Mesenchymal stem cells (MSCs) have been intensively investigated for their therapeutic potentials. In addition to their ability to differentiate into multiple cell types, they can orchestrate immune responses and modulate tissue microenvironments, resulting in tissue regeneration1,2. However, therapeutic inconsistency is one of the major obstacles to clinical application.
This therapeutic inconsistency may result from the population heterogeneity, tissue origin, passage number or in vitro expansion system of MSCs. Human platelet lysate (PL) has been demonstrated to support human MSCs’ expansion and used as the standard culture medium for MSCs’ clinical applications3. However, batch variation in human PL preparations may affect the phenotype and function of MSCs. Indeed, it has been noted that human PL products from different commercial suppliers show different therapeutic efficacies in treating a mouse model of systemic lupus erythematosus (SLE)4. Furthermore, previous investigations have shown that PL upregulates the expression of the costimulatory factors CD40 and CD865,6, which may induce immunorecognition and rejection by the host immune system.
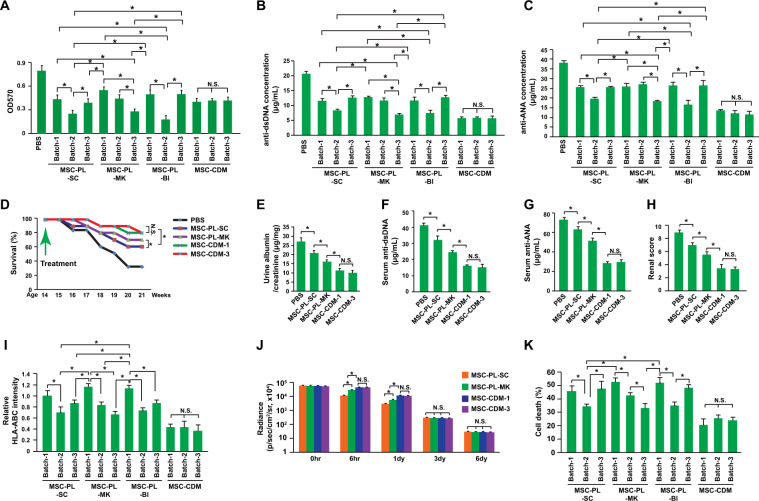
Thus, we conducted the present study to investigate whether human PL contributes to MSCs’ therapeutic inconsistency in treating a mouse model of SLE and to evaluate potential mechanisms. Human MSCs were isolated from a single donor and expanded with culture media containing 9 different batches of human PL from 3 different commercial suppliers (3 different batches from each supplier) or a commercial chemically defined medium (CDM, 3 different batches). Splenocyte coculture and proliferation analysis showed that the MSCs expanded with different batches of PL had different immunosuppressive efficacies (Fig. 1a). In contrast, the MSCs expanded with 3 different batches of CDM showed comparable levels of splenocyte proliferation suppression (Fig. 1a). Furthermore, the PL batches variation also conferred different autoantibody suppression efficacies on MSCs in experiments with PBMCs isolated from MRL/lpr mice, while the CDM batches did not (Fig. 1b, c). Thus, human PL may contribute to the variation in the immunosuppressive efficacy of MSCs in vitro. CDM produced a more consistent performance.
Fig. 1.
Improved therapeutic consistency and efficacy of MSCs expanded with CDM for SLE. a A proliferation assay was performed with splenocytes derived from MRL/lpr mice and treated with MTT after a coculture with MSCs expanded with PL (platelet lysate) from different preparation batches and suppliers or chemically defined medium (n = 3). b, c The levels of the autoantibodies, anti-dsDNA antibodies and anti-ANAs (anti-nuclear antibodies), produced by PBMCs from MRL/lpr mice were determined by ELISA after a coculture with MSCs expanded with PL from different preparation batches and suppliers or chemically defined medium (n = 3). d The survival kinetics of MRL/lpr mice transplanted with MSCs expanded with MSC-PL–SC, MSC-PL–MK, or MSC-CDM. PBS was used as a negative control (n = 40). The treatments were performed in 14-week-old MRL/lpr mice. MSCs were transplanted every two weeks. *P < 0.05 for both the Kruskal-Wallis test and Kaplan-Meier analysis. e Urine protein concentrations were measured with ELISA kits at the age of 18 weeks, which was 4 weeks after the first treatment (n = 8). f Serum levels of anti-DNA antibodies were measured by ELISA at the age of 18 weeks, which was 4 weeks after the first treatment (n = 8). g Serum levels of anti-ANAs were measured by ELISA at the age of 18 weeks, which was 4 weeks after the first treatment (n = 8). h Renal pathology scoring was conducted at the age of 18 weeks, which was 4 weeks after the first treatment (n = 8). The renal pathology scoring was calculated according to the levels of glomerular proliferation, inflammation, necrosis, interstitial changes, vasculitis, and crescent formation, and each feature was scored on a scale from 0 to 3. The final renal score was the sum of these scores. i Flow cytometry was used to analyze the expression of HLA-ABC on MSCs expanded with PL from different preparation batches and suppliers or chemically defined medium (n = 3). j The average radiance of luciferase-labeled MSCs (n = 8) is shown. The engraftment efficiency of MSCs after transplantation into MRL/lpr mice was analyzed by luciferase reporter tracing. Transplantation was performed at the age of 14 weeks. The treatments were performed only once. MSCs were expanded with PL from different preparation batches and suppliers or chemically defined medium. k Cytotoxicity was detected by measuring LDH (lactate dehydrogenase) secretion. CD8+ T cells were purified from SLE mice and cocultured with MSCs expanded with PL from different preparation batches and suppliers or chemically defined medium (n = 3). *P < 0.05; N.S. indicates no significance; MSC-PL–SC: MesenCult™-hPL Medium Kit from STEMCELL Technology; MSC-PL–MK: PLTMax Human Platelet Lysate from Merck; MSC-PL–BI: LTMax Human Platelet Lysates from Biological Industries; and MSC-CDM: TheraPEAKTM Mesenchymal Stem Cell Chemically Defined Growth Medium from Lonza
Then, therapeutic effects on a mouse model of SLE were evaluated. In accordance with the in vitro data, the MSCs expanded with different culture media had different therapeutic effects on the overall survival of MRL/lpr mice, the urine protein concentration, autoantibody production, and a renal pathology scoring analysis (Fig. 1d–h). Therefore, MSCs expanded with CDM showed improved therapeutic consistency and efficacy in treating the mouse model of SLE.
The in vitro and in vivo data presented here showed that human PL from different prepared batches or suppliers might cause MSCs to have different therapeutic efficacies, which might explain some controversies regarding MSCs’ therapeutic applications in both preclinical and clinical investigations. The potential underlying mechanisms of these inconsistencies may be different engraftment efficiencies resulting from immune rejection or different levels of immunoregulator expression. It has been demonstrated that PL upregulates the expression of the costimulatory genes CD405 and CD866. Thus, we measured the expression levels of HLA-ABC, HLA-DP, HLA-DQ, HLA-DR, CD40, CD80, and CD86 on MSCs expanded with PL or CDM. The data showed that different batches of PL did not affect the percentage of HLA-ABC-positive cells (82.4 ± 3.6%). However, they did affect the expression level of HLA-ABC in HLA-ABC-positive cells (Fig. 1i). The MSCs expanded with CDM expressed lower and more stable levels of HLA-ABC (Fig. 1i). However, the expression of HLA-DP, HLA-DQ, HLA-DR, CD80, and CD86 were not detected in the current study. As HLA-ABC is tightly related to immune rejection7, the engraftment efficiencies of the MSCs expanded with the different culture media were assessed. The data showed that the human MSCs expanded with PL had a lower engraftment efficiency than those expanded with CDM, with batch variations (Fig. 1j). However, these differences existed only during the first few days, and the difference disappeared as early as day 3 after transplantation (Fig. 1j). One of the explanations might be that HLA-ABC, HLA-II and other costimulatory factors might be stimulated by the inflammatory microenvironment in SLE mice4. However, the relatively rapid elimination of MSCs during the early stage after transplantation could already reduce MSC therapeutic efficacy.
MHC-I can induce CD8+ T cells to eliminate an allograft, and NK cells eliminate cells lacking MHC-I7,8. To further confirm that the variation in HLA-ABC expression contributes to the inconsistencies in the engraftment efficiency of MSCs expanded with PL, a cytolytic assay was conducted in vitro. CD8+ T and NK cells were purified from SLE mice and cocultured with MSCs. Cytotoxicity was detected by measuring LDH (lactate dehydrogenase) secretion. The data showed that the CD8+ T cells induced MSC death at different levels (Fig. 1k) and that T cell cytotoxic activity was tightly correlated with HLA-ABC expression levels (P < 0.01). However, the NK cells induced comparable cytotoxicity among different groups (3.8 ± 1.3%), indicating that the expression level of HLA-ABC regulated by PL variation did not decrease enough to trigger NK cell activation.
Although the low immunogenicity of MSCs has been widely demonstrated and is accepted by most researchers, the immunogenic characteristics of MSCs should be reconsidered because immune rejection is one of the major issues that needs to be addressed in clinical studies7,8. It has been demonstrated that MHC I- and MHC II-mismatched allogenic MSCs can undergo immune-mediated rejection in mice7,8. Allogenic MSCs even induce immune memory, resulting in more rapid elimination of MSCs during a 2nd MSCs transplantation7,8. Knocking out MHC-I, MHC-II or both can significantly suppress immune rejection and prolong graft survival9.
In summary, human MSCs expanded with CDM were relatively therapeutically consistent across different batches, indicating that fully CDM for expanding human MSCs is critical for both basic biology studies and clinical applications.
Supplementary information
Acknowledgements
This work was supported by the Natural Science Foundation of Shenzhen (JCYJ20180305163407913 and KQJSCX20180328093434771), Guangdong Provincial Science and Technology Program (No. 2019B030301009) and the Medical Foundation of Guangdong (A2018308).
Author contributions
J.X. designed and supervised the project, interpreted the data, and wrote the manuscript; W.L. conducted the cell culture experiments; H.W. and X.W. conducted the animal studies; J.C., L.Y. and X.Z. collected the clinical samples and conducted the animal studies; and L.L. and Z.H. analyzed the data and corrected the manuscript.
Competing interests
The authors declare no competing interests.
Ethics
This study was approved by the ethics committee of Shenzhen University and followed the tenets of the Declaration of Helsinki.
Informed consent
Informed consent was obtained from participants prior to study participation.
Supplementary information
The online version of this article (10.1038/s41423-020-0364-4) contains supplementary material.
References
- 1.Shi Y, et al. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat. Rev. Nephrol. 2018;14:493–507. doi: 10.1038/s41581-018-0023-5. [DOI] [PubMed] [Google Scholar]
- 2.Xu, J. Immunue modulation by mesenchymal stem cells. Cell Prolif.53, e12712 (2020). [DOI] [PMC free article] [PubMed]
- 3.Wuchter P, et al. Standardization of Good Manufacturing Practice-compliant production of bone marrow-derived human mesenchymal stromal cells for immunotherapeutic applications. Cytotherapy. 2015;17:128–139. doi: 10.1016/j.jcyt.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Xu J, et al. Additive therapeutic effects of mesenchymal stem cells and IL-37 for systemic lupus erythematosus. J. Am. Soc. Nephrol. 2020;31:54–65. doi: 10.1681/ASN.2019050545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reis M, et al. Global phenotypic characterisation of human platelet lysate expanded MSCs by high-throughput flow cytometry. Sci. Rep. 2018;8:3907. doi: 10.1038/s41598-018-22326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kong CM, Lin HD, Biswas A, Bongso A, Fong CY. Manufacturing of human Wharton’s jelly stem cells for clinical use: selection of serum is important. Cytotherapy. 2019;21:483–495. doi: 10.1016/j.jcyt.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, et al. Understanding the immunological mechanisms of mesenchymal stem cells in allogeneic transplantation: from the aspect of major histocompatibility complex class I. Stem Cells Dev. 2019;28:1141–1150. doi: 10.1089/scd.2018.0256. [DOI] [PubMed] [Google Scholar]
- 8.Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat. Biotechnol. 2014;32:252–260. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deuse T, et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat. Biotechnol. 2019;37:252–258. doi: 10.1038/s41587-019-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.