Abstract
The goal of this study was to use transcranial direct current stimulation (tDCS) to examine the role of the prefrontal cortex (PFC) in neural oscillatory activity associated with proactive cognitive control in schizophrenia. To do so, we tested the impact of PFC-targeted tDCS on behavioral and electrophysiological markers of proactive cognitive control engagement in individuals with schizophrenia. Using a within-participants, double-blinded, sham-controlled crossover design, we recorded EEG while participants with schizophrenia completed a proactive cognitive control task (the Dot Pattern Expectancy (DPX) Task), after receiving 20 min of active prefrontal stimulation at 2 mA or sham stimulation. We hypothesized that active stimulation would enhance proactive cognitive control, leading to changes in behavioral performance on the DPX task and in activity in the gamma frequency band during key periods of the task designed to tax proactive cognitive control. The results showed significant changes in the pattern of error rates and increases in EEG gamma power as a function of tDCS condition (active or sham), that were indicative of enhanced proactive cognitive control. These findings, considered alongside our previous work in healthy adults, provides novel support for the role gamma oscillations in proactive cognitive control and they suggest that frontal tDCS may be a promising approach to enhance proactive cognitive control in schizophrenia.
Subject terms: Schizophrenia, Translational research, Cognitive control, Electrophysiology
Introduction
Schizophrenia is a disorder characterized by psychotic symptoms and cognitive deficits [1–3]. Schizophrenia is particularly associated with impairment in the abilities that support goal-directed cognition, or “cognitive control” [4], including proactive cognitive control, which refers to the maintenance and representation over time of goals, context or rules that are relevant to the task at hand. For example, proactive cognitive control enables participants use the rules of an experimental task to prepare an upcoming response (e.g., preparing to respond to a target after seeing a cue). Proactive cognitive control stands in contrast to reactive control, which refers to adjustments in behavior made in response to increased cognitive demands. In short, proactive cognitive control involves preparation for upcoming cognitive demands, and reactive control involves after-the-fact responses to cognitive demands. Individuals with schizophrenia have impairments in both proactive and reactive cognitive control; our focus in the current paper is on proactive cognitive control in particular. Proactive cognitive control is thought to be supported by the dorsolateral prefrontal cortex (PFC), a key hub in the larger frontal-parietal cognitive control network [5]. Deficits in proactive cognitive control have been consistently reported in schizophrenia, accompanied by dysfunction in the underlying neural circuitry, including the dorsolateral PFC (see Lesh et al. [4] for a review).
Recent work in both healthy adults and in schizophrenia has suggested that targeting neural circuits known to support cognitive control processes with noninvasive brain stimulation may enhance behavioral performance and neural markers of cognitive control [6–9]. For example, in a study of healthy adults, we found that stimulating the PFC with transcranial direct current stimulation (tDCS) prior to a proactive cognitive control task increased gamma band activity related to goal maintenance (a key element of proactive cognitive control), compared with sham stimulation [10]. Given that proactive control is impaired in schizophrenia, the goal of the current study was to determine if frontal tDCS could be used to enhance this ability in patients.
Electrophysiological recordings (EEG) provide a high temporal resolution tool to examine cognitive processing in schizophrenia. Specifically, studies of scalp-recorded EEG have found that, compared with healthy adult participants, participants with schizophrenia show reduced gamma band activity (30–80 Hz) in response to task conditions that are demanding of proactive cognitive control [11, 12]. High-frequency activity in the gamma band has been linked to higher-order cognitive functions, and sustained dorsolateral PFC gamma activity has been observed in response to increased cognitive control demands using intracranial EEG recordings [13].
We tested the impact of PFC-targeted tDCS on behavioral and neural markers of proactive cognitive control using a within-participants, double-blinded, sham-controlled crossover design. To examine proactive cognitive control, we recorded EEG while participants with schizophrenia completed the dot-pattern expectancy (DPX) task following tDCS administration (see Fig. 1 for task overview). This task is designed to engage proactive cognitive control by showing cue-probe sequences that require participants to maintain cue context over a delay in order to correctly respond to probes [14, 15]. This task is adapted from the AX Continuous Performance task (AX-CPT), which uses letters as cues and probes rather than the dot patterns used in the DPX task. Compared with healthy controls, patients with schizophrenia show deficits in performance on both versions of this task [16–18].
Fig. 1. Sample stimuli and timing information for the Dot Pattern Expectancy (DPX) Task.
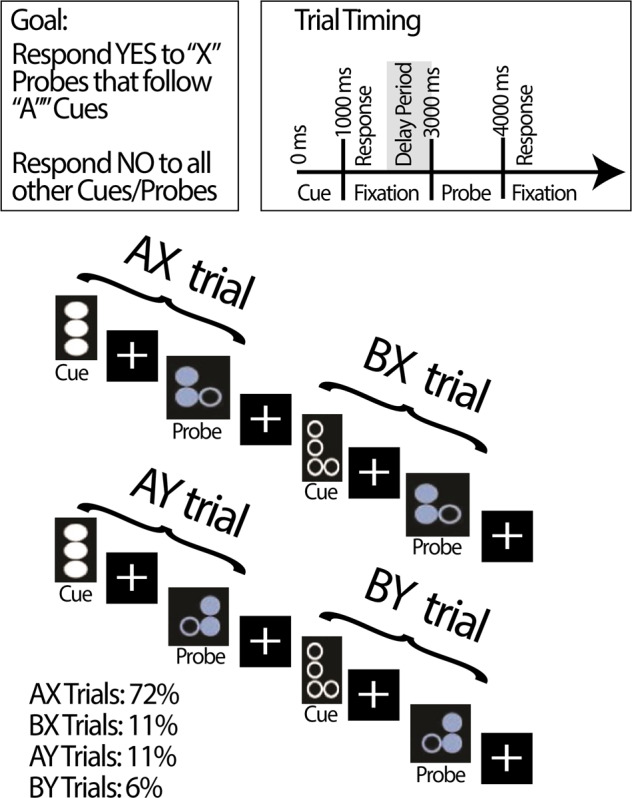
The Dot Pattern Expectancy (DPX) task is a modification of the AX continuous performance task (AX-CPT). In the DPX, dot patterns are used as stimuli. The version used here was developed by the CNTRACS initiative and is freely available online (http://cntracs.ucdavis.edu/dpx). The task consists of 144 trials across 4 blocks (36 trials per block). Each trial consisted of a cue-probe pair, in four conditions: AX (72%), AY (11%), BX (11%), and BY (6%). AX trials serve as targets, and all other cue-probe combinations are nontargets. Only trials on which participants responded correctly to the cues were analyzed.
In the DPX task, cues and probes are classified as targets or nontargets. Targets consist of a particular dot pattern probe (“X”) that is preceded by a particular dot pattern cue (“A”), known as an “AX” trial. All other stimuli are nontargets. AX trials comprise the majority of stimuli, which leads participants to develop an expectation to make a target response to probes after seeing an “A” cue, and when seeing “X” probes generally. Successful proactive cognitive control is associated with a AY > BX error pattern [16–20]. This is because proactive cognitive control engagement in the form of anticipation of an “X” probe after an “A” cue leads to an increased error rate on AY trials. In contrast, on BX trials, proactive control engagement to maintain “B” cue context and preparing to respond to a nontarget leads to a reduced error rate. Thus, successful use of proactive cognitive control is a disadvantage on AY trials, but an advantage on BX trials and on overall trial performance.
Compared with sham stimulation, we predicted that active stimulation of the PFC would enhance proactive cognitive control, leading to changes in both behavioral performance on the DPX task and activity in the gamma frequency band. Behaviorally, we predicted that active stimulation would decrease BX errors and conversely increase AY errors, compared with sham. For the EEG data, we predicted that consistent with the results reported by Boudewyn et al. [10] in healthy adults, gamma power would be increased for active stimulation compared with sham, and specifically, that active stimulation would enhance the gamma power in the delay period between cue and probe. We focused on the delay period between cue and probe because this is when proactive cognitive control demands are highest, as the cue context must be maintained in order to guide responding to the upcoming probe. Last, we predicted that active stimulation may enhance delay period gamma power for relatively more demanding B cue trials compared with A cue trials (on which additional proactive cognitive control is needed to prepare to make a relatively rare, nontarget response), as reported in healthy adults by Boudewyn et al. [10].
Methods
Participants
In total, 37 participants (12 female) with schizophrenia or a schizophrenia spectrum disorder were enrolled in this experiment. Participants were recruited through the Early Diagnosis and Preventive Treatment of Psychosis clinic at the University of California, Davis Medical Center. Participants were recruited with the following exclusion criteria: (a) IQ below 70, as measured by the Wechsler Abbreviated Scale of Intelligence; (b) history of neurological illness, including head injury; (c) substance-related disorder in the previous 6 months; (d) uncontrolled medical illness; (e) history of electroconvulsive therapy; (f) pacemakers, implants or other metal in the body; (g) corrected vision that does not achieve 20/30. All participants provided informed consent, with approval from the Institutional Review Board at the University of California, Davis. Participants were compensated at a rate of $15 per hour for completing this study.
Diagnoses were established using the Structured Clinical Interview for DSM-IV Axis 1 Disorders, with experienced clinicians conducting all diagnostic evaluations. Diagnoses were confirmed by consensus conference. All clinicians demonstrated reliability, defined by >0.8 intraclass correlation coefficients for continuous measures, and by kappa >0.7 for categorical measures. All clinicians participated in monthly reliability checks to prevent drift from these standards. Clinical symptom scores were measured using the Brief Psychiatric Rating Scale (BPRS) and the Scales for the Assessment of Positive and Negative Symptoms (SAPS and SANS).
Of the 37 participants enrolled in the study, 4 did not return for the second session, 1 did not have data at both sessions because of technical problems during EEG recording, and 5 were excluded because they had too few artifact-free trials on the EEG task (artifact correction and rejection procedures are described below). Thus, 27 participants (8 female) had complete usable datasets. All analyses reported in this manuscript reflect this final sample. Demographic information and clinical symptom scores are summarized in Table 1.
Table 1.
Demographics, symptom severity, and medication information for all participants.
| Measure | Mean | Range | Standard deviation |
|---|---|---|---|
| Age | 22.76 | 18–30 | 3.65 |
| IQ (WASI) | 105.41 | 73–138 | 15.43 |
| Education (Self, Years) | 13.15 | 11–16 | 1.43 |
| Education (Parental, Years) | 12.98 | 2–18 | 3.48 |
| Years since First Episode of Psychosis | 2.96 | 0.71–6.33 | 1.26 |
| BPRS total | 43.19 | 27–72 | 13.12 |
| SAPS total | 16.41 | 0–45 | 14.33 |
| SANS total | 29.63 | 5–70 | 17.78 |
| Medication | N | Percentage | |
| Unmedicated | 4 | 14.81% | |
| Antipsychotics | 21 | 77.78% | |
| Antidepressants | 6 | 22.22% | |
| Anticholinergics | 1 | 3.7% | |
| Mood Stabilizers | 2 | 7.41% |
Protocol overview
We used a within-participants, double-blinded, sham-controlled crossover design in this study. All participants completed both stimulation condition (sham and active) on different days. The order of sessions was randomized across participants (average interval between sessions: 8.93 days; range: 2–28 days). Participants and the primary experimenter were blinded to experiment condition. A secondary experimenter not blinded to experiment condition operated the neurostimulation software. Each session started with administration of tDCS, during which participants completed the N-back task, which has been shown to engage the prefrontal circuits targeted by our active stimulation protocol [21, 22]. The use of task-engaged stimulation was motivated by previous tDCS work suggesting enhanced effects of protocol when stimulation is administered while participants performed a relevant task [23]. Both stimulation conditions (sham and active) were administered and EEG was recorded using a StarStim32 neurostimulator (neuroelectrics.com) and corresponding NIC1.0 software. The StarStim32 system includes electrodes capable of both recording and stimulation. Thus, cap preparation and electrode placement for both tDCS and EEG were completed in one step prior to the start of the tDCS protocol, and EEG data collection began directly after stimulation. Participants completed entrance and exit mood questionnaires to screen for possible changes in mood due to stimulation (none reported), and completed three tolerability questionnaires during stimulation, rating sensations of itching, heat and tingling on a scale of 1–10. Participants were informed that ratings of 7 or higher on any of those sensations corresponded to painful levels, and our protocol called for immediate ramp-down and discontinuation of stimulation should a rating of 7 or higher be recorded (none reported; average rating was 2.18 on a scale of 1–10 where 1 was none/very mild, 7 was so bothersome that you would like to stop, and 10 was maximally uncomfortable/painful). Immediately following stimulation, participants completed the DPX task. Sample stimuli and timing information for the DPX task are shown in Fig. 1.
tDCS and EEG
Direct current was administered using Neuroelectrics Pistim electrodes, which have a π cm2 contact area on an Ag/AgCI pellet with a 12 mm diameter (anode placement: over left dorsolateral PFC (F3); cathode placement: right supraorbital site (FP2)). This montage is commonly used to target the dorsolateral PFC [10, 24]. Stimulation parameters were informed by our previous study in healthy young adults [10]: during active stimulation, current was administered for 20 min at 2 mA, with a 30-s ramp-up and ramp-down. Sham stimulation followed the same procedure, except that current ramped down and remained off after the 30-s ramp-up at the beginning of the 20-min period.
Time-frequency analysis
EEG recording and pre-processing details are described in the Supplemental Materials. EEG spectral power was calculated by convolving single-trial epochs with 7-cycle complex Morlet wavelets (as in Boudewyn et al., [10]). Power for 154 log-spaced frequencies from 3 to 80 Hz was averaged across trials within a condition and log-transformed. For analysis, average power in the gamma band (30–80 Hz) was computed and averaged across electrodes in a cluster. Electrodes were grouped spatially into seven clusters: Left Frontal (F3, F7, FC5), Mid Frontal (AF4, AF3, Fz), Right Frontal (F4, F8, FC6), Central (FC2, Cz, CP2, FC1, CP1), Left Posterior (P3, CP5, P7), Mid Posterior (O1, Oz, O2), and Right Posterior (P4, CP6, P8).
Statistical analyses
To analyze both the behavioral and EEG data, we conducted (rANCOVA), with the within-participants factor of Protocol (Sham, Active), between-participants factor of Run Order (2 levels: Sham First, Active First), and the number of days between sessions as a covariate (Days Between). Run Order and Days Between were added to examine order effects, based on pilot testing that indicated the presence of such effects in similar protocols, and as has been done in previous tDCS studies [25–28]. The EEG analysis also included the within-participants factors of Trial Type and Electrode Cluster. Significant interactions with Protocol were followed up with additional analyses, and the Greenhouse-Geisser correction was applied to all analyses with more than one degree of freedom in the numerator.
Results
Behavior
DPX error rate data
As noted above, AY and BX trials were our primary focus in this study, and specifically, the AY > BX contrast (indicative of proactive cognitive control engagement). Thus the error rate for AY minus BX trials was used as the dependent measure in a rANCOVA with the factors Protocol, Run Order and Days Between, as described above. There was a significant main effect of Protocol (F(1,24) = 5.474; p = 0.028; η2 = 0.157), such that the AY-BX contrast was less negative for Active than Sham stimulation. As can be seen in Fig. 2, this was driven by a reduction in the BX error rate for Active stimulation compared with Sham, paired with the reverse error rate pattern for AY trials. The interactions of Protocol with either Days Between (F(1,24) = 3.2; p = 0.086) or Run Order (F(1,24) = 2.15; p = 0.156) did not reach statistical significance.
Fig. 2. Behavioral results.
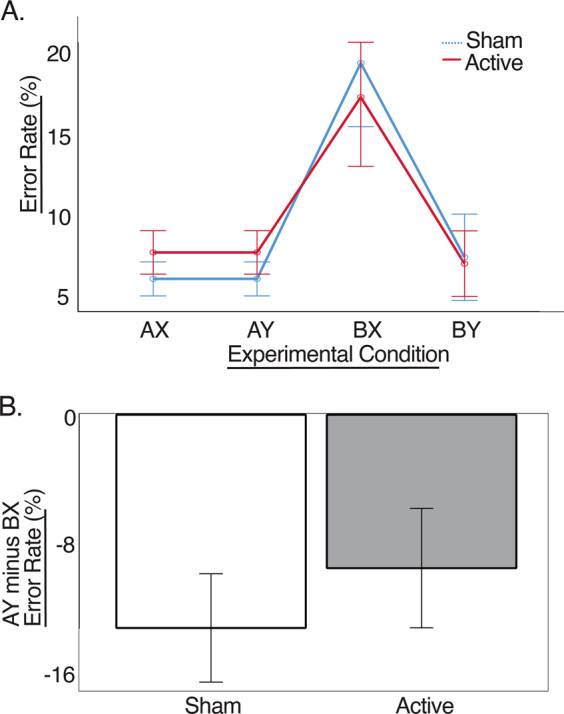
a error rate (%) for all trial types, for active DLPFC-targeted stimulation and sham stimulation. b AY minus BX error rate (%) for active DLPFC-targeted stimulation and sham stimulation. Error bars reflect the standard error of the mean, with the covariate Number of Days evaluated at 8.93 (mean). *p < 0.05.
EEG (DPX Task)
Our primary focus in this study was on gamma activity during the delay period between the cue and the probe on the DPX task that followed tDCS administration. We hypothesized that active stimulation would enhance proactive cognitive control compared with sham stimulation, leading to an increase in gamma power during the delay period.
We conducted a repeated measures analysis of covariance (rANCOVA) of delay period gamma power adding the within-participants factor of Trial Type (2 levels: A Cue, B Cue) and Electrode Cluster (7 levels: Left Frontal, Mid Frontal, Right Frontal, Central, Left Posterior, Mid Posterior, Right Posterior) to the factors Protocol, Run Order, and Days Between that are described above. Results are summarized below and in Fig. 3.
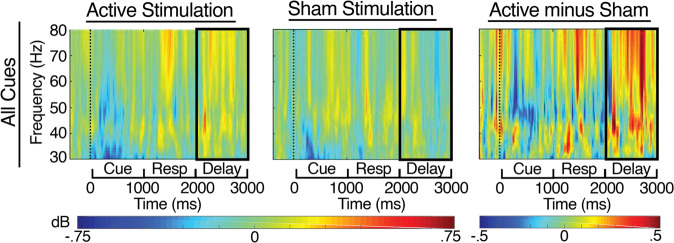
Fig. 3. Time-frequency results at the Left Frontal electrode cluster for Active, Sham and Active>Sham stimulation, time-locked to the cues, and extending through the delay period (2000–3000 ms), for all cues combined.
The black boxes indicate delay period gamma band activity (30–80 Hz from 2000 to 3000 ms post cue onset).
Consistent with our primary hypothesis, the omnibus rANCOVA showed a significant main effect of Protocol (F(1,24) = 6.55; p = 0.017), such that Active stimulation yielded increased delay period gamma power compared with Sham stimulation, overall. This effect was characterized by significant interactions with Run Order, meaning that there were different patterns of results for participants who completed the Active before the Sham session and those who completed the Sham session before the Active session (See Supplemental Table 1 for complete omnibus results). Follow-up analyses to break down this interaction revealed that participants who completed the Sham session first showed an overall enhancement of delay period gamma power during the Active session, as compared with the Sham session (F(1,11) = 7.94; p = 0.017), and that delay period gamma power was higher for A Cues than for B Cues (F(1,11) = 14.9; p = 0.003). In contrast, participants who received active stimulation first showed no significant overall effect of tDCS Protocol, but did show significant interactions of Protocol by Trial Type by Cluster (F(6,72) = 3.26; p = 0.018) and Protocol by Trial Type by Cluster by Days Between (F(6,72) = 2.8; p = 0.034). Additional follow-ups by Cluster showed that the Active > Sham pattern for delay period gamma power was significant at all electrode clusters except Left and Mid Posterior for participants who received Sham First, but not present for any individual Cluster for participants who received Active First (See Supplemental Tables 2 and 3 for complete follow-up results). These results showed that the Active > Sham pattern was driven primarily by participants who received Sham First.
Discussion
In this study, we used a within-participants, double-blinded, sham-controlled crossover design to test the impact of PFC targeted tDCS on behavioral and gamma band markers of proactive cognitive control in schizophrenia. Results showed that active stimulation significantly enhanced proactive cognitive control, as indicated by the increased tendency to show AY, relative to BX, errors. As most trials wereAX trials, good use of cue context (successful proactive cognitive control) led participants to prepare to make a target response to an anticipated X trial. Thus, poor use of cue context to prepare for probe response actually aided performance on AY trials, as it minimized an individual’s tendency to prepare to make a target response on an expected X probe that is then not received (i.e., poor proactive cognitive control should reduce the AY error rate). In contrast, poor use of cue context to prepare for probe response negatively impacted performance on BX trials, as it minimized an individual’s ability to prepare to inhibit the prepotent target response when encountering an X probe (i.e., poor proactive cognitive control should increase BX error rate). Indeed, previous studies have found that individuals with schizophrenia make more errors on BX trials than on AY trials on the DPX task or its variant, the AX-CPT task [16–19]. In contrast, healthy adults tend to show either the reverse error pattern, or else similar error rates on these two trial types; in either case, compared with individuals with schizophrenia, an increased AY > BX error rate ratio is typically observed in healthy adults, indicative of intact proactive cognitive control processes [16–20]. In the current study, the finding that active prefrontal stimulation significantly reduced the BX error rate and increased the AY error rate (i.e., increased the AY > BX error rate ratio) compared with sham indicates that PFC-targeted tDCS led to stronger use of the context provided by the cues on this task, compared with sham, an indication of improved cognitive control.
The EEG results confirmed that prefrontally focused stimulation enhanced delay related gamma activity compared with sham stimulation. Our analysis demonstrated a robust active>sham effect for delay period gamma power across all trial types. Although the B > A contrast can be used to isolate a particularly challenging proactive cognitive control condition (B cue delay period) by subtracting a less challenging condition (A cue delay period), all cues require proactive cognitive control during the delay period in order to maintain cue context and prepare to make a correct response to the probe. Indeed, our pattern of behavioral results, with increased AY errors and decreased BX errors as a function of active stimulation compared with sham, is consistent with this interpretation. This result demonstrates that PFC-targeted tDCS can increase gamma power related to proactive cognitive control engagement in schizophrenia.
Our results are consistent with the results of Hoy et al. [29], who found evidence that PFC-targeted stimulation may increase activity in the gamma band and improve performance on the N-back task. In that study, behavioral performance and gamma band activity measured 40 min after 2 mA PFC-targeted stimulation was significantly increased when compared with 0 min after stimulation [29]. Although not compared directly to sham stimulation, this finding provided valuable initial evidence of tDCS-induced gamma band effects in schizophrenia during a task known to engage the PFC. The current results are also consistent with our previous work in healthy adults, in which we also observed greater gamma band power during the delay period on this task for active PFC-targeted tDCS compared with sham stimulation [10]. In that study, we additionally observed greater gamma band activity in the delay period following B cues compared with A cues in healthy adults [10]. A similar pattern has been reported in healthy adults using a different proactive cognitive control task, in which increased gamma power was found during the delay period following cues indicating that a relatively difficult probe was coming next than following cues indicating that a relatively easy probe was coming next [11, 12]. This association between intact proactive cognitive control and greater delay period gamma power for B cues compared with A cues informed our initial predictions in the current study. Specifically, we hypothesized that this pattern might be found for active stimulation compared with sham, although it is important to note that these studies also found that participants with schizophrenia did not show this pattern of gamma activity compared with healthy adults [11, 12]. The current results are therefore consistent with the previously reported pattern in patients. While our results indicate that single-session tDCS to PFC yielded increased proactive cognitive control engagement and overall greater delay period gamma power compared with sham, it is possible that it was not sufficient to produce the gamma power effect for the more challenging B cue trials compared with A cue trials. It is also possible that the relatively small number of B cue trials in this study limited our ability to capture this contrast. This would be an important question to address in future studies.
The results of the current study have both translational and theoretical implications. Executive control deficits are a core feature of cognitive impairment in the disorder with significant impact on real-world functioning [1–3], and the current results provide initial evidence to motivate future studies of tDCS as a potential means of enhancing proactive cognitive control in schizophrenia. While it is premature to conclude that tDCS is a viable means of improving proactive cognitive control in schizophrenia, the current results suggest that it may be possible to enhance behavioral and EEG markers of proactive cognitive control in schizophrenia, in the context of a single-session experiment. It will be important for future work to replicate and extend these results to address questions such as the duration and generalizability of the effects of stimulation.
In addition, the current results provide new evidence for the hypothesized role of the PFC and gamma activity in supporting proactive cognitive control. The PFC is part of a distributed frontal-parietal cognitive control system, and is thought to be critical for proactive cognitive control processes such as goal maintenance, referring to the maintenance and representation of task-relevant goals and rules over time [5, 30–32]. Proactive cognitive control and specifically goal maintenance have been consistently shown to be impaired in schizophrenia, and this impairment has been linked to dysfunction of the PFC [18, 32]. These findings have informed the interpretation of EEG studies of schizophrenia that have demonstrated deficits in proactive cognitive control and linked them to disrupted neural oscillations in the gamma band [11, 12, 33, 34]. In the current study, we used tDCS to directly manipulate neural activity in the PFC, which yielded causal evidence connecting PFC-modulated gamma band activity to proactive cognitive control.
Gamma band activity is a high-frequency signal strongly linked to the BOLD response measured by fMRI [35, 36]. Based on these findings and on observations of gamma band activity with intracranial recordings, gamma activity is thought to be a marker of local cortical activity, rather than a marker of long-distance neural communication across cortical regions, as lower frequency oscillations in the theta band (4–7 Hz) are thought to be [37]. Neural oscillations in the gamma band are hypothesized to be essential to neural computation [38], and while gamma has been primarily studied in relation to perceptual processing, changes in gamma activity have been reported in response to increased cognitive demands in a range of domains, including working memory and cognitive control [11, 12, 39–41]. The current results demonstrate that gamma band effects related to proactive cognitive control in schizophrenia are sensitive to PFC-targeted tDCS.
Although the tDCS-mediated gamma power enhancement was maximal at frontal sites, we cannot firmly conclude that this activity was generated by the dorsolateral PFC, given the limited spatial precision afforded by scalp EEG. Although this scalp topography is compatible with a dorsolateral PFC generator, which would also fit with available neuroimaging evidence from fMRI implicating the dorsolateral PFC in proactive control [e.g., 5, 33], scalp-recorded EEG does not allow us to draw such a specific spatial inference, or to rule out the contribution of other regions to the signal recorded at the scalp. Instead, we can conclude from these results that PFC-targeted stimulation led to an increase in gamma band power associated with proactive control, whether or not the gamma was directly or exclusively generated by the dorsolateral PFC.
The mechanisms of action underlying tDCS are not fully understood. The available data suggest that tDCS likely does not directly influence neural firing rates, at least at the currents typically used in studies of human cognition (~1–2 mA) [42–44]. Instead, current strengths at these levels modulate electric fields in cortex [e.g. 45, 46]. The relatively weak modulation of cortical electric fields by tDCS may influence cognition through changes in membrane potentials, the probability and timing of neural firing, or neural plasticity mechanisms such as long-term potentiation [44]. Consistent with this idea, we found evidence of tDCS-induced changes in both behavior and scalp-recorded EEG on a proactive cognitive control task completed within an hour of stimulation.
Furthermore, we found that run order had a robust impact on the pattern of results. Specifically, the effects of active prefrontal stimulation on gamma band activity during the delay period of our proactive cognitive control task were most robust when participants received sham stimulation prior to active stimulation, as opposed to when active stimulation was administered first. This outcome was surprising, as most tDCS studies assume short-lived effects from single-session stimulation, in some cases even conducting multiple tDCS protocol conditions in the same day. Indeed, very few studies have included a direct test of Run Order in their analyses [25–28], and it is possible that similar results might have present, but undetected in some previous studies. In the current study, sessions were separated by at least 2 days, and yet receiving active stimulation in the first session appears to have had a significant impact on the effects observed in session two. As the design was counterbalanced, such that Run Order was randomly assigned, this result cannot be attributed to a practice effect in which practice in the sham stimulation session led to benefits in the active stimulation session. Instead, it appears that when the active stimulation was administered first, it had some significant impact on performance during the next (sham) session. In addition, the significant role of the covariate Number of Days indicated that this effect was most robust when sessions were closer together.
We can think of two possible mechanisms for this carryover effect. First, despite what might be expected given typical study design, it is possible that active stimulation affected gamma activity related to proactive control two or more days later, essentially conferring a lingering enhancement of proactive control on the subsequent session. This would be consistent with a relatively long-duration mechanism for tDCS, such as the idea that tDCS has the potential to induce neuroplastic changes in brain activity. It is also possible that completing the task for the first time in the active stimulation condition enhanced the benefit participants obtained from practicing the task more than completing the task for the first time in the sham condition. While we cannot adjudicate between these two possibilities with the current data (and they are not mutually exclusive), we favor the latter explanation, as it does not require that the effects of active stimulation linger per se, but instead suggests that part of the impact of a single-session of active stimulation was to enhance the typical effect of practice. These results suggest that it may be important in future work to increase the number of days between sessions, to minimize the effect of run order. Ultimately, determining the duration of such tDCS-induced effects will be an important goal for future studies.
Limitations
One limitation of the current study is that, while the results provide evidence for the enhancement of proactive control following active stimulation of the PFC in participants with schizophrenia, we did not include a healthy control group for comparison. A similar study conducted by our group using the same paradigm in healthy young adults offers a useful comparison [10], as noted in the discussion, but we acknowledge that the lack of a healthy control group in the current design limits the ability to make a direct comparison. An additional limitation of the current study is that we did not examine the possible impact of medication on the tDCS effects. Finally, in this study we compared PFC-targeted active stimulation to sham stimulation, which limits our ability to rule out the possibility that the effects were due to arousal or another generic mechanism, or that active stimulation targeting another brain region would yield a similar effect when compared with sham. This would require the inclusion of an additional tDCS condition (active control condition); such a study is currently underway.
Conclusions
In this study, we observed significant enhancement of both behavioral and neural oscillatory markers of proactive cognitive control in individuals with schizophrenia after a single session of PFC-targeted tDCS, compared with sham stimulation. These results were consistent with our hypothesis that proactive cognitive control processes, specifically goal maintenance, are supported by PFC-mediated neural oscillations in the gamma frequency band. Furthermore, our data demonstrate that gamma activity associated with goal maintenance in schizophrenia can be enhanced with a single session of noninvasive neurostimulation.
Funding and disclosure
This work was supported by: National Institute of Mental Health (5R01MH059883) to CSC; National Institute of Mental Health (5R01MH119546) to CSC; California Center for Behavioral Health Excellence to CR; Vannevar Bush Fellowship (Office of Naval Research Grant N00014-15-1-0033) to CR. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the Office of Naval Research or the U.S. Department of Defense. The authors have no competing financial interests in relation to the work described to disclose.
Supplementary information
Author contributions
MAB drafted the manuscript; MAB, KS, CR, and CSC critically revised the manuscript; MAB and KS contributed substantially to the acquisition and analysis of the work, and MAB, KS, CR, and CSC contributed to the conception, design and interpretation of the work.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41386-020-0750-8).
References
- 1.Carter CS. Understanding the glass ceiling for functional outcome in schizophrenia. Am J Psychiatry. 2006;163:356–8. doi: 10.1176/appi.ajp.163.3.356. [DOI] [PubMed] [Google Scholar]
- 2.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? THe American Journal of Psychiatry. (1996) 10.1176/ajp.153.3.321. [DOI] [PubMed]
- 3.Green MF, Nuechterlein KH. Should schizophrenia be treated as a neurocognitive disorder? Schizophrenia Bull. 1999;25:309. doi: 10.1093/oxfordjournals.schbul.a033380. [DOI] [PubMed] [Google Scholar]
- 4.Lesh TA, Niendam TA, Minzenberg MJ, Carter CS. Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharmacology. 2011;36:316–38. doi: 10.1038/npp.2010.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–8. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 6.Hoy KE, Arnold SL, Emonson MR, Daskalakis ZJ, Fitzgerald PB. An investigation into the effects of tDCS dose on cognitive performance over time in patients with schizophrenia. Schizophrenia Res. 2014;155:96–100. doi: 10.1016/j.schres.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Reinhart RM, Zhu J, Park S, Woodman GF. Synchronizing theta oscillations with direct-current stimulation strengthens adaptive control in the human brain. Proc Natl Acad Sci USA. 2015;112:9448–53. doi: 10.1073/pnas.1504196112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith RC, Boules S, Mattiuz S, Youssef M, Tobe RH, Sershen H, et al. Effects of transcranial direct current stimulation (tDCS) on cognition, symptoms, and smoking in schizophrenia: A randomized controlled study. Schizophrenia Research. 2015;168:260–6. doi: 10.1016/j.schres.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Vercammen A, Rushby JA, Loo C, Short B, Weickert CS, Weickert TW. Transcranial direct current stimulation influences probabilistic association learning in schizophrenia. Schizophrenia Research. 2011;131:198–205. doi: 10.1016/j.schres.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 10.Boudewyn M, Roberts BM, Mizrak E, Ranganath C, Carter CS. Prefrontal transcranial direct current stimulation (tDCS) enhances behavioral and EEG markers of proactive control. Cogn Neurosci. 2019;10:57–65. doi: 10.1080/17588928.2018.1551869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci USA. 2006;103:19878–83. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minzenberg MJ, Firl AJ, Yoon JH, Gomes GC, Reinking C, Carter CS. Gamma oscillatory power is impaired during cognitive control independent of medication status in first-episode schizophrenia. Neuropsychopharmacology. 2010;35:2590–9. doi: 10.1038/npp.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartoli E, Conner CR, Kadipasaoglu CM, Yellapantula S, Rollo MJ, Carter CS, et al. Temporal dynamics of human frontal and cingulate neural activity during conflict and cognitive control. Cerebral Cortex, 2017;28:1–15. [DOI] [PMC free article] [PubMed]
- 14.Jones JA, Sponheim SR, MacDonald AW., 3rd The dot pattern expectancy task: reliability and replication of deficits in schizophrenia. Psychol Assess. 2010;22:131–141. doi: 10.1037/a0017828. [DOI] [PubMed] [Google Scholar]
- 15.MacDonald AW, Carter CS, Flory JD, Ferrell RE, Manuck SB. COMT val158Met and executive control: a test of the benefit of specific deficits to translational research. J Abnorm Psychol. 2007;116:306. doi: 10.1037/0021-843X.116.2.306. [DOI] [PubMed] [Google Scholar]
- 16.Barch DM, Carter CS, MacDonald AW, Braver TS, Cohen JD. Context-processing deficits in schizophrenia: diagnostic specificity, 4-week course, and relationships to clinical symptoms. J Abnorm Psychol. 2003;112:132. doi: 10.1037/0021-843X.112.1.132. [DOI] [PubMed] [Google Scholar]
- 17.Cohen JD, Barch DM, Carter C, Servan-Schreiber D. Context-processing deficits in schizophrenia: converging evidence from three theoretically motivated cognitive tasks. J Abnorm Psychol. 1999;108:120. doi: 10.1037/0021-843X.108.1.120. [DOI] [PubMed] [Google Scholar]
- 18.MacDonald AW, Carter CS, Kerns JG, Ursu S, Barch DM, Holmes AJ, et al. Specificity of prefrontal dysfunction and context processing deficits to schizophrenia in never-medicated patients with first-episode psychosis. American Journal of Psychiatry. 2005;162:475–84. doi: 10.1176/appi.ajp.162.3.475. [DOI] [PubMed] [Google Scholar]
- 19.Henderson D, Poppe AB, Barch DM, Carter CS, Gold JM, Ragland JD, et al. Optimization of a goal maintenance task for use in clinical applications. Schizophrenia Bulletin. 2012;38:104–13. doi: 10.1093/schbul/sbr172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez-Garcia P, Lesh TA, Salo T, Barch DM, MacDonald AW, Gold JM, et al. The neural circuitry supporting goal maintenance during cognitive control: A comparison of expectancy AX-CPT and dot probe expectancy paradigms. Cognitive, Affective, &. Behavioral Neuroscience. 2016;16:164–75. doi: 10.3758/s13415-015-0384-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Owen AM, McMillan KM, Laird AR, Bullmore E. N‐back working memory paradigm: a meta‐analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perlstein WM, Dixit NK, Carter CS, Noll DC, Cohen JD. Prefrontal cortex dysfunction mediates deficits in working memory and prepotent responding in schizophrenia. Biol Psychiatry. 2003;53:25–38. doi: 10.1016/S0006-3223(02)01675-X. [DOI] [PubMed] [Google Scholar]
- 23.Filmer HL, Varghese E, Hawkins GE, Mattingley JB, Dux PE. Improvements in Attention and Decision-Making Following Combined Behavioral Training and Brain Stimulation. Cerebral Cortex. 2017;27:3675–82. [DOI] [PubMed]
- 24.Laakso I, Tanaka S, Mikkonen M, Koyama S, Sadato N, Hirata A. Electric fields of motor and frontal tDCS in a standard brain space: A computer simulation study. Neuroimage. 2016;137:140–51. doi: 10.1016/j.neuroimage.2016.05.032. [DOI] [PubMed] [Google Scholar]
- 25.Fregni F, Boggio PS, Nitsche M, Bermpohl F, Antal A, Feredoes E, et al. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Experimental Brain Research. 2005;166:23–30. doi: 10.1007/s00221-005-2334-6. [DOI] [PubMed] [Google Scholar]
- 26.Fregni F, Boggio PS, Santos MC, Lima M, Vieira AL, Rigonatti SP, et al. Noninvasive cortical stimulation with transcranial direct current stimulation in Parkinson’s disease. Movement Disorders. 2006;21:1693–702. doi: 10.1002/mds.21012. [DOI] [PubMed] [Google Scholar]
- 27.Martin DM, Moffa A, Nikolin S, Bennabi D, Brunoni AR, Flannery W, et al. Cognitive effects of transcranial direct current stimulation treatment in patients with major depressive disorder: An individual patient data meta-analysis of randomised, sham-controlled trials. Neurosci Biobehav Rev. 2018;90:137–45. doi: 10.1016/j.neubiorev.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Scocchia L, Bolognini N, Convento S, Stucchi N. Cathodal transcranial direct current stimulation can stabilize perception of movement: evidence from the two-thirds power law illusion. Neurosci Lett. 2015;609:87–91. doi: 10.1016/j.neulet.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 29.Hoy KE, Bailey NW, Arnold SL, Fitzgerald PB. the effect of transcranial direct current stimulation on gamma activity and working memory in schizophrenia. Psychiatry Res. 2015;228:191–6. doi: 10.1016/j.psychres.2015.04.032. [DOI] [PubMed] [Google Scholar]
- 30.D’Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M. The neural basis of the central executive system of working memory. Nature. 1995;378:279. doi: 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- 31.Esposito M. From cognitive to neural models of working memory. Philos Trans R Soc B: Biol Sci. 2007;362:761. doi: 10.1098/rstb.2007.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacDonald AW, Carter CS. Event-related FMRI study of context processing in dorsolateral prefrontal cortex of patients with schizophrenia. J Abnorm Psychol. 2003;112:689. doi: 10.1037/0021-843X.112.4.689. [DOI] [PubMed] [Google Scholar]
- 33.Uhlhaas PJ, Pipa G, Lima B, Melloni L, Neuenschwander S, Nikolić D, et al. Neural synchrony in cortical networks: History, concept and current status. Frontiers in Integrative Neuroscience, 3. (2009). [DOI] [PMC free article] [PubMed]
- 34.Uhlhaas PJ, Pipa G, Neuenschwander S, Wibral M, Singer W. A new look at gamma? High-(>60áHz)[gamma]-band activity in cortical networks: function, mechanisms and impairment. Progr Biophys Mol Biol. 2010;105:14–8. [DOI] [PubMed]
- 35.Magri C, Schridde U, Murayama Y, Panzeri S, Logothetis NK. The amplitude and timing of the BOLD signal reflects the relationship between local field potential power at different frequencies. J Neurosci. 2012;32:1395–407. doi: 10.1523/JNEUROSCI.3985-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mukamel R, Gelbard H, Arieli A, Hasson U, Fried I, Malach R. Coupling between neuronal firing, field potentials, and FMRI in human auditory cortex. Science. 2005;309:951–4. doi: 10.1126/science.1110913. [DOI] [PubMed] [Google Scholar]
- 37.Cavanagh JF, Frank MJ. Frontal theta as a mechanism for cognitive control. Trends Cogn Sci. 2014;18:414–21. doi: 10.1016/j.tics.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci. 2009;32:209–24. doi: 10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- 39.Howard MW. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 2003;13:1369–74. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- 40.Roux F, Wibral M, Mohr HM, Singer W, Uhlhaas PJ. Gamma-band activity in human prefrontal cortex codes for the number of relevant items maintained in working memory. J Neurosci. 2012;32:12411–20. doi: 10.1523/JNEUROSCI.0421-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Vugt MK, Sederberg PB, Kahana MJ. Comparison of spectral analysis methods for characterizing brain oscillations. J Neurosci methods. 2007;162:49–63. doi: 10.1016/j.jneumeth.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Esmaeilpour Z, Marangolo P, Hampstead BM, Bestmann S, Galletta E, Knotkova H, et al. Incomplete evidence that increasing current intensity of tDCS boosts outcomes. Brain Stimulation. 2018;11:310–21. doi: 10.1016/j.brs.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Voroslakos M, Takeuchi Y, Brinyiczki K, Tamas Z, Oliva A, Fernandez-Ruiz A, et al. Direct effects of transcranial electric stimulation on brain circuits in rats and humans. Nature Communications, 2018:9. 10.1038/s41467-018-02928-3. [DOI] [PMC free article] [PubMed]
- 44.Chase HW, Boudewyn MA, Carter CS, Phillips ML. Transcranial direct current stimulation: a roadmap for research, from mechanism of action to clinical implementation. Mol Psychiatry. (2019) 10.1038/s41380-019-0499-9. [DOI] [PMC free article] [PubMed]
- 45.Huang Y, Dmochowski JP, Su Y, Datta A, Rorden C, Parra LC. Automated MRI segmentation for individualized modeling of current flow in the human head. Journal of Neural Engineering. 2013;10:066004. doi: 10.1088/1741-2560/10/6/066004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reato D, Rahman A, Bikson M, Parra LC. Low-intensity electrical stimulation affects network dynamics by modulating population rate and spike timing. J Neurosci. 2010;30:15067–79. doi: 10.1523/JNEUROSCI.2059-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.