition, a double stranded RNA interference (RNAi) construct (MdHSFA8a-RNAi) containing 315 bp of the 3′ coding sequence (CDS) was also transformed into the wild type (Fig. 2A). Both OE and RNAi transgenic lines were evaluated, of which OE lines 7 and 41 and RNAi lines 13 and 39 showed substantially changed expression of MdHSFA8a at both the transcript and protein levels (Fig. 2, B and C). We then treated the transgenic apple plants with PEG-simulated drought stress and evaluated flavonoid and anthocyanin production. After drought treatment, especially in 40% PEG, the growth parameters of MdHSFA8a-OE apple plants were significantly higher than those of MdHSFA8a-RNAi, which suggested that MdHSFA8a-RNAi might inhibit the growth of apple plants (Fig. 2D). In addition, it was observed that MdHSFA8a-OE plants were reddish owing to anthocyanin accumulation (Fig. 2A). The MdHSFA8a-OE plants accumulated higher contents of flavonoids and anthocyanins than the wild-type control under drought treatment, whereas MdHSFA8a-RNAi plants accumulated lower contents (Fig. 2, E and F). The expression levels of flavonoid biosynthetic pathway genes were quantified, of which DIHYDROFLAVONOL REDUCTASE (MdDFR), MdFLS, and MdANS showed the same pattern as the change in flavonoid accumulation (Fig. 3). These results demonstrated that MdHSFA8a is required for drought-induced flavonoid biosynthesis and suggested that MdHSFA8a might regulate the transcription of MdDFR, MdFLS, and MdANS.
Figure 2.
MdHSFA8a is involved in drought-induced accumulation of flavonoids and anthocyanins. A, Wild-type (WT) apple ‘GL-3’ plants, MdHSFA8a-OE (OE), and MdHSFA8a-RNAi (RNAi) transgenic apple plants cultured under simulated drought stress. Bars = 1 cm. B, Detection of MdHSFA8a protein expression in wild-type, OE, and RNAi transgenic apple plants by immunoblotting with specific MdHSFA8a antibody. C, Transcript levels of MdHSFA8a in wild-type, OE, and RNAi transgenic apple plants under simulated drought treatments. MdActin was used as an internal control gene. Wild-type apple plants treated with 0% PEG were used as an internal standard. D, Growth parameters of wild-type, OE, and RNAi transgenic apple plants under simulated drought treatment. E and F, Anthocyanin and flavonoid contents in wild-type, OE, and RNAi transgenic apple plants under simulated drought treatment. FW, Fresh weight. Values are means ± sd of three independent biological replicates. Asterisks indicate statistical significance by Tukey’s test using DPS software (*P < 0.05 and **P < 0.01).
Figure 3.
Transcript levels of flavonoid biosynthesis pathway genes in apple plants under simulated drought stress. A, Transcript levels of MdDFR in wild-type (WT), OE, and RNAi transgenic apple plants under simulated drought treatment. B, Transcript levels of MdFLS in wild-type, OE, and RNAi transgenic apple plants under simulated drought treatment. C, Transcript levels of MdANR in wild-type, OE, and RNAi transgenic apple plants under simulated drought treatment. ANR, Anthocyanidin reductase. D, Transcript levels of MdLAR in wild-type, OE, and RNAi transgenic apple plants under simulated drought treatment. LAR, Leucoanthocyanidin reductase. E, Transcript levels of MdANS in wild-type, OE, and RNAi transgenic apple plants under simulated drought treatment. F, Transcript levels of MdUFGT in wild-type, OE, and RNAi transgenic apple plants under simulated drought treatment. MdActin was used as an internal control gene. Wild-type apple plants treated with 0% PEG were used as an internal standard. Values are means ± sd of three independent biological replicates. Asterisks indicate statistical significance by Tukey’s test using DPS software (*P < 0.05 and **P < 0.01).
MdHSFA8a Promotes Drought Tolerance and Flavonoid Accumulation and Stimulates ROS Scavenging under Natural Drought Conditions
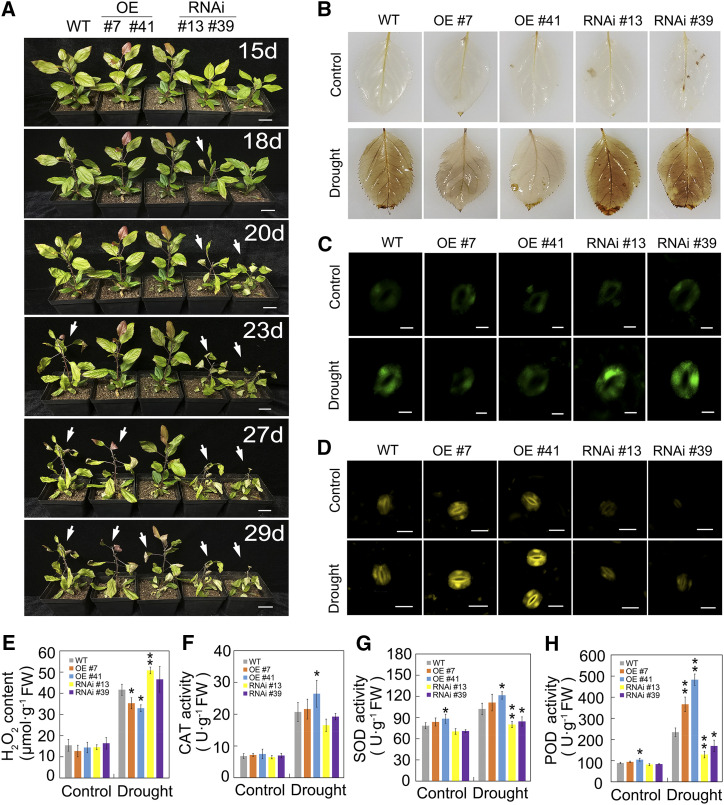
To further verify whether MdHSFA8a regulated flavonoid accumulation and drought tolerance, wild-type, MdHSFA8a-OE, and MdHSFA8a-RNAi apple plants cultured in pots were subjected to natural drought treatment. The initial weight of 400 g of saturated soil attained 70% water loss after 18 d and entered the drought state (Supplemental Fig. S4). The MdHSFA8a-RNAi plants wilted at 18 and 20 d under natural drought treatment, the wild-type plants wilted at 23 d, whereas the MdHSFA8a-OE plants wilted at 27 and 29 d (Fig. 4A). MdHSFA8a-OE plants showed the strongest drought tolerance, which demonstrated that HSFA8a played an important role in plant drought tolerance. Consistent with the tissue culture results, the MdHSFA8a-OE pot culture plants also accumulated higher contents of flavonoids and anthocyanins than the wild-type plants under drought stress, whereas MdHSFA8a-RNAi plants accumulated lower contents (Supplemental Fig. S5). In addition, MdHSFA8a-RNAi lines were distinctly smaller than the other lines after 15 d of growth under normal conditions. These results suggested that MdHSFA8a-RNAi might inhibit the growth of apple plants, which was consistent with previous reports (Liu et al., 2011).
Figure 4.
MdHSFA8a promotes drought tolerance and stimulates ROS scavenging under natural drought. A, Wild-type (WT), OE, and RNAi apple plants cultured in pots were subjected to natural drought treatment. Plants were photographed 15 d after water was withheld. Arrows indicate the appearance of a drought phenotype, such as leaf curling. Bars = 3 cm. B, DAB staining of wild-type, OE, and RNAi apple plants. Staining with DAB was performed on leaves 15 d after natural water loss. Leaves from normally watered plants served as the control. C, Fluorescence detection of ROS in guard cells of wild-type, OE, and RNAi apple plants using H2DCF-DA after natural drought treatment. Bars = 10 μm. D, Diphenylboric acid 2-aminoethylester (DPBA) staining in wild-type, OE, and RNAi apple leaves. Yellow fluorescence represents the relative flavonol contents in guard cells. Bars = 20 μm. E, H2O2 content in wild-type, OE, and RNAi apple plants under drought stress. F to H, CAT, SOD, and POD activities in wild-type, OE, and RNAi apple plants under drought stress. Values are means ± sd of three independent biological replicates. Asterisks indicate statistical significance by Tukey’s test using DPS software (*P < 0.05 and **P < 0.01). FW, Fresh weight.
Flavonoids, as an antioxidant, have been widely reported to scavenge ROS to protect plants from diverse abiotic stresses (Tattini et al., 2004; Li et al., 2017b; Xie et al., 2018). We evaluated ROS accumulation in wild-type, MdHSFA8a-OE, and MdHSFA8a-RNAi plants after drought treatment. Histochemical staining with 3,3′-diaminobenzidine tetrahydrochloride (DAB), which visualized ROS accumulation, revealed that MdHSFA8a-OE leaves accumulated less ROS than wild-type leaves, whereas MdHSFA8a-RNAi accumulated a higher abundance of ROS (Fig. 4B). Consistent with these observations, visualization of ROS in guard cells using 2,7-dihydrofluorescein diacetate (H2DCF-DA) and quantification of the hydrogen peroxide (H2O2) content showed the same pattern as DAB staining (Fig. 4, C and E). In contrast, the flavonol content in guard cells was significantly higher in MdHSFA8a-OE plants than in wild type and MdHSFA8a-RNAi plants, thus demonstrating that MdHSFA8a promoted ROS scavenging by regulating flavonoid synthesis (Fig. 4D). In addition, we detected the activities of catalase (CAT), superoxide dismutase (SOD), and peroxidase (POD), which are typical antioxidant enzymes that scavenge ROS (Gill and Tuteja, 2010). Activities of the antioxidant enzymes under drought stress were higher in MdHSFA8a-OE plants and lower in MdHSFA8a-RNAi plants compared with those of the wild type. This finding suggested that, in addition to flavonoid synthesis, MdHSFA8a may also promote ROS scavenging by modulating the activities of antioxidant enzymes (Fig. 4, F–H).
Maintaining the intracellular ion balance is an important characteristic of plants to tolerate drought stress. For example, K+ plays an important role in regulating osmotic balance, enzyme activity, and stomatal closure (Minguet-Parramona et al., 2016; Jezek and Blatt, 2017). We measured the accumulation of K+ and Na+ ions in leaves of transgenic and wild-type apple plants grown under control or drought stress conditions. Drought stress disrupted the ion balance of apple leaves and reduced the K+/Na+ ratio significantly. Compared with wild-type and MdHSFA8a-RNAi plants, MdHSFA8a-OE plants were more conducive to maintenance of the ion balance under drought stress and, thus, showed stronger drought tolerance (Supplemental Fig. S6).
MdHSFA8a Enhances the Transcription of MdMYB12, MdANS, and MdFLS by Binding to Their Promoters
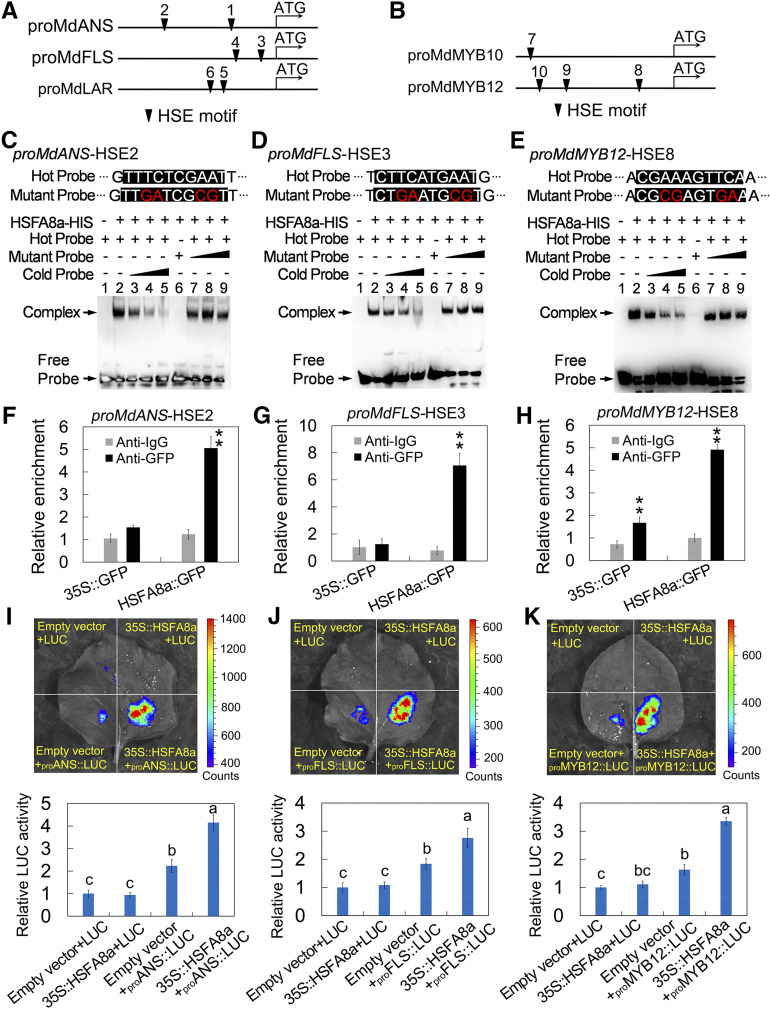
In addition to the genes encoding crucial enzymes, flavonoid synthesis is predominantly regulated by a variety of TFs, among which MYB TFs are considered to be important regulators. MdMYB1 (MdMYB10), MdMYB9, MdMYB11, MdMYB12, MdMYB22, MdMYB23, and MdMYBPA1 have been reported to participate in flavonoid synthesis in apple (Takos et al., 2006; Espley et al., 2009; An et al., 2015, 2018, Wang et al., 2017a, 2018). Thus, we conducted a yeast one-hybrid assay to investigate the binding of MdHSFA8a to the promoter of crucial enzyme genes and MYB TFs. MdHSFA8a bound to the promoters of MdMYB12, MdFLS, MdANS, and MdLAR (Supplemental Fig. S7). Next, we analyzed the HSE cis-elements in the promoter of flavonoid pathway genes and observed that MdANS (H1, −462; H2, −1,245), MdFLS (H3, −166; H4, −446), and MdLAR (H5, −518; H6, −642) each contained two HSEs (Fig. 5A). The promoters of MdMYB10 and MdMYB12 contained one HSE (H7, −1,716) and three HSEs (H8, −407; H9, −1,327; H10, −1,472; Fig. 5B). To confirm these results, we purified the MdHSFA8a protein and performed an electrophoretic mobility shift assay (EMSA). The EMSA results showed that MdHSFA8a combined with the promoters of MdANS (H2), MdFLS (H3), and MdMYB12 (H8; Supplemental Fig. S8). When the unlabeled probe was added as a competitor, the binding of MdHSFA8a to the promoter was distinctly outcompeted, whereas when the competitive probe contained two mutated nucleotides, the binding of MdHSFA8a was not affected (Fig. 5, C–E).
Figure 5.
MdHSFA8a enhances the transcription of MdMYB12, MdANS, and MdFLS by binding to their promoters. A and B, Characteristics of HSEs in the promoters of crucial enzyme genes (A) and MYB TFs (B) associated with flavonoid synthesis. C to E, EMSA showing the binding of MdHSFA8a to the candidate HSE motif in MdANS (C), MdFLS (D), and MdMYB12 (E) promoters. Hot probe was a biotin-labeled fragment containing the HSE motif. Cold probe was a nonlabeled competitive probe (100-fold that of hot probe). Mutant probe contained two nucleotide mutations. F to H, ChIP-qPCR assay showing the binding of MdHSFA8a to the candidate HSE motif in the promoters of MdANS (F), MdFLS (G), and MdMYB12 (H) in vivo. Cross-linked chromatin samples were extracted from MdHSFA8a::GFP transgenic calli and precipitated with anti-GFP. The ChIP assay was repeated three times, and enriched DNA fragments in each ChIP were used as one biological replicate for qPCR. Values are means ± sd of three independent biological replicates. Asterisks indicate statistical significance by Tukey’s test using DPS software (**P < 0.01). I to K, Effects of MdHSFA8a on the promoter activities of MdANS (I), MdFLS (J), and MdMYB12 (K) as demonstrated by a dual-luciferase reporter assay in tobacco leaves. Values are means ± sd of three independent biological replicates. Different lowercase letters indicate significant differences by Tukey’s test using DPS software (P < 0.05).
To verify in vivo binding of MdHSFA8a to the promoters of MdMYB12, MdANS, and MdFLS, a chromatin immunoprecipitation (ChIP)-qPCR assay was performed using transgenic apple calli overexpressing MdHSFA8a fused to a GFP tag. Apple calli expressing the empty vector carrying the GFP tag were used as a negative control. The regions containing HSE cis-elements in the promoter of MdMYB12, MdANS, and MdFLS and their enrichment levels in the DNA coimmunoprecipitated with the GFP antibody or a nonspecific antibody (preimmune mouse IgG) were examined. MdHSFA8a substantially enhanced the enrichment levels of the promotor regions containing HSE cis-elements (Fig. 5, F–H), which indicated that MdHSFA8a bound to the candidate HSEs in the promoters of MdMYB12, MdANS, and MdFLS in vivo.
Next, we validated the effects of MdHSFA8a on the promoter activity of MdMYB12, MdANS, and MdFLS using a transient luciferase (LUC) complementation imaging assay in tobacco (Nicotiana tabacum) leaves. The promoter sequences of MdMYB12, MdANS, and MdFLS were inserted individually into the pGreenII 0800-LUC vector, which was fused to a LUC reporter gene. The CDS of MdHSFA8a was fused to the pGreenII 62-SK vector as an effector. Luminescence detection revealed that coexpression of 35S::MdHSFA8a with proMdANS::LUC exhibited remarkably stronger luminescence intensity compared with the individual expression of proMdANS::LUC and the negative controls (Fig. 5I). Identical results were obtained for the detection of the promoter activity of proMdFLS::LUC and proMdFMYB12::LUC (Fig. 5, J and K). These results indicated that MdHSFA8a positively activated the expression of MdMYB12, MdANS, and MdFLS.
MdHSFA8a Interacts with MdHSP90 and MdRAP2.12
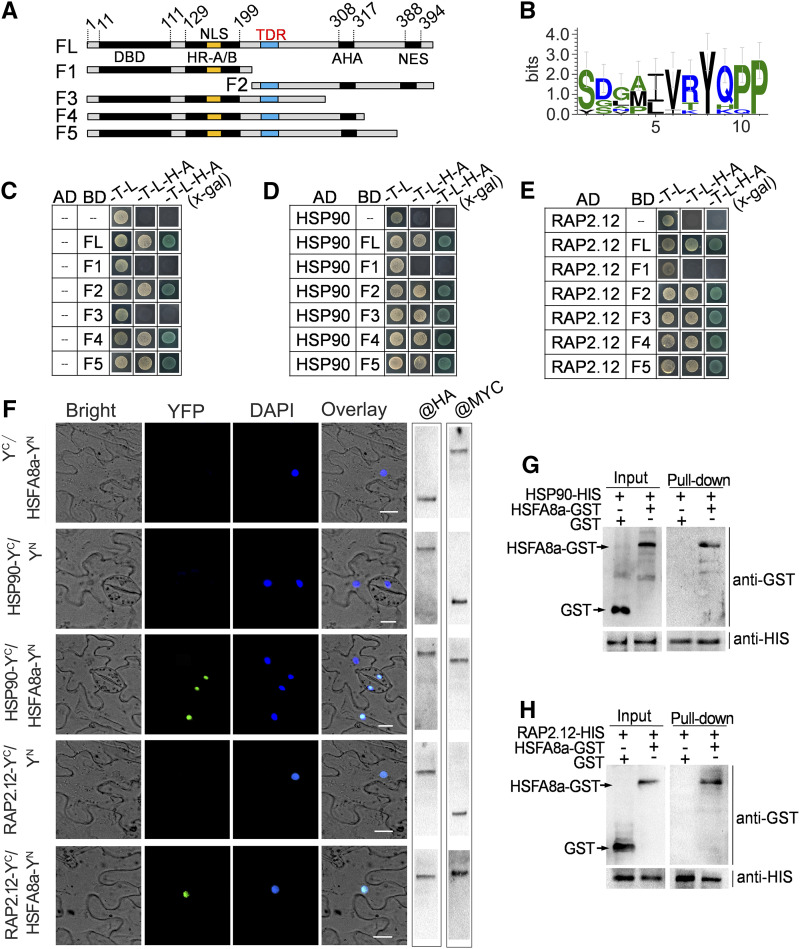
The HSF TFs typically interact with transcriptional cofactors and/or chaperones for transcriptional regulation (Scharf et al., 1998; Díaz-Martín et al., 2005; Ohama et al., 2016). To explore potential cofactors that interact with MdHSFA8a, a yeast two-hybrid (Y2H) screening assay through a cDNA library was performed. MdHSP90 (XM_008381972.3) and MdRAP2.12 (NP_001280975.1) were identified as potential MdHSFA8a-interacting proteins.
To verify the interaction between MdHSFA8a, MdHSP90, and MdRAP2.12, Y2H assays were performed. The recombinant pGBKT7 plasmids containing the full-length MdHSFA8a showed self-activating activities. We then divided MdHSFA8a into five fragments (F1–F5) and observed that F1 and F3 showed no self-activating activities, which indicated that the AHA motif (rich in aromatic, hydrophobic, and acidic amino acid residues) may be the self-activation domain (Fig. 6A). The Y2H results showed that F3 interacted with MdHSP90 and MdRAP2.12, whereas F1 showed no interaction (Fig. 6, C–E). The corresponding immunoblot analysis was performed to verify the expression of the interaction partner (Supplemental Fig. S9). The results indicated that the missing fragment of F1 compared with F3 contained the interaction domain of MdHSFA8a, which may determine its interaction with other proteins. Interestingly, this interaction domain also contained a conserved sequence (I/LVRYQPP; Fig. 6B; Supplemental Fig. S10), which was similar to the previously reported TDR domain (Ohama et al., 2016).
Figure 6.
Interaction of MdHSFA8a with chaperone MdHSP90 and the AP2/ERF TF MdRAP2.12. A, The full-length (FL) CDS of MdHSFA8a was divided into five fragments (F1–F5): AHA, Motif rich in aromatic, hydrophobic, and acidic amino acid residues; DBD, DNA-binding domain; HR-A/B, oligomerization domain; NES, nuclear export; NLS, nuclear import. The amino acid positions of fragments are numbered. B, Conserved sequence analysis of the interaction domain in MdHSFA8a, which is similar to the previously reported temperature-dependent repression (TDR) domain. C to E, Y2H assays showing the interaction of MdHSFA8a with MdHSP90 and MdRAP2.12. The F1 and F3 domains showed no self-activating activity (C). F3 interacted with MdHSP90 (D) and MdRAP2.12 (E), whereas F1 showed no interaction. F, BiFC assay showing the interaction of MdHSFA8a with MdHSP90 and MdRAP2.12 in vivo. The YFP field indicates fluorescence signals; the DAPI (4', 6-diamidino-2-phenylindole) field indicates the locations of nuclei. The right gels show the expression of the fusion protein examined by western blotting with anti-MYC and anti-HA antibodies. Bars = 10 μm. G and H, Pull-down assay was performed by copurifying recombinant HSP90-HIS and RAP2.12-HIS fusion proteins with HSFA8a-GST and GST empty vector. Western blotting with a GST antibody showed that HSFA8a was pulled down by HSP90-HIS and RAP2.12-HIS.
Bimolecular fluorescence complementation (BiFC) assays were performed to confirm the interactions in vivo. The corresponding immunoblot analysis was performed to examine the expression of the fusion protein with anti-MYC and anti-HA antibodies. MdHSFA8a interacted with both MdHSP90 and MdRAP2.12 (Fig. 6F). Pull-down assays by copurification of the reconstructed MdHSP90- and MdRAP2.12-His (HIS) fusion proteins with MdHSFA8a-GST and the GST empty vector were conducted. MdHSFA8a was observed to be pulled down by MdHSP90 and MdRAP2.12, which indicated that MdHSFA8a interacts with MdHSP90 and MdRAP2.12 (Fig. 6, G and H).
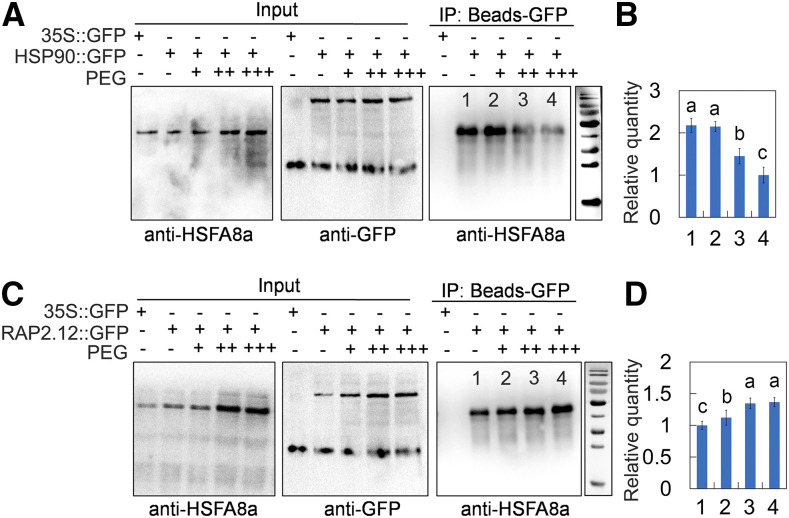
Drought Induces Degradation of the MdHSFA8-MdHSP90 Protein Complex
To investigate whether the MdHSFA8-MdHSP90 and MdRAP2.12-MdHSFA8a protein complexes were mediated by drought stress, an in vivo coimmunoprecipitation (CoIP) assay was conducted. The transgenic apple calli overexpressing MdHSP90 and MdRAP2.12 fused to a GFP tag were used for immunoadsorption of a GFP antibody, and the specific MdHSFA8a antibody (Abmart) was used to detect the immunoprecipitated MdHSFA8a protein. MdHSFA8a proteins in calli were immunoprecipitated by MdHSP90-GFP and MdRAP2.12-GFP, which was consistent with the Y2H, BiFC, and pull-down results. However, with the increase in PEG concentration, fewer MdHSFA8a proteins were immunoprecipitated by MdHSP90-GFP under drought, which indicated that drought induced degradation of the MdHSFA8a-MdHSP90 protein complex (Fig. 7, A and B). By contrast, a greater quantity of MdHSFA8a proteins were immunoprecipitated by MdRAP2.12-GFP under drought (Fig. 7, C and D). However, because the MdHSFA8a protein itself was induced by drought, it cannot be concluded that drought induced the formation of the MdHSFA8a-MdRAP2.12 protein complex.
Figure 7.
Effects of drought on MdHSFA8a-MdHSP90 and MdHSFA8a-MdRAP2.12 protein complexes. A, CoIP detection of the interaction between MdHSFA8a and MdHSP90 in vivo under drought treatment. MdHSP90::GFP transgenic calli were used. Immunoprecipitation (IP) samples were assayed using specific anti-HSFA8a. B, Relative quantitative analysis of immunoblotting proteins. Bars 1 to 4 correspond to channels 1 to 4 in A, respectively. Channel 1, MdHSP90::GFP calli cultured under normal conditions; channels 2 to 4, MdHSP90::GFP calli cultured under 10%, 20%, and 40% PEG treatment. C, CoIP detection of the interaction between MdHSFA8a and MdRAP2.12 in vivo under drought treatment. MdRAP2.12::GFP transgenic calli were used. CoIP was conducted as in A. D, Relative quantitative analysis of western-blotting proteins was conducted. Bars 1 to 4 correspond to channels 1 to 4 in C, respectively. Channel 1, MdRAP2.12::GFP calli cultured under normal conditions; channels 2 to 4, MdRAP2.12::GFP calli cultured under 10%, 20%, and 40% PEG treatment. In B and D, values are means ± sd of three independent biological replicates. Different lowercase letters indicate significant differences by Tukey’s test using DPS software (P < 0.05).
To further clarify the regulatory effect of MdHSFA8a-MdHSP90 on flavonoid accumulation and drought stress, we overexpressed and RNAi silenced MdHSP90 in OE-MdHSFA8a calli and obtained OE-MdHSFA8a+OE-MdHSP90 and OE-MdHSFA8a+RNAi-MdHSP90 cotransgenic calli. Through direct interaction with MdHSFA8a, MdHSP90 inhibited flavonoid synthesis and drought tolerance regulated by MdHSFA8a (Supplemental Fig. S11). In addition, the subcellular localization of MdHSFA8a and MdHSP90 was analyzed. The results showed that MdHSFA8a was predominantly expressed in the nucleus of epidermal and guard cells and MdHSP90 was mainly expressed in the plasma membrane and nucleus of epidermal and guard cells (Supplemental Fig. S12).
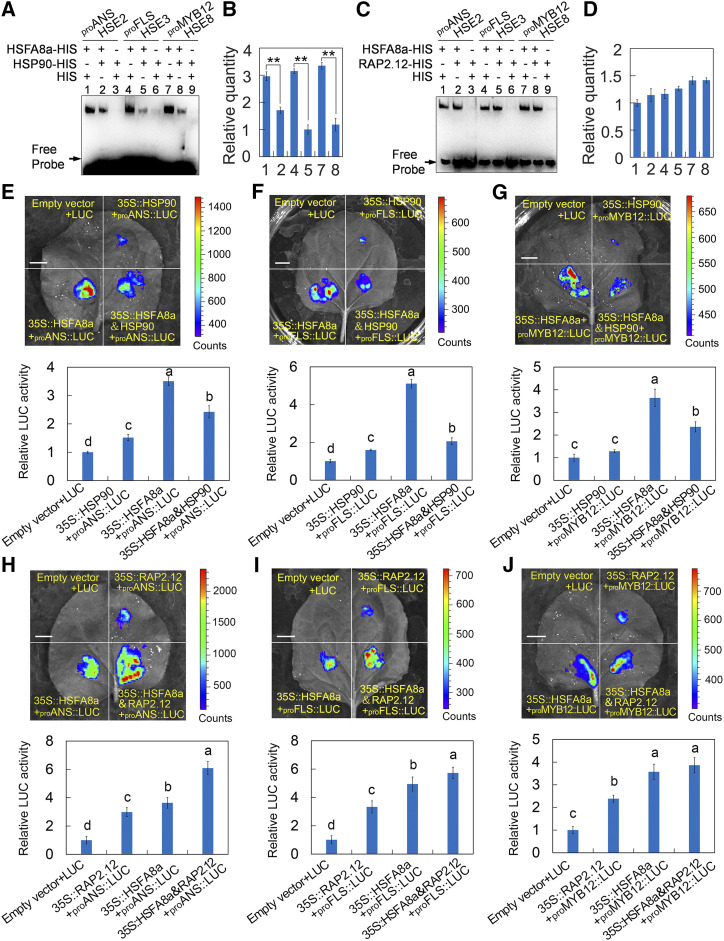
MdHSP90 and MdRAP2.12 Mediate the DNA Binding and Transcriptional Activity of MdHSFA8
The function of HSP90 as a negative regulator of HSFs has been reported in animals and plants (Zou et al., 1998; Hahn et al., 2011). We therefore examined the MdHSFA8-MdHSP90 complex for binding and transcriptional activity of MdHSFA8a. To validate the binding activity, an EMSA was performed using MdHSFA8a-HIS and MdHSP90-HIS fusion proteins. The HIS protein alone was used to standardize the amount of protein in each tube. The binding strength of MdHSFA8a to the promoters of MdMYB12, MdANS, and MdFLS was significantly inhibited with the addition of MdHSP90 (Fig. 8, A and B), which suggested that MdHSP90 suppressed the binding activity of MdHSFA8a. To validate the transcriptional activity, a transient LUC complementation imaging assay was performed. The CDS of MdHSP90 was fused to the pGreenII 62-SK vector as a coeffector. Luminescence detection revealed that coexpression of 35S::MdHSFA8+35S::MdHSP90 with proMdANS::LUC exhibited remarkably weaker luminescence intensity compared with the individual expression of 35S::MdHSFA8a with proMdANS::LUC (Fig. 8E). Identical results were obtained for the detection of the promoter activity of proMdFLS::LUC and proMdFMYB12::LUC (Fig. 8, F and G). These results indicated that MdHSP90, through direct interaction with MdHSFA8a, not only inhibited the latter’s DNA-binding activity but also inhibited its transcriptional activation of downstream target genes.
Figure 8.
Effects of drought on MdHSFA8a-MdHSP90 and MdHSFA8a-MdRAP2.12 protein complexes. A, EMSA showing that the binding strength of MdHSFA8a to the MdMYB12, MdANS, and MdFLS promoters was significantly inhibited with the addition of MdHSP90. Empty HIS protein was used to ensure that each tube contained the same amount of protein. B, Relative quantitative analysis of proteins bound to the probe. Values are means ± sd of three independent repeated experiments. Asterisks indicate statistical significance by Tukey’s test using DPS software (** P < 0.01). C, EMSA showing the binding strength of MdHSFA8a to the MdMYB12, MdANS, and MdFLS promoters with the addition of MdRAP2.12. EMSA was conducted as in A. D, Relative quantitative analysis of proteins bound to the probe. Values are means ± sd of three independent repeated experiments. E to G, Dual-luciferase reporter assay showing the transcriptional activation of MdHSFA8a to the MdANS (E), MdFLS (F), and MdMYB12 (G) promoters with the addition of MdHSP90. H to J, Dual-luciferase reporter assay showing the transcriptional activation of MdHSFA8a to the MdANS (H), MdFLS (I), and MdMYB12 (J) promoters with the addition of MdRAP2.12. Values are means ± sd of three independent biological replicates. Different lowercase letters indicate significant differences by Tukey’s test using DPS software (P < 0.05). Bars = 1 cm.
In the same manner, we also determined the effect of MdRAP2.12 on the DNA-binding and transcriptional activation activities of MdHSFA8a. The EMSA revealed that the binding strength of MdHSFA8a to the promoters of MdMYB12, MdANS, and MdFLS was unchanged with the addition of MdRAP2.12 (Fig. 8, C and D), which indicated that MdRAP2.12 did not affect the DNA-binding activity of MdHSFA8a. In contrast, a transient LUC complementation imaging assay showed that MdRAP2.12 significantly enhanced the transcriptional activation by MdHSFA8a of the downstream target genes MdMYB12, MdANS, and MdFLS (Fig. 8, H–J).
MdHSFA8a Participates in ABA-Mediated Stomatal Movement to Regulate Drought Response
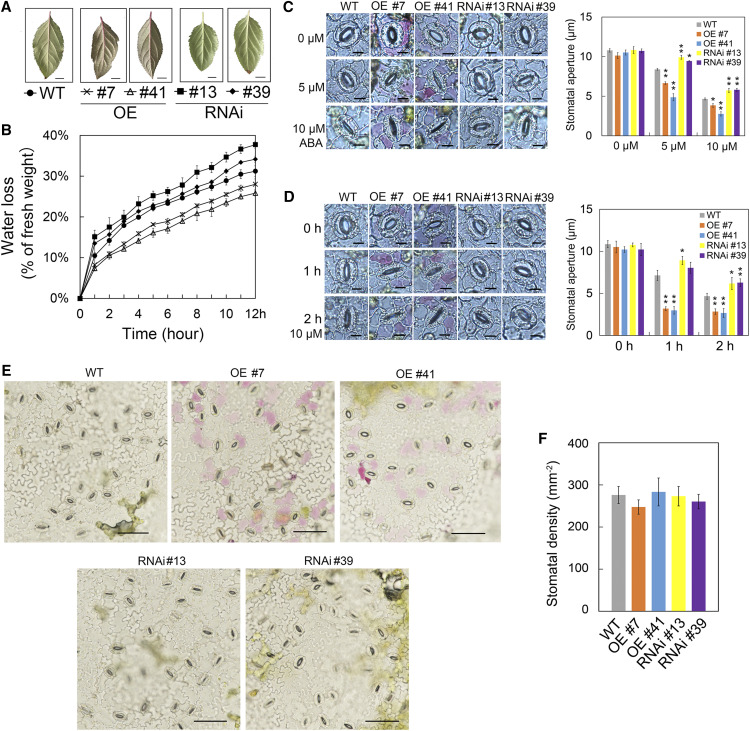
In the regulatory network for plant drought tolerance, the ABA signaling pathway plays an important role (Cutler et al., 2010). Given that MdHSFA8a enhanced drought tolerance by promoting the accumulation of flavonoids and anthocyanins, we further investigated whether MdHSFA8a can participate in the ABA-mediated drought response. By means of natural water loss assays, we observed that detached leaves of MdHSFA8a-OE plants lost water more slowly than leaves of the wild type, whereas detached leaves of MdHSFA8a-RNAi plants lost water more quickly (Fig. 9, A and B). Given that leaf water loss is mainly controlled by stomata (Verslues et al., 2006), we explored the effect of MdHSFA8a on ABA-induced stomatal movement. Detached leaves with preopened stomata were treated with 0, 5, or 10 μm ABA before the stomatal apertures were observed and measured. In addition, we monitored the time course of stomatal apertures of plant leaves treated with 10 μm ABA for 0, 1, and 2 h. The results showed that the leaves of MdHSFA8a-OE plants responded more quickly to ABA, and the stomata closed completely after ABA treatment for 1 h. By contrast, MdHSFA8a-RNAi leaves were significantly slower to respond to ABA, and the stomata were still not completely closed after ABA treatment for 2 h (Fig. 9, C and D). Stomatal density did not differ significantly between MdHSFA8a transgenic plants and wild-type plants, which suggested that MdHSFA8a did not affect stomatal density (Fig. 9, E and F). We incidentally observed that anthocyanin accumulation in MdHSFA8a-OE leaves was generally concentrated around guard cells and not in other mesophyll cells. We speculated that this might be due to overexpression around the guard cells of MdHSFA8a induced by drought and ABA, which then promoted anthocyanin accumulation. Whether anthocyanins around guard cells participate in stomatal movement requires further study (Supplemental Fig. S13).
Figure 9.
MdHSFA8a participates in ABA-mediated stomatal movement to regulate drought response. A, Detached leaves of wild-type (WT), OE, and RNAi apple plants. Bars = 1 cm. B, Natural water loss assays in detached leaves of wild-type, OE, and RNAi apple plants at 24°C for 12 h. Three leaves from three plants were mixed for each measurement. Values are means ± sd of three independent biological replicates. C, Stomatal apertures of wild-type, MdHSFA8a-OE, and MdHSFA8a-RNAi apple plants in response to exogenous ABA treatment. Stomatal apertures were observed and measured after treatment with 0, 5, or 10 μm ABA for 2 h and were calculated from 100 stomata from leaves of three different apple plants. D, Time-course data for stomatal apertures of different plant leaves treated with 10 μm ABA for 0, 1, and 2 h. The stomatal aperture was calculated for 100 stomata from leaves of three different apple plants. Values are means ± sd of three independent biological replicates. Asterisks indicate statistical significance by Tukey’s test using DPS software (*P < 0.05 and **P < 0.01). Bars = 10 μm. E, Images showing the number of stomata in wild-type, MdHSFA8a-OE, and MdHSFA8a-RNAi apple plants. Bars = 50 μm. F, Stomatal density in the leaves of wild-type, MdHSFA8a-OE, and MdHSFA8a-RNAi apple plants. Stomatal density was calculated from 10 images taken from different parts of the leaf epidermis. Values are means ± sd of three independent biological replicates.
To further investigate the role of MdHSFA8a in the ABA signaling pathway, we examined the expression of ABA signaling-related genes and drought-responsive marker genes (Verslues et al., 2006; Harb et al., 2010). MdHSFA8a had no effect on the expression level of MdNCED1, a crucial gene in ABA synthesis (Xiong and Zhu, 2003), or on the expression level of ABA-responsive factors (e.g. ABI1, ABI2, ABF3, and ABI5). However, MdHSFA8a significantly affected the expression levels of the ABA receptor genes MdPLY3 and MdPLY4 (Supplemental Fig. S14). In addition, MdHSFA8a significantly promoted the expression of the Ser-Thr kinase OPEN STOMATA1, which encodes a SnRK2-type protein kinase that participates in ABA-mediated stomatal closure (Lee et al., 2009). These results suggested that MdHSFA8a may be involved in ABA-mediated stomatal closure and drought response.
DISCUSSION
Flavonoids are secondary metabolites that have interacted with biotic and abiotic stresses throughout the evolution of plants; consequently, the synthesis of flavonoids is induced by diverse environmental stresses (Winkel-Shirley, 2002). Conversely, flavonoid accumulation can lead to feedback regulation of plant stress tolerance (Hernández et al., 2009). In the past decade, studies on drought-induced flavonoid synthesis have achieved substantial progress in understanding physiological phenotypes to molecular mechanisms. In this study, we identified an HSF, MdHSFA8a, from apple that was significantly induced by drought stress. Overexpression of MdHSFA8a significantly increased flavonoid and anthocyanin accumulation and enhanced drought stress tolerance in transgenic apple plants.
HSFs are important elements for plants to tolerate various abiotic stresses and to respond to stress signals (von Koskull-Döring et al., 2007). The HSFs HSFA1b, HSFA3, HSFA4, HSFA6, and HSFA9 have been reported to be involved in drought tolerance (Sakuma et al., 2006; Bechtold et al., 2013; Hwang et al., 2014; Personat et al., 2014; Lang et al., 2017), but their functions and regulatory mechanisms are complex and variable among plant species. For example, the response of HSFA3 to drought and heat stress is determined by DREB2A, a member of the AP2/ERF TF family (Sakuma et al., 2006; von Koskull-Döring et al., 2007). In contrast, AtHSFA6a is involved in the response to high salinity and dehydration stress as a transcription activator via the ABA-dependent signaling pathway (Hwang et al., 2014). BnHSFA4a regulates the expression of BnGolS and BnRS2 and affects the accumulation of galactinol and raffinose to enhance desiccation tolerance in seeds (Lang et al., 2017). Moreover, the same HSF TF may show different or even opposite regulatory functions in different species, as is the case for HSFB2b. Overexpression of OsHSFB2b in rice significantly decreased tolerance to drought and salt stress, whereas overexpression of GmHSFB2b in soybean (Glycine max) promoted salt tolerance by enhancing flavonoid accumulation and inhibiting the repressor gene GmNAC2 (Xiang et al., 2013; Bian et al., 2020).
In this study, we demonstrated that MdHSFA8a can improve drought tolerance through the promotion of flavonoid accumulation, which differed from the regulatory models of HSFA3, HSFA4, and HSFA6 reported previously (Sakuma et al., 2006; Hwang et al., 2014; Lang et al., 2017). The regulation of flavonoid synthesis by HSFs can only be captured by a transcriptome analysis (Li et al., 2017a; Wang et al., 2017b). Recently, soybean HSFB2b was shown to promote salt tolerance by regulating flavonoid synthesis (Bian et al., 2020), which is consistent with the results here for MdHSFA8a regulating drought tolerance. HSFB2b can bind to the HSE cis-elements and promotes the expression of GmCHS and GmFLS (Bian et al., 2020). Similarly, in this study, we observed that HSFA8a regulated the transcriptional activity of MdANS and MdFLS, which indicated that HSFs exhibit the function of directly binding to and regulating structural genes of the flavonoid synthesis pathway. In addition, we observed that MdHSFA8a bound to and regulated the transcription activity of MdMYB12, which we identified as a critical regulator of flavanol synthesis in a previous study (Wang et al., 2017a).
In plants, the regulatory network of HSF response to abiotic stress is cooperatively controlled by different HSF members and the interaction between HSFs and other chaperones (von Koskull-Döring et al., 2007; Scharf et al., 2012). Among HSFs, HSPs, such as HSP70, HSP90, HSP101, and small HSPs, can directly interact with HSFs and interfere with their biological functions (Port et al., 2004; Hahn et al., 2011). It is generally considered that under normal conditions, HSFs are kept inactive by interaction with HSPs. However, when stress triggers an imbalance in protein homeostasis in plant cells, HSPs will be recruited by denatured and misfolded proteins to act as chaperones, thus releasing the previously inhibited HSFs and activating the expression of downstream stress-response genes (Hahn et al., 2011; Scharf et al., 2012; Ohama et al., 2016). In tomato, under a nonstress temperature, HSFA1 interacts with HSP70 to remain inactive, whereas HSFB1 interacts with HSP90 and is degraded by the proteasome to be maintained at a low concentration. However, after heat shock treatment, HSP70 and HSP90 are recruited competitively by denatured proteins, resulting in the release and activation of HSFA1 and the rapid accumulation of HSFB1, which coregulates the expression of heat response-related genes (Hahn et al., 2011). In Arabidopsis, the central region of HSFA1d contains a crucial regulatory domain, designated the TDR domain, which inhibits the activation of HSFA1d by interacting with HSP70 and HSP90 (Ohama et al., 2016). In this study, we screened an HSP, MdHSP90, which interacted with MdHSFA8a. Based on the segmental analysis of HSFA8a, we observed that the missing fragment of F1 compared with F3 contained the interaction domain of MdHSFA8a, which determined its interaction with MdHSP90. Interestingly, this domain is similar to the previously reported TDR domain and also contains a conserved sequence (I/LVRYQPP). Through direct interaction with MdHSFA8a, MdHSP90 also inhibited the binding activity and transcription activity of MdHSFA8a on downstream target genes, which is consistent with previous research (Ohama et al., 2016). Similar to degradation of the HSF-HSP complex caused by heat stress, we observed that drought stress can also induce MdHSP90 to be competitively bound, leading to dissociation of the MdHSFA8a-MdHSP90 complex.
In addition to binding with molecular chaperone HSPs, the interaction between HSFs and other factors is rarely reported in the HSF regulatory network, among which the AP2/ERF family of TFs are the most typical. In Arabidopsis, AtHSFA3, which is the only HSF family member that can be activated by the AP2/ERF family TF DREB2A, regulates and participates in the response to heat stress (Sakuma et al., 2006; Schramm et al., 2008). In sunflower (Helianthus annuus), HaHSFA9 and DREB2 form a transcription complex to synergistically regulate the transcription of HaHSP17.6G1, so as to ensure normal growth and development of embryos (Díaz-Martín et al., 2005). In loquat (Eriobotrya japonica), EjHSF3 can directly interact with EjAP2-1, thus inhibiting the combination of EjMYB1 and EjMYB2 with the downstream lignin synthesis genes, thereby reducing lignin accumulation and lignification of fruit in response to low temperature (Zeng et al., 2016). In this study, we screened an AP2/ERF family TF, MdRAP2.12, which interacted with MdHSFA8a. In contrast to the function of MdHSP90, MdRAP2.12 did not affect the DNA-binding activity of MdHSFA8a but significantly enhanced the transcriptional activation by MdHSFA8a of the downstream target genes MdMYB12, MdANS, and MdFLS.
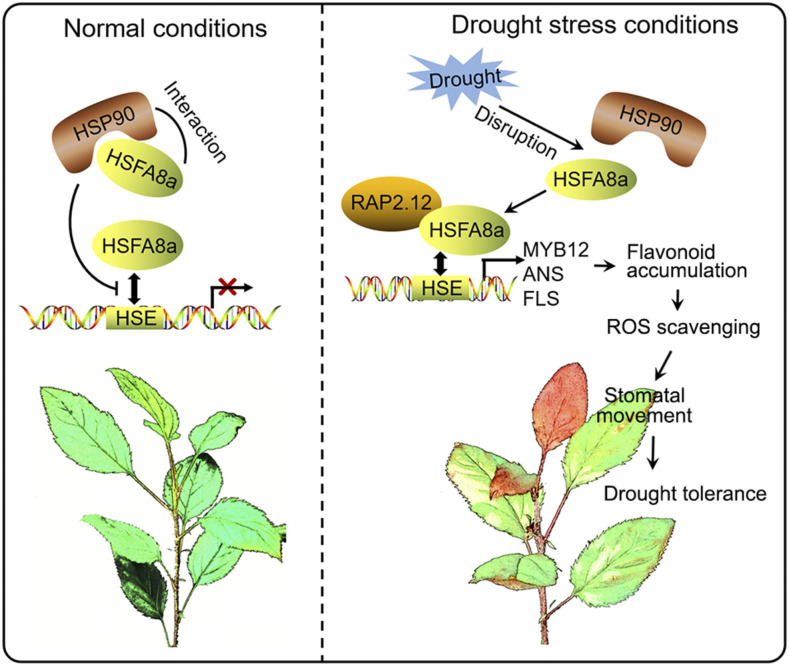
In addition to regulating the expression of stress-related genes, the regulatory mechanism of HSFs involved in stress responses also includes the perception of stress signals and signal transduction. HSFA4 was first identified as a sensor of the H2O2 signal, which can transmit ROS signal induced by stress to the downstream ZAT, WRKY, and other families of TFs, and then induce the response network to reduce the damage caused by ROS (Davletova et al., 2005; Miller and Mittler, 2006). In addition, HSFA8a is considered as a possible ROS and/or redox sensor in plants and is more sensitive to oxidative stress than heat stress (Miller and Mittler, 2006; Giesguth et al., 2015). In Arabidopsis, AtHSFA6a is induced by exogenous ABA, drought, and salt stress and is transcriptionally regulated by ABF2 (AREB1), ABF3, and AREB3, which are critical regulators of the ABA signaling pathway (Hwang et al., 2014). AtHSFA6b, however, is not only regulated by AREB1 but also can directly bind to the promoter of DREB2A and enhance its expression, thereby connecting ABA signaling and ABA-mediated heat responses (Huang et al., 2016). In this study, we observed that MdHSFA8a positively regulates ABA-mediated stomatal closure and drought-responsive genes to promote plant survival during drought. Figure 10 summarizes the proposed function of MdHSFA8a in drought response and tolerance in apple by modulating flavonoid synthesis and the ABA signaling pathway.
Figure 10.
Proposed model for MdHSFA8a modulation of flavonoid synthesis to regulate drought tolerance in apple. Under normal conditions, MdHSP90 interacts with MdHSFA8a to inhibit the binding activity and transcription activity of MdHSFA8a on downstream target genes. Under drought stress, the MdHSP90-MdHSFA8a complex dissociates and the released MdHSFA8a further interacts with the AP2/ERF family TF MdRAP2.12 to activate downstream gene activity. Solid arrows show positive regulation.
In summary, this study provides insight into the function of MdHSFA8a in flavonoid accumulation and drought response. MdHSFA8a directly binds to the promoters of MdMYB12, MdANS, and MdFLS and enhances their expression, participates in ABA-induced stomatal closure, and promotes ROS scavenging and plant survival under drought. MdHSP90 interacts with MdHSFA8a to inhibit its binding activity and transcriptional activation, but under drought stress the MdHSP90-MdHSFA8a complex dissociates and the released MdHSFA8a further interacts with the AP2/ERF family TF MdRAP2.12 to activate downstream gene activity. The identification of the interaction partners and target genes of MdHSFA8a is helpful to further understand the mechanisms by which HSFs control flavonoid synthesis and drought responses.
MATERIALS AND METHODS
Plant Materials and Treatments
An offspring of apple (Malus domestica) ‘Royal Gala’ that gives higher regeneration capacity, named ‘GL-3’, and calli induced from apple ‘Orin’ were used for genetic transformation. The cv GL-3 plants were grown on MS medium containing 0.2 mg L−1 indoleacetic acid and 0.5 mg L−1 6-benzylaminopurine under long-day conditions (16 h of light/8 h of dark) at 24°C and were subcultured every 30 d. The cv Orin calli were grown on MS medium containing 0.2 mg L−1 indoleacetic acid and 0.5 mg L−1 6-benzylaminopurine in the dark at 24°C and were subcultured every 20 d.
Solid MS medium supplemented with different concentrations of PEG was used to simulate drought stress (Gopal and Iwama, 2007). In addition, rooted wild-type and transgenic apple plants were transferred to soil and used for drought treatment by natural dehydration. For each drought-treated sample, at least three biological replicates were used. All experiments were performed at least three times.
Phylogenetic Analysis
A phylogenetic tree was constructed from protein sequences for 21 Arabidopsis (Arabidopsis thaliana) HSFs and 30 apple HSFs. The apple HSFs were obtained from the Genome Database for Rosaceae (http://www.rosaceae.org/) or National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/) database, and the Arabidopsis HSFs were obtained from the TAIR database (http://www.arabidopsis.org/). MEGA-X software (https://www.megasoftware.net/) was used to construct the phylogenetic tree with 1,000 bootstrap replicates after aligning sequences with ClustalW (opening = 10, extension = 0.2).
Determination of Anthocyanin and Flavonoid Contents
The determination of anthocyanins and flavonoids was based on a previously described method (Wang et al., 2017a, 2018). Flavonoids were extracted from 1 g of powdered apple plants and then incubated in 10 mL of 1% (v/v) HCl-methanol for 4 h at 4°C. The absorbance of the solution was measured using a spectrophotometer (UV-2450; Shimadzu) at 510 nm. Rutin (Sigma) was used as the master standard. Anthocyanins were extracted from 0.5 g of powdered apple plants and then incubated in 5 mL of 1% (v/v) HCl-methanol at 4°C in the dark for 24 h. The absorbance of the solution was determined at 510 and 700 nm. The anthocyanin content was calculated using the pH-differential method.
Transformation of Apple Plants
To generate overexpression plants, the CDS of MdHSFA8a was cloned and introduced into the pRI101-AN plasmid harboring the cauliflower mosaic virus 35S promoter and a GFP tag. To generate RNAi constructs, 420-bp sense and antisense C-terminal fragments of MdHSFA8a were cofused into the pFGC-1008 plasmid. The leaves of apple plants were infected by incubation with transformed Agrobacterium tumefaciens for 20 to 30 min and cocultured on MS medium without antibiotics in the dark at 24°C for 5 to 10 d. The infected leaves containing the pRI101-AN plasmid were placed on screening medium containing 250 mg L−1 carbenicillin (Solarbio) and 50 mg L−1 kanamycin (Solarbio). The infected leaves containing the pFGC-1008 plasmid were placed on screening medium containing 250 mg L−1 carbenicillin (Solarbio) and 15 mg L−1 hygromycin B (Roche). The primers used are listed in Supplemental Table S1.
RNA Extraction and RT-qPCR
The isolation of total RNA from wild-type and transgenic apple plants and the synthesis of first-strand cDNA were performed as previously described (Wang et al., 2018). The primers for RT-qPCR were synthesized by Sangon Biotech (Supplemental Table S2). RT-qPCR consisted of three biological and three technical replicates and was performed using an iCycler iQ5 system (Bio-Rad). Transcript levels were calculated using the 2−ΔΔCt method.
DAB Staining and Determination of H2O2 Content and POD, CAT, and SOD Activities
Detached leaves were placed in a culture dish containing DAB staining solution with a concentration of 1 mg mL−1 (0.01% [v/v] Triton X-100, pH 3.8) and vacuum infiltrated for 10 to 20 min. The leaves were incubated in the dark for 6 to 8 h and then in the light for 1 h until reddish-brown spots appeared. The leaves were washed with distilled, deionized water, decolorizing solution (ethanol:lactic acid:glycerin, 3:1:1) was added, and decolorization was performed in an 80°C water bath until the leaves no longer contained chlorophyll.
The determination of H2O2 content and POD, CAT, and SOD activities was conducted using assay kits following the manufacturer’s instructions (Solarbio; catalog nos. BC3595, BC0200, BC0090, and BC0170).
DPBA Staining of Flavonol
Detached leaves were placed in a culture dish containing DPBA (Sigma) staining solution with a concentration of 2.52 mg mL−1 (0.02% Triton X-100) for 3 h. The leaves were then washed with distilled, deionized water for 1 min. The fluorescence was detected using an epifluorescence microscope (Olympus BX53F) with an excitation wavelength of 340 to 380 nm.
Fluorescence Detection of ROS
A fluorogenic ROS sensor, H2DCF-DA, was used to detect ROS content in guard cells. Lower epidermal strips were transferred to 5 mL of loading buffer (containing 10 mmol L−1 Tris and 50 mmol L−1 KCl, pH 6.5), to which 5 μL of H2DCF-DA was added to make a final concentration of 50 μmol L−1, and incubated in the dark for 30 to 60 min. The leaves were then washed with loading buffer three times. The fluorescence was detected using an epifluorescence microscope (Olympus BX53F) with an excitation wavelength of 488 nm.
Yeast One-Hybrid Assay
The CDS of MdHSFA8a was inserted into the pGADT7 plasmid (Clontech). The promoter sequences of MdFLS, MdANS, MdANR, MdLAR, MdUFGT, MdMYB1 (MdMYB10), MdMYB9, MdMYB11, and MdMYB12 were inserted into the pHIS2 plasmid (Wang et al., 2017a, 2018). The background leakiness of the pHIS2 plasmid was suppressed using 3-amino-1,2,4-triazole. The Y187 (Clontech) yeast strain harboring the recombinant pGADT7 and pHIS2 plasmids was spotted onto medium lacking Trp, His, and Leu to determine the interactions. The empty pGADT7 plasmid was used as the control. The primers used are listed in Supplemental Table S1.
EMSA
The CDSs of MdHSFA8a, MdHSP90, and MdRAP2.12 were cloned and introduced into the pET32a plasmid. The recombinant plasmids were transformed into Escherichia coli strain BL21 (Tiangen) for protein induction and purification. EMSA was conducted using the LightShift Chemiluminescent EMSA Kit (Thermo Scientific) following the manufacturer’s instructions. The biotin-labeled promoter sequences are listed in Supplemental Table S3. Unlabeled probes were used as competitors. The mutant probes were labeled and contained two mutated nucleotides.
ChIP-qPCR Analysis
The transgenic apple calli harboring MdHSFA8a fused to a GFP tag were prepared for the ChIP-qPCR assay. The ChIP assays were performed using the EZ-ChIP 244 Chromatin Immunoprecipitation Kit (Upstate) as previously described (Wang et al., 2018). The enrichment levels of the promoter regions containing the HSE cis-element in the DNA coimmunoprecipitated with the GFP antibody or a nonspecific antibody (preimmune mouse IgG) were determined by qPCR. The primers used are listed in Supplemental Table S2.
Dual-Luciferase Reporter Assay
The CDSs of MdHSFA8a, MdHSP90, and MdRAP2.12 were inserted into the pGreenII 62-SK plasmid as effectors. The promoter sequences of MdMYB12, MdANS, and MdFLS were recombined into the pGreenII 0800-LUC plasmid as reporters. Recombinant plasmids were cotransformed into tobacco (Nicotiana tabacum) leaves by A. tumefaciens-mediated genetic transformation. A living imaging analysis system (NightOWL II LB983; Berthold) was used for luminescence detection. The primers used are listed in Supplemental Table S1.
Y2H Assay
The CDS of MdHSFA8a was divided into five fragments (F1–F5). The full-length CDS and each of the five fragments were separately recombined into the pGBKT7 plasmid. The CDSs of MdHSP90 and MdRAP2.12 were recombined into the pGADT7 plasmid. The Y2HGold yeast strain harboring the recombinant pGADT7 and pGBKT7 plasmids was grown on selective medium lacking Trp and Leu and lacking Trp, Leu, His, and adenine. Expression of the fusion protein was examined by western blotting with anti-HA and anti-FLAG antibodies. The primers used are listed in Supplemental Table S1.
BiFC Assay
The CDS of MdHSFA8a without stop codons was recombined into the pSPYCE-35S plasmid. The CDSs of MdHSP90 and MdRAP2.12 were recombined into the pSPYNE-35S plasmid. The BiFC assays were conducted as previously described (Wang et al., 2018). Expression of the fusion protein was examined by western blotting with anti-HA and anti-MYC antibodies. The primers used are listed in Supplemental Table S1.
Pull-Down Assay
The CDS of MdHSFA8a was inserted into the GST-tag pGEX-4T-1 plasmid. The CDSs of MdHSP90 and MdRAP2.12 were recombined into the HIS-tag pET-32a (+) plasmid. The pull-down assays were conducted using the His-Tagged Protein Purification Kit (Clontech) as previously described (Wang et al., 2018). The primers used are listed in Supplemental Table S1.
CoIP Assay
The transgenic apple calli harboring MdHSP90 and MdRAP2.12 fused to GFP tags were prepared for the CoIP assay. Total proteins were extracted from each transgenic callus cultured under nonstress and drought conditions using the Plant Protein Extraction Reagent (ComWin Biotech). The extracted protein was mixed with anti-GFP (Abmart) and incubated for 2 h before the addition of agarose beads (Sigma). After incubation for 2 h, the beads were rinsed four times with immunoprecipitation buffer and analyzed with a GFP antibody and a specific HSFA8 antibody (Abmart).
Analysis of Stomatal Characteristics
For ABA-induced stomatal closure, apple leaves were floated on stomatal opening buffer (containing 10 mm CaCl2, 50 mm KCl, and 5 mm MES, pH 6.15) for 3 h in the light to preopen stomata. The leaves were subsequently transferred to control buffer (0.1% [v/v] ethanol) or buffers containing either 0, 5, or 10 μm ABA for 0, 1, and 2 h. Lower epidermal strips were collected for measurement of stomatal apertures. The stomatal aperture was calculated for 100 stomata from leaves of three different apple plants. The stomatal density was determined using young leaves of transgenic and wild-type plants. Stomatal density was calculated from 10 images captured from different parts of the leaf epidermis, and the average per leaf was calculated. All measurements were made using ImageJ software.
Statistical Analysis
All experiments were performed in triplicate. Error bars show the sd of three replicates. Values are means ± sd of three independent biological replicates. Significant differences were detected by Tukey’s test using DPS software. Differences were considered significant at *P < 0.05 and **P < 0.01.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL and National Center for Biotechnology Information databases under the following accession numbers: MdHSFA8a (MDP0000191541), MdMYB12 (MDP0000887107), MdMYB10 (MDP0000259614), MdANS (NM_001293838.1), MdFLS (NM_001319250.1), MdHSF90 (XM_008381972.3), and MdRAP2.12 (NP_001280975.1).
Supplemental Data
The following supplemental materials are available
Supplemental Figure S1. General structure of flavonoids.
Supplemental Figure S2. Phylogenetic tree constructed from protein sequences for 21 Arabidopsis HSFs and 30 apple HSFs.
Supplemental Figure S3. Expression levels of 30 screened HSFs in apple under simulated drought treatment.
Supplemental Figure S4. Natural water loss assays of 400 g of saturated soil at 24°C for 30 d.
Supplemental Figure S5. MdHSFA8a participates in flavonoid synthesis in apple plants.
Supplemental Figure S6. Ion content in leaves of wild-type, MdHSFA8a-OE, and MdHSFA8a-RNAi apple plants.
Supplemental Figure S7. Yeast one-hybrid assay showing binding of HSFA8a to the promoters of MdMYB12, MdFLS, MdANS, and MdLAR.
Supplemental Figure S8. EMSA showing the binding of MdHSFA8a to the HSE motif in the promoters of crucial enzyme genes and MYB TFs.
Supplemental Figure S9. Expression of the fusion protein examined by immunoblotting with anti-HA and anti-FLAG antibodies.
Supplemental Figure S10. Protein sequence alignment of HSFA8 from different species.
Supplemental Figure S11. MdHSFA8a and MdHSP90 participate in flavonoid synthesis in apple calli.
Supplemental Figure S12. Subcellular localization of MdHSFA8a and MdHSP90 in tobacco leaves.
Supplemental Figure S13. Anthocyanin accumulation in MdHSFA8a-OE leaves was generally concentrated around guard cells.
Supplemental Figure S14. Expression of ABA signaling-related genes and drought-responsive marker genes.
Supplemental Table S1. Primers used for gene isolation and construction of plasmids containing coding and promoter sequences.
Supplemental Table S2. Primers used for RT-qPCR and ChIP-qPCR analyses.
Supplemental Table S3. Primers used to synthesize probes for EMSA.
Acknowledgments
We thank the Yujin Hao laboratories for providing the plasmids. We thank Shujing Wu and Liwen Bianji (Edanz Group China) for editing the English text of a draft of this article.
Footnotes
This work was supported by the Agricultural Variety Improvement Project of Shandong Province (grant no. 2019LZGC007), the National Key Research Project (grant no. 2016YFC0501505), and the National Natural Science Foundation of China (grant no. 31730080).
References
- Agati G, Tattini M(2010) Multiple functional roles of flavonoids in photoprotection. New Phytol 186: 786–793 [DOI] [PubMed] [Google Scholar]
- An JP, Li R, Qu FJ, You CX, Wang XF, Hao YJ(2018) R2R3-MYB transcription factor MdMYB23 is involved in the cold tolerance and proanthocyanidin accumulation in apple. Plant J 96: 562–577 [DOI] [PubMed] [Google Scholar]
- An XH, Tian Y, Chen KQ, Liu XJ, Liu DD, Xie XB, Cheng CG, Cong PH, Hao YJ(2015) MdMYB9 and MdMYB11 are involved in the regulation of the JA-induced biosynthesis of anthocyanin and proanthocyanidin in apples. Plant Cell Physiol 56: 650–662 [DOI] [PubMed] [Google Scholar]
- André CM, Schafleitner R, Legay S, Lefèvre I, Aliaga CAA, Nomberto G, Hoffmann L, Hausman JF, Larondelle Y, Evers D(2009) Gene expression changes related to the production of phenolic compounds in potato tubers grown under drought stress. Phytochemistry 70: 1107–1116 [DOI] [PubMed] [Google Scholar]
- Baniwal SK, Chan KY, Scharf KD, Nover L(2007) Role of heat stress transcription factor HsfA5 as specific repressor of HsfA4. J Biol Chem 282: 3605–3613 [DOI] [PubMed] [Google Scholar]
- Bechtold U, Albihlal WS, Lawson T, Fryer MJ, Sparrow PA, Richard F, Persad R, Bowden L, Hickman R, Martin C, et al. (2013) Arabidopsis HEAT SHOCK TRANSCRIPTION FACTORA1b overexpression enhances water productivity, resistance to drought, and infection. J Exp Bot 64: 3467–3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian XH, Li W, Niu CF, Wei W, Hu Y, Han JQ, Lu X, Tao JJ, Jin M, Qin H, et al. (2020) A class B heat shock factor selected for during soybean domestication contributes to salt tolerance by promoting flavonoid biosynthesis. New Phytol 225: 268–283 [DOI] [PubMed] [Google Scholar]
- Busch W, Wunderlich M, Schöffl F(2005) Identification of novel heat shock factor-dependent genes and biochemical pathways in Arabidopsis thaliana. Plant J 41: 1–14 [DOI] [PubMed] [Google Scholar]
- Castellarin SD, Matthews MA, Di Gaspero G, Gambetta GA(2007) Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta 227: 101–112 [DOI] [PubMed] [Google Scholar]
- Choudhury FK, Rivero RM, Blumwald E, Mittler R(2017) Reactive oxygen species, abiotic stress and stress combination. Plant J 90: 856–867 [DOI] [PubMed] [Google Scholar]
- Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR(2010) Abscisic acid: Emergence of a core signaling network. Annu Rev Plant Biol 61: 651–679 [DOI] [PubMed] [Google Scholar]
- Daccord N, Celton JM, Linsmith G, Becker C, Choisne N, Schijlen E, van de Geest H, Bianco L, Micheletti D, Velasco R, et al. (2017) High-quality de novo assembly of the apple genome and methylome dynamics of early fruit development. Nat Genet 49: 1099–1106 [DOI] [PubMed] [Google Scholar]
- Davletova S, Rizhsky L, Liang H, Shengqiang Z, Oliver DJ, Coutu J, Shulaev V, Schlauch K, Mittler R(2005) Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 17: 268–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Martín J, Almoguera C, Prieto-Dapena P, Espinosa JM, Jordano J(2005) Functional interaction between two transcription factors involved in the developmental regulation of a small heat stress protein gene promoter. Plant Physiol 139: 1483–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espley RV, Brendolise C, Chagné D, Kutty-Amma S, Green S, Volz R, Putterill J, Schouten HJ, Gardiner SE, Hellens RP, et al. (2009) Multiple repeats of a promoter segment causes transcription factor autoregulation in red apples. Plant Cell 21: 168–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedoroff NV, Battisti DS, Beachy RN, Cooper PJ, Fischhoff DA, Hodges CN, Knauf VC, Lobell D, Mazur BJ, Molden D, et al. (2010) Radically rethinking agriculture for the 21st century. Science 327: 833–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Chinnusamy V, Rodrigues A, Rubio S, Antoni R, Park SY, Cutler SR, Sheen J, Rodriguez PL, Zhu JK(2009) In vitro reconstitution of an abscisic acid signalling pathway. Nature 462: 660–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furihata T, Maruyama K, Fujita Y, Umezawa T, Yoshida R, Shinozaki K, Yamaguchi-Shinozaki K(2006) Abscisic acid-dependent multisite phosphorylation regulates the activity of a transcription activator AREB1. Proc Natl Acad Sci USA 103: 1988–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesguth M, Sahm A, Simon S, Dietz KJ(2015) Redox-dependent translocation of the heat shock transcription factor AtHSFA8 from the cytosol to the nucleus in Arabidopsis thaliana. FEBS Lett 589: 718–725 [DOI] [PubMed] [Google Scholar]
- Gilbert N.(2012) Drought devastates US crops. Nature 11065 [Google Scholar]
- Gill SS, Tuteja N(2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48: 909–930 [DOI] [PubMed] [Google Scholar]
- Giorno F, Guerriero G, Baric S, Mariani C(2012) Heat shock transcriptional factors in Malus domestica: Identification, classification and expression analysis. BMC Genomics 13: 639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal J, Iwama K(2007) In vitro screening of potato against water-stress mediated through sorbitol and polyethylene glycol. Plant Cell Rep 26: 693–700 [DOI] [PubMed] [Google Scholar]
- Hahn A, Bublak D, Schleiff E, Scharf KD(2011) Crosstalk between Hsp90 and Hsp70 chaperones and heat stress transcription factors in tomato. Plant Cell 23: 741–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harb A, Krishnan A, Ambavaram MM, Pereira A(2010) Molecular and physiological analysis of drought stress in Arabidopsis reveals early responses leading to acclimation in plant growth. Plant Physiol 154: 1254–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández I, Alegre L, Van Breusegem F, Munné-Bosch S(2009) How relevant are flavonoids as antioxidants in plants? Trends Plant Sci 14: 125–132 [DOI] [PubMed] [Google Scholar]
- Huang YC, Niu CY, Yang CR, Jinn TL(2016) The heat stress factor HSFA6b connects ABA signaling and ABA-mediated heat responses. Plant Physiol 172: 1182–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SM, Kim DW, Woo MS, Jeong HS, Son YS, Akhter S, Choi GJ, Bahk JD(2014) Functional characterization of Arabidopsis HsfA6a as a heat-shock transcription factor under high salinity and dehydration conditions. Plant Cell Environ 37: 1202–1222 [DOI] [PubMed] [Google Scholar]
- Ikeda M, Mitsuda N, Ohme-Takagi M(2011) Arabidopsis HsfB1 and HsfB2b act as repressors of the expression of heat-inducible Hsfs but positively regulate the acquired thermotolerance. Plant Physiol 157: 1243–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezek M, Blatt MR(2017) The membrane transport system of the guard cell and its integration for stomatal dynamics. Plant Physiol 174: 487–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HS, Crisp PA, Estavillo GM, Cole B, Hong F, Mockler TC, Pogson BJ, Chory J(2013) Subset of heat-shock transcription factors required for the early response of Arabidopsis to excess light. Proc Natl Acad Sci USA 110: 14474–14479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn M, Peterek S, Mock HP, Heyer AG, Hincha DK(2008) Heterosis in the freezing tolerance, and sugar and flavonoid contents of crosses between Arabidopsis thaliana accessions of widely varying freezing tolerance. Plant Cell Environ 31: 813–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasensky J, Jonak C(2012) Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J Exp Bot 63: 1593–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang S, Liu X, Xue H, Li X, Wang X(2017) Functional characterization of BnHSFA4a as a heat shock transcription factor in controlling the re-establishment of desiccation tolerance in seeds. J Exp Bot 68: 2361–2375 [DOI] [PubMed] [Google Scholar]
- Lee SC, Lan W, Buchanan BB, Luan S(2009) A protein kinase-phosphatase pair interacts with an ion channel to regulate ABA signaling in plant guard cells. Proc Natl Acad Sci USA 106: 21419–21424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenka SK, Katiyar A, Chinnusamy V, Bansal KC(2011) Comparative analysis of drought-responsive transcriptome in Indica rice genotypes with contrasting drought tolerance. Plant Biotechnol J 9: 315–327 [DOI] [PubMed] [Google Scholar]
- Lewis DR, Ramirez MV, Miller ND, Vallabhaneni P, Ray WK, Helm RF, Winkel BSJ, Muday GK(2011) Auxin and ethylene induce flavonol accumulation through distinct transcriptional networks. Plant Physiol 156: 144–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Ou-Lee TM, Raba R, Amundson RG, Last RL(1993) Arabidopsis flavonoid mutants are hypersensitive to UV-B irradiation. Plant Cell 5: 171–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Ren L, Gao Z, Jiang M, Liu Y, Zhou L, He Y, Chen H(2017a) Combined transcriptomic and proteomic analysis constructs a new model for light-induced anthocyanin biosynthesis in eggplant (Solanum melongena L.). Plant Cell Environ 40: 3069–3087 [DOI] [PubMed] [Google Scholar]
- Li P, Li YJ, Zhang FJ, Zhang GZ, Jiang XY, Yu HM, Hou BK(2017b) The Arabidopsis UDP-glycosyltransferases UGT79B2 and UGT79B3, contribute to cold, salt and drought stress tolerance via modulating anthocyanin accumulation. Plant J 89: 85–103 [DOI] [PubMed] [Google Scholar]
- Littlefield O, Nelson HC(1999) A new use for the ‘wing’ of the ‘winged’ helix-turn-helix motif in the HSF-DNA cocrystal. Nat Struct Biol 6: 464–470 [DOI] [PubMed] [Google Scholar]
- Liu HC, Charng YY(2013) Common and distinct functions of Arabidopsis class A1 and A2 heat shock factors in diverse abiotic stress responses and development. Plant Physiol 163: 276–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HC, Liao HT, Charng YY(2011) The role of class A1 heat shock factors (HSFA1s) in response to heat and other stresses in Arabidopsis. Plant Cell Environ 34: 738–751 [DOI] [PubMed] [Google Scholar]
- Loreti E, Povero G, Novi G, Solfanelli C, Alpi A, Perata P(2008) Gibberellins, jasmonate and abscisic acid modulate the sucrose-induced expression of anthocyanin biosynthetic genes in Arabidopsis. New Phytol 179: 1004–1016 [DOI] [PubMed] [Google Scholar]
- Ma D, Sun D, Wang C, Li Y, Guo T(2014) Expression of flavonoid biosynthesis genes and accumulation of flavonoid in wheat leaves in response to drought stress. Plant Physiol Biochem 80: 60–66 [DOI] [PubMed] [Google Scholar]
- Miao Y, Lv D, Wang P, Wang XC, Chen J, Miao C, Song CP(2006) An Arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses. Plant Cell 18: 2749–2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Mittler R(2006) Could heat shock transcription factors function as hydrogen peroxide sensors in plants? Ann Bot 98: 279–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minguet-Parramona C, Wang Y, Hills A, Vialet-Chabrand S, Griffiths H, Rogers S, Lawson T, Lew VL, Blatt MR(2016) An optimal frequency in Ca2+ oscillations for stomatal closure is an emergent property of ion transport in guard cells. Plant Physiol 170: 33–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakabayashi R, Yonekura-Sakakibara K, Urano K, Suzuki M, Yamada Y, Nishizawa T, Matsuda F, Kojima M, Sakakibara H, Shinozaki K, et al. (2014) Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J 77: 367–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nover L, Bharti K, Döring P, Mishra SK, Ganguli A, Scharf KD(2001) Arabidopsis and the heat stress transcription factor world: How many heat stress transcription factors do we need? Cell Stress Chaperones 6: 177–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohama N, Kusakabe K, Mizoi J, Zhao H, Kidokoro S, Koizumi S, Takahashi F, Ishida T, Yanagisawa S, Shinozaki K, et al. (2016) The transcriptional cascade in the heat stress response of Arabidopsis is strictly regulated at the level of transcription factor expression. Plant Cell 28: 181–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Salamó I, Papdi C, Rigó G, Zsigmond L, Vilela B, Lumbreras V, Nagy I, Horváth B, Domoki M, Darula Z, et al. (2014) The heat shock factor A4A confers salt tolerance and is regulated by oxidative stress and the mitogen-activated protein kinases MPK3 and MPK6. Plant Physiol 165: 319–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Personat JM, Tejedor-Cano J, Prieto-Dapena P, Almoguera C, Jordano J(2014) Co-overexpression of two Heat Shock Factors results in enhanced seed longevity and in synergistic effects on seedling tolerance to severe dehydration and oxidative stress. BMC Plant Biol 14: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Port M, Tripp J, Zielinski D, Weber C, Heerklotz D, Winkelhaus S, Bublak D, Scharf KD(2004) Role of Hsp17.4-CII as coregulator and cytoplasmic retention factor of tomato heat stress transcription factor HsfA2. Plant Physiol 135: 1457–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richey A, Barik MG (2016) Quantifying the role of groundwater for drought mitigation in Washington State. American Geophysical Union Fall Meeting, abstract H53C-1725
- Sakuma Y, Maruyama K, Qin F, Osakabe Y, Shinozaki K, Yamaguchi-Shinozaki K(2006) Dual function of an Arabidopsis transcription factor DREB2A in water-stress-responsive and heat-stress-responsive gene expression. Proc Natl Acad Sci USA 103: 18822–18827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf KD, Berberich T, Ebersberger I, Nover L(2012) The plant heat stress transcription factor (Hsf) family: Structure, function and evolution. Biochim Biophys Acta 1819: 104–119 [DOI] [PubMed] [Google Scholar]
- Scharf KD, Heider H, Höhfeld I, Lyck R, Schmidt E, Nover L(1998) The tomato Hsf system: HsfA2 needs interaction with HsfA1 for efficient nuclear import and may be localized in cytoplasmic heat stress granules. Mol Cell Biol 18: 2240–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf KD, Rose S, Zott W, Schöffl F, Nover L(1990) Three tomato genes code for heat stress transcription factors with a region of remarkable homology to the DNA-binding domain of the yeast HSF. EMBO J 9: 4495–4501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm F, Larkindale J, Kiehlmann E, Ganguli A, Englich G, Vierling E, von Koskull-Döring P(2008) A cascade of transcription factor DREB2A and heat stress transcription factor HsfA3 regulates the heat stress response of Arabidopsis. Plant J 53: 264–274 [DOI] [PubMed] [Google Scholar]
- Singh R, Singh S, Parihar P, Mishra RK, Tripathi DK, Singh VP, Chauhan DK, Prasad SM(2016) Reactive oxygen species (ROS): Beneficial companions of plants’ developmental processes. Front Plant Sci 7: 1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soon FF, Ng LM, Zhou XE, West GM, Kovach A, Tan MH, Suino-Powell KM, He Y, Xu Y, Chalmers MJ, et al. (2012) Molecular mimicry regulates ABA signaling by SnRK2 kinases and PP2C phosphatases. Science 335: 85–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takos AM, Jaffé FW, Jacob SR, Bogs J, Robinson SP, Walker AR(2006) Light-induced expression of a MYB gene regulates anthocyanin biosynthesis in red apples. Plant Physiol 142: 1216–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattini M, Galardi C, Pinelli P, Massai R, Remorini D, Agati G(2004) Differential accumulation of flavonoids and hydroxycinnamates in leaves of Ligustrum vulgare under excess light and drought stress. New Phytol 163: 547–561 [DOI] [PubMed] [Google Scholar]
- Van Breusegem F, Dat JF(2006) Reactive oxygen species in plant cell death. Plant Physiol 141: 384–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verslues PE, Agarwal M, Katiyar-Agarwal S, Zhu J, Zhu JK(2006) Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J 45: 523–539 [DOI] [PubMed] [Google Scholar]
- von Koskull-Döring P, Scharf KD, Nover L(2007) The diversity of plant heat stress transcription factors. Trends Plant Sci 12: 452–457 [DOI] [PubMed] [Google Scholar]
- Walia H, Wilson C, Condamine P, Liu X, Ismail AM, Zeng L, Wanamaker SI, Mandal J, Xu J, Cui X, et al. (2005) Comparative transcriptional profiling of two contrasting rice genotypes under salinity stress during the vegetative growth stage. Plant Physiol 139: 822–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Qu C, Jiang S, Chen Z, Xu H, Fang H, Su M, Zhang J, Wang Y, Liu W, et al. (2018) The proanthocyanidin-specific transcription factor MdMYBPA1 initiates anthocyanin synthesis under low-temperature conditions in red-fleshed apples. Plant J 96: 39–55 [DOI] [PubMed] [Google Scholar]
- Wang N, Xu H, Jiang S, Zhang Z, Lu N, Qiu H, Qu C, Wang Y, Wu S, Chen X(2017a) MYB12 and MYB22 play essential roles in proanthocyanidin and flavonol synthesis in red-fleshed apple (Malus sieversii f. niedzwetzkyana). Plant J 90: 276–292 [DOI] [PubMed] [Google Scholar]
- Wang Z, Cui Y, Vainstein A, Chen S, Ma H(2017b) Regulation of fig (Ficus carica L.) fruit color: Metabolomic and transcriptomic analyses of the flavonoid biosynthetic pathway. Front Plant Sci 8: 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins JM, Chapman JM, Muday GK(2017) Abscisic acid-induced reactive oxygen species are modulated by flavonols to control stomata aperture. Plant Physiol 175: 1807–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins JM, Hechler PJ, Muday GK(2014) Ethylene-induced flavonol accumulation in guard cells suppresses reactive oxygen species and moderates stomatal aperture. Plant Physiol 164: 1707–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CA, Grayer RJ(2004) Anthocyanins and other flavonoids. Nat Prod Rep 21: 539–573 [DOI] [PubMed] [Google Scholar]
- Winkel-Shirley B.(2002) Biosynthesis of flavonoids and effects of stress. Curr Opin Plant Biol 5: 218–223 [DOI] [PubMed] [Google Scholar]
- Wrzaczek M, Brosché M, Kangasjärvi J(2013) ROS signaling loops: Production, perception, regulation. Curr Opin Plant Biol 16: 575–582 [DOI] [PubMed] [Google Scholar]
- Xiang J, Ran J, Zou J, Zhou X, Liu A, Zhang X, Peng Y, Tang N, Luo G, Chen X(2013) Heat shock factor OsHsfB2b negatively regulates drought and salt tolerance in rice. Plant Cell Rep 32: 1795–1806 [DOI] [PubMed] [Google Scholar]
- Xie Y, Chen P, Yan Y, Bao C, Li X, Wang L, Shen X, Li H, Liu X, Niu C, et al. (2018) An atypical R2R3 MYB transcription factor increases cold hardiness by CBF-dependent and CBF-independent pathways in apple. New Phytol 218: 201–218 [DOI] [PubMed] [Google Scholar]
- Xiong L, Zhu JK(2003) Regulation of abscisic acid biosynthesis. Plant Physiol 133: 29–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Fujita Y, Sayama H, Kidokoro S, Maruyama K, Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K(2010) AREB1, AREB2, and ABF3 are master transcription factors that cooperatively regulate ABRE-dependent ABA signaling involved in drought stress tolerance and require ABA for full activation. Plant J 61: 672–685 [DOI] [PubMed] [Google Scholar]
- Zeng JK, Li X, Zhang J, Ge H, Yin XR, Chen KS(2016) Regulation of loquat fruit low temperature response and lignification involves interaction of heat shock factors and genes associated with lignin biosynthesis. Plant Cell Environ 39: 1780–1789 [DOI] [PubMed] [Google Scholar]
- Zhang J, Xu H, Wang N, Jiang S, Fang H, Zhang Z, Yang G, Wang Y, Su M, Xu L, et al. (2018) The ethylene response factor MdERF1B regulates anthocyanin and proanthocyanidin biosynthesis in apple. Plant Mol Biol 98: 205–218 [DOI] [PubMed] [Google Scholar]
- Zhu JK.(2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53: 247–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JK.(2016) Abiotic stress signaling and responses in plants. Cell 167: 313–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Guo Y, Guettouche T, Smith DF, Voellmy R(1998) Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell 94: 471–480 [DOI] [PubMed] [Google Scholar]