Abstract
Management of scoliosis in young children needs a comprehensive approach because of its complexity. There are many debatable points; however, only serial casting, growing rods (including traditional and magnetically controlled) and anterior vertebral body tethering will be discussed in this article.
Serial casting is a time-gaining method for postponing surgical interventions in early onset scoliosis, despite the fact that it has some adverse effects which should be considered and discussed with the family beforehand.
Use of growing rods is a growth-friendly surgical technique for the treatment of early onset spine deformity which allows chest growth and lung development. Magnetically controlled growing rods are effective in selected cases although they sometimes have a high number of unplanned revisions.
Anterior vertebral body tethering seems to be a promising novel technique for the treatment of idiopathic scoliosis in immature cases. It provides substantial correction and continuous curve control while maintaining mobility between spinal segments. However, long-term results, adverse effects and their prevention should be clarified by future studies.
Cite this article: EFORT Open Rev 2020;5:753-762. DOI: 10.1302/2058-5241.5.190087
Keywords: casting, early onset, growing rods, scoliosis, vertebral body tethering, young children
Introduction
Scoliosis is a three-dimensional deformity of spine. Although it is seen predominantly in the adolescent age group, scoliosis in the young age group occasionally exists and requires sophisticated management. Traditionally, idiopathic scoliosis has been classified based on initial age at which the pathology is first identified. If the age at onset of scoliosis is younger than three years, it is defined as infantile. If the age at onset is between four and 10 years, it is described as juvenile and finally, if the curve is detected later than 10 years, it is classified as adolescent idiopathic scoliosis (AIS).1 After the development of better understanding of the relationship of spinal growth to thorax and lung development, a new description was developed. Early onset scoliosis (EOS) now refers to a coronal plain curvature of more than 10 degrees with onset earlier than 10 years of age including all types of aetiologies such as idiopathic, congenital, neuromuscular and also syndromic.2 This heterogeneous group of aetiologies helps in predicting outcome of EOS cases. Recently, Willliams et al developed a new classification system (C-EOS) which includes four domains: aetiology of deformity, magnitude of major curve, presence of kyphosis and annual progression ratio.3 The reliability and validity of C-EOS has been already demonstrated.4
The basic principle in the treatment of EOS is to create a well-developed chest and lung with an optimal overall pulmonary function rather than to have a straight spine. Thus, growth-friendly treatment methods allow surgeons to achieve a maximum T1–T12 length which is crucial for optimizing lung volume. Growth-friendly treatment consists of both conservative methods, including bracing and serial casting, and surgical methods, including distraction-based, guided-growth and compression-based techniques.5 Distraction-based techniques which are traditional/magnetically controlled growing rods and vertical expandable prosthetic titanium rib (VEPTR ) correct deformity by distractive forces applied across the deformity with anchors which are attached to the spine, rib or pelvis. Guided-growth techniques including Luque trolley and Shilla technique allow spinal growth while controlling deformity with pedicle screws which are able to slide over the rods. Compression-based techniques including staples and tethers correct scoliosis by means of convex growth inhibition created by a compression force.
Because of possible pulmonary complications, EOS is a potentially life-threatening condition that requires early intervention to prevent severe deformity and pulmonary compromise. There are many debatable points and diverse approaches to scoliosis in young children. However, we will focus on three popular methods: serial casting, growing rods and vertebral body tethering in this review article.
Serial casting
The management of EOS in young children is challenging. Conservative options such as bracing or serial casting should be the initial treatment of choice. Infantile idiopathic scoliosis (IIS) was first recognized as a distinct clinical episode in a 1951 case series by James and comprises < 1% of all idiopathic scoliosis cases in the United States.6 Although the definition of IIS was recognized in 1951, treatment of scoliosis goes back to the 19th century with use of plaster of Paris (POP) for this purpose.7 In 1893, Bradford and Brackett presented five cases along with a detailed description of their traction device and cast application method. One of their cases was a four-year-old child and a straight spine was achieved after one year of cast treatment.8
In the 20th century, surgery was introduced. Initially in situ fusion with bone grafting, and plaster as a corrective tool to maintain correction was performed until bony fusion was achieved. But posterior fusion techniques resulted in a short trunk, a disproportionate body and, more importantly, lung development problems in EOS patients.
In 1964, a report by Cotrel and Morel suggested that serial casting might be used as a corrective measure in EOS. In their report they detailed the ‘EDF’ casting technique in which E referred to elongation, D to derotation and F to flexion.9 In 1979 the first formal report of serial casting specifically addressing idiopathic EOS was presented by Mehta and Morel.10
Problems such as the ‘law of diminishing returns’, autofusion and short trunk in young children treated with growth-friendly implants resulted in revitalization of casting.11,12 Sanders et al advocated Mehta’s casting technique, and in the last 10 years we have seen a significant rise in the use of the serial casting technique for younger EOS patients, and it is in many countries the most common choice of treatment.13,14
Indications for serial casting
Several studies have shown that age is a significant predictor for serial casting outcome. Once the diagnosis of progressive scoliosis is made, based on either a curve of Cobb > 25 degrees and progressive Cobb angle, or an RVAD (rib-vertebral angle difference) of more than 20 degrees8 rib phase 2, or a double curve, cast correction is recommended. With curves less than 60 degrees a full recovery of the scoliosis may result. But in many cases it is a delaying technique, postponing surgery. The response to casting is not predictable, not even when based on rib-vertebral angle measurements. Traditionally, serial casting has been used for idiopathic cases. However, recent studies have shown that serial casting is an alternative technique to slow down progression in severe deformities even in patients with congenital, syndromic or neuromuscular deformities.14–16 Its efficacy in congenital cases was demonstrated in two retrospective studies. A total of 19 congenital cases were followed and it was concluded that serial casting is a time-buying method to delay surgical treatment.14,17 As already mentioned, both studies were conducted based on a retrospective study, and this might have caused bias by picking up cases from flexible curves. It is difficult to know whether the same results can be achieved with more rigid deformities.
The technique of serial casting
A designated casting table is very important. Patients are intubated due to the negative effect cast moulding may have on thoracic pressure, making ventilation temporarily difficult. A head halter and pelvic traction assists in stabilizing the patient. Traction alone can correct the curve while applied, but it cannot be retained in the cast once traction is released and the body recoils. If there is a lumbar curve, flex the hips slightly to decrease lumbar lordosis and facilitate curve correction. Plaster, which is easy moulded, is applied. The foundation of the plaster is the pelvic portion. Then the posteriorly rotated ribs are rotated anteriorly to create a more normal chest configuration with counter rotation applied through the pelvic mould and upper torso. Some centres use an over-the-shoulder cast (described as the Cotrel–Morel technique) but others stay below the shoulders (most infantile scoliosis cases will have more caudal apices T9–T11). Further windows in the cast are made anterior (to relieve chest and abdominal pressure) and posterior (on the concave side giving the ribs space to move posteriorly). It is important to have an anterior mirror to see the anterior handling of the rotational manoeuvre.
Each jacket is worn for 8 to 16 weeks to allow the spine sufficient time to grow in a progressively improved direction and shape. When the radiographs show restoration of the symmetry of the rib cage, de-rotation of the apical vertebra and a complete or almost complete correction of the curve itself, the jackets are relinquished. If after six months the spine remains corrected, the brace is gradually discarded, and treatment may cease.
There is a discussion regarding using muscle relaxing medication or not. At the Oslo University Hospital we use muscle relaxation medication and, in our hands, it works well.
Results
In Mehta’s prospective study performed in 2005, spinal deformity was completely corrected if treatment with casting started early in children with moderate curves.18 Cast treatment did not resolve the curves in older children with severe curves.
Sanders et al reported in 2009 on 55 patients with progressive infantile scoliosis, of whom all but six responded to treatment at a minimum one-year follow-up.13 The cohort included syndromic patients as well as idiopathic scoliosis patients. Their results were for: initiation of cast correction at a younger age, moderate curve size (< 60 degrees), and an idiopathic diagnosis, which carry a better prognosis than curves at an older age of initiation, curves of more than 60 degrees and with a non-idiopathic diagnosis.
In a recent study, 38 patients with minimum five-year follow-up were reviewed and predictors for sustained deformity resolution were defined. Initial Cobb angle, first-cast Cobb angle, rib-vertebral angle difference and traction Cobb angle were identified as predicting factors.19 Hassanzadeh et al reviewed 45 patients with infantile idiopathic scoliosis with a relatively short follow-period for radiographic predictors of sustained scoliosis correction. Concave and convex height ratios of the apical three vertebra at initial casting were found to be significant for predicting prognosis. They also demonstrated that Mehta casting is an effective method for the treatment of infantile idiopathic scoliosis.20 On the other hand, Welborn et al found that the major curve Cobb angle at the end of casting was the most reliable predictive factor in their case series. However, initial Cobb angle was not a predictive factor for the outcome.21 Iorio et al identified another predictive factor for outcome which was body mass index and demonstrated that high body mass index was a predictor for Cobb angle improvement.22
Anaesthetic concerns and complications of serial casting
Some studies have demonstrated that, in animal models, the extent of any neurological deficit is time dependent, and greatest at the stage of development where synaptogenesis or neurodevelopment is at its peak. This can trigger widespread apoptotic neurodegeneration.23 But even in humans, several studies of prolonged or repeated exposure to general anaesthesia have demonstrated a modest adverse effect on learning and behaviour.24 This is a concern and should be addressed before initiating serial casting. On the other hand, recent studies have demonstrated that serial casting under general anaesthesia combined with neuromuscular blocking agents provides more effective prevention of progression when it is compared with only general anaesthesia.25 The reason for that could be that maximum muscle relaxation allows better initial correction to be achieved in three plains of deformity.
Casting may potentially cause pressure sores, particularly in patients with very large curves that have more prominent bony projections. Rib or mandibular deformities secondary to serial casting were also reported. Besides these problems, vascular complications such as superior mesenteric artery syndrome and subclavian vein thrombosis also can be seen.26 Dhawale et al demonstrated that serial cast application causes increased peak inspiratory pressure and it does not completely improve after cutting out the abdominal window. This is a significant problem, especially for patients who have pulmonary co-morbidities.27 A child with a torso cast will have a very limited mobilization ability. Although the hip, knee, and ankle joints can move completely in their full range of motion, upper body swinging becomes limited. This may result in some type of waddling during the gait and this may then cause pressure sores on the iliac crests from the rigid cast material. Therefore, casting may have psychological effects on children, and the literature has not shed light on these unknown effects yet.28
Growing rods
The introduction of spinal instrumentation by Paul Harrington changed the treatment strategy even in the early-onset population. Instrumentation and fusion surgery of deformity with correction of the spine gained wide acceptance, regardless of age.29 In the last three decades the increased understanding of EOS, the importance of spinal growth and pulmonary function has changed the surgical strategy from spinal fusion to growth-friendly implants.30,31 Magnetically controlled growing rods (MCGR) were developed recently for reducing the time of lengthening operations.5 Traditional and magnetically controlled growing rods will be discussed separately since both implants have specific features.
Traditional growing rods
Deformity control and preservation of growth have become the targets in treating EOS patients. Significant gains in T1–S1 distance can be achieved using growth-friendly implants. Growing rods are one of the most well-known and widely used growth-friendly implants which are utilized for several years in order to control deformity progression in EOS until the patients reach an appropriate maturity for definitive fusion. Although there are many modifications of growing rod constructs, the technique was originally applied as a single rod construct by using the Harrington Rod device. Then, it was proved that double rod constructs provide better biomechanical properties.32 Thus, this section will mainly focus on the dual rod technique.
Indications for traditional growing rods
A patient younger than eight years old who has a curve magnitude of more than 60 degrees and a flexible curve is a good candidate for a growing rod. Besides these indications, patients with pulmonary problems which prevent serial cast application, intolerance to cast application and syndromic scoliosis can be other indications (Fig. 1).33 However, there should be more specific indications and patient-dependent factors should be considered for final decision making. The dual growing rod technique effectively maintains thoracic cage growth. Nevertheless, growth rate decreases in parallel with the increased number of lengthening procedures. This phenomenon is called the ‘law of diminishing returns’.12,34
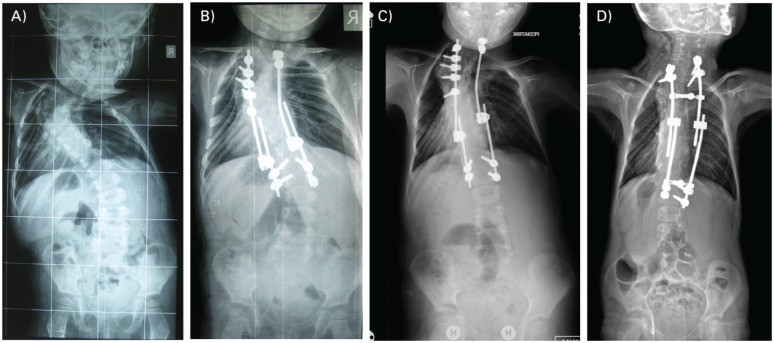
Fig. 1.
(A) A two-year-old girl with complex congenital scoliosis, (B) was operated on with posterior convex hemi-epiphysiodesis and growing rod. (C) Decompensation developed four years postoperatively and revision was carried out with multiple Schwab Type-2 osteotomies at the apex and changing of the growing rod construct. (D) Lengthening procedures are still ongoing at six-years postoperatively.
Surgical technique for growing rods
The authors’ preferred method is the subfascial or submuscular technique in which two separate incisions are made for proximal and distal fixation. After determining instrumentation levels according to Harrington’s stable zone, proximal and distal claws are applied by using either hooks or pedicle screws to make foundations. Two bilateral rods are introduced subfascially without opening the skin and dissecting muscles between two ends. This precludes spontaneous fusion. Then, both titanium rods are connected to each other with tandem connectors (Fig. 1C and 1D). These connectors allow lengthening procedures every six to nine months. Cross-links are usually mounted on each side, especially if hooks are applied. Limited fusion is preferred around the foundations for preventing pull-outs. A recent study showed that type of proximal foundation does not change outcome and rate of complications.35 A thoracolumbosacral orthosis is used at for least six months postoperatively. If the patient is too active, orthosis can be maintained whilst lengthening the growing rod. Distraction devices are used during lengthening procedures with limited incisions on the tandem connectors. This can be carried out as day surgery if revision of foundations or rods are not necessary. Graduation of the growing rods can be considered if the patient has reached sufficient maturity (acetabular cartilage should be closed, and also age should be at least 10 years for girls, 12 years for boys).36 After achieving optimal correction final fusion should be carried out with further curve correction if needed.
Results
Helenius et al compared the outcomes of growing rod surgery performed in a total of 107 EOS patients including two groups, severe and moderate curves. They found that even severe curves can be treated with growing rods, although this group of patients had higher complication rates. Severely scoliotic patients have significantly higher neurological complications. Risk factors are correction/distraction during the index surgery, difficulties in placing screws during index surgery and screw pull-outs.37
Magnetically controlled growing rods
MCGR were launched a decade ago in order to reduce the number of lengthening procedures performed in the operating room and concerns regarding general anaesthesia.38 A remote distracting device which allows distractions of rods outside of the body is used as an out-patient procedure. However, many studies have reported unplanned surgeries and revisions due to implant failures such as anchor pull-outs.39
Indications for MCGR
This special device needs specific indications especially for use in young children. Technically it is not possible to bend the shaft of the distraction component. This important detail limits usage of the device, especially for kyphotic patients. Another concern about this implant is metallosis around the distraction device.40 Rod breakage is not accepted as a complication in traditional growing rods. When the rod breaks in the traditional technique this prevents proximal anchor pull-out which sometimes causes devastating problems. However, MCGR hardly ever breaks because of its larger diameter. This potentially comes with a risk of proximal anchor pull-out.41
Surgical and distraction technique of MCGR
The surgical technique of MCGR is similar to that with traditional growing rods. After inserting the proximal and distal anchors, rods are introduced subfascially. An interval under the fascia should be created by dissection with scissors or a clamp for facilitating the rod placement. The lengthening procedure is performed using an external magnet in the out-patient clinic. This procedure is not painful for the patient. There is no consensus for lengthening frequency, timing and amount of distraction. The authors of this article prefer lengthening every two to three months using the maximum amount of lengthening in every session.
Results
MCGR were found to be an effective method for the treatment of primary EOS cases. However, lengthening rate declined in conversion cases according to a multicentre study.42 Magnetically controlled and traditional growing rod patients were compared in terms of psychosocial health status in a cross-sectional case control study. The aim was to evaluate the impact of surgical stress on psychosocial health in EOS patients who treated two different surgical implants. They found no improvement regarding psychosocial stress with the less invasive lengthening procedure applied in the out-patient setting.43 The same group demonstrated that the positive effect of MCGR on health-related quality of life decreased with the passage of time. So, this newer technology is not a ‘magic fix-all’ device and the traditional technique is still valid as an option for the treatment of EOS.44
Comparison of growing rods and serial casting
The growing rod has some of significant advantages over casting. A child with a torso cast will have a very limited mobilization ability. Although the hip, knee, and ankle joints can move completely in their full range of motion, upper body swinging becomes limited. This limitation of upper body movement also prevents the patient from reaching several points on his/her body by hand for scratching or cleaning. For all these reasons, a very young child with a body cast needs very concentrated attention from their carers.28 Serial casting should be applied every two to four months, whereas growing rods should be lengthened only every 6–12 months. More frequent general anaesthesia exposure comes with greater possibility of side effects. On the other hand, starting the growing rod surgery at a very young age may result in a spontaneous fusion problem.11 Thus, serial casting is still an important time-gaining method for delaying operations in the management of EOS.
Anterior vertebral body tethering
Treatment of spinal deformity on a growing spine should aim for 3-D correction of scoliosis and continued growth.45 In producing a long-term sustainable outcome spinal fusion has been the only procedure achieving this goal. Currently, pedicle screw instrumentation with posterior spinal fusion represents the ‘gold-standard’ of treatment for idiopathic scoliosis, when a minimum 22 cm thoracic height can be been reached.31,45,46 Additional length obtained from spinal fusion averages about 25 mm in a normal AIS.47 Spinal fusion is a permanent stage which cannot be reversed. Additionally, it will decrease spinal mobility48 and will strain the remaining mobile segments.49,50
The vertebral body grows via endochondral ossification (length) and intramembranous (circumferential) growth.51 Compression of the growth plate will inhibit growth and distraction will promote growth (Hueter–Volkmann principle).52 Asymmetrical growth plate inhibition using stapling and unilateral plates with screws has been used for decades in the growing lower extremities to address mechanical axis deviations.53 Paediatric orthopaedic surgeons have tried for decades to control scoliosis by using asymmetrical hemiepiphysiodesis, but this remained unpredictable.51,54,55 Betz and colleagues tried to produce asymmetric hemiepiphysiodesis using stapling over the disc and growth plates, but this worked properly only in thoracic curves of less than 35 degrees, which typically are treated using a brace on a growing child.56
Recently, advances in the understanding of spine biomechanics, development of minimally invasive surgical techniques, and improved device design have led to a new generation of fusionless implants for growth modulation of the spine: ‘spinal tethering’.57–59 Spinal tethering is carried out using an anterior approach (thoracoscopic, mini-open or open surgery). Each vertebral body is instrumented with a single typically bicortical screw and between the screws a cable is tightened. Correction of the deformity will occur during the initial surgery, but also during continued spinal growth according to the Hueter–Volkmann principle.
Indications for and technical considerations of spinal tethering
The most well documented indication for spinal tethering is a Lenke 1 or 2 curve with flexible lumbar and proximal thoracic curve.59 Skeletal growth is assessed using a hand radiograph and Sanders’ classification.60 Currently, the ideal patient for this procedure should have a relatively flexible right thoracic curve (bending to 30 degrees or below), a reasonable rib hump (less than 20 degrees), and a suitable amount of growth remaining (Sanders between 3 and 4). If the procedure is performed too early the curve may sometimes reverse into an overcorrection (right-sided curve will become left-sided curve), and if performed too late the remaining growth modulation will not correct the curve enough and a tethering rupture may result. In the thoracic spine, anterior shortening may help with the restoration of thoracic kyphosis, but according to the literature this seems to be minimal. Thoracolumbar idiopathic scoliosis might be an option for spinal tethering, but loss of lumbar lordosis might be an issue and there are no studies on spinal tethering in this area.
The procedure should be carried out using a strict lateral decubitus position and single lung ventilation. Instrumentation is typically carried from end vertebra to end vertebra. Spinal tethering is carried out using an anterior approach (thoracoscopic, mini-open or open surgery).59 Most surgeons favour either thoracoscopic or mini-open approaches to minimize the chest wall violation and the associated deleterious effects on pulmonary function.53 Preoperative initial screw trajectory planning with fluoroscopy helps in the portal planning.
During the surgery, the right lung should be easily collapsed. The parietal pleura is opened over the spine. Segmental vessels are ligated on the convex side. Staples and bicortical screws are positioned using fluoroscopic control. The polyethylene tether is tightened especially over the apical segments, while the upper two screws and the lower end are left relatively loose to prevent screw pull-out. A chest drainage tube is typically placed and set to suction.
The mini-open technique (small thoracotomy) allows segmental vessels to be mobilized especially in the apical area, whilst with the thoracoscopic technique all the segmental vessels need to be ligated. Spinal manipulation through the open wound in terms of de-rotation and tightening of the cord seems to be easier as well. The thoracoscopic technique might reduce postoperative pain and maintain pulmonary function as no chest distraction is needed. However, there are no studies comparing these two approaches.
Results
As stated above, the premise of anterior vertebral body tethering (AVBT) includes the modulation of vertebral growth in AIS patients in a way to promote deformity correction in all planes through a first correction achieved at the time of surgery together with subsequent improvements through asymmetrical vertebral growth through the Hueter–Volkman principle.60 Although a similar technique may be used from the posterior aspect, i.e. tethering by the insertion of tethers (mostly flexible bands), the anterior technique has been proven to be more effective in 3-D deformity correction61,62 in a finite element model. Another finite element model has demonstrated that the important factors for an ideal correction are the tensioning of the cable (100 N vs. 200 N) and the location of the screws on the lateral sides of the vertebral bodies (lateral/anterior/triangulated), a 200 N tightening and an anterior location providing better correction rates in all three planes.63 All in all, AVBT appears to be an effective modality in these models. As for clinical data regarding its efficacy and safety, we have witnessed some reports appearing in peer-reviewed journals as well as presentations in meetings all of which demonstrated the efficacy of the technique with some different views with regard to its safety. The following sections will summarize the theoretical advantages of AVBT, as well as disadvantages and the available clinical data.
Advantages
The main premise of AVBT is correction of the scoliotic deformity in all three planes without reverting to spinal fusion. Implants inserted through an open or minimally invasive thoracotomy assume the role of initial correctors, tethers enabling further correction through the axial growth of the patient and maintainers of the achieved correction in the long run. In terms of correction, AVBT has the potential to correct coronal plane deformity by compression and further inhibition of growth at the convexity of the curve(s), correcting the hypokyphosis as the compression and further tethering is placed at the anterior column and correcting axial rotation as these forces are applied from the side (laterally).
Further advantages may be listed as a more rapid return to normal daily life, especially if a minimally invasive technique such as thoracoscopy is used. Thoracoscopy is expected to provide minimal respiratory problems as well as minimal blood loss.64 Another advantage is claimed to be the achievement of correction in a way that the mobility of the spinal column at the involved segments is preserved, at least to some extent. These advantages, taken together, suggest that AVBT appears to be a ‘dream come true’ in the surgical treatment of AIS.
Clinical results
A limited number of clinical case series demonstrating the efficacy of AVBT is available in the literature. Of these, Samdani et al58 in their analysis of the two-year results of 11 consecutive patients, reported a correction of the thoracic Cobb angle from 44.2±9.0 degrees to 20.3±11.0 degrees at the time of first correction which further improved to 13.5±11.6 degrees at the last follow-up (70% correction rate). In this series, lumbar curves were also demonstrated to undergo spontaneous correction from 25.1±8.7 degrees to 14.9±4.9 degrees and 7.2±5.1 degrees respectively (71% correction rate). Axial rotation measured by scoliometer was found to be improved from 12.4±3.3 degrees to 6.9±3.4 degrees at latest follow-up. In a more recent report, Alanay et al,65 reporting the results of 14 patients who had been followed for at least two years into skeletal maturity, demonstrated mean initial correction rates of 34%, 54% and 49%, and last follow-up correction rates of 44%, 78% and 83% for upper thoracic (UT), main thoracic (MT) and lumbar (L) curves, respectively. In their series, the mean rib hump of 12 degrees was found to be corrected to 5.4 degrees at final follow-up. Finally, Parent et al,66 reporting on 53 patients with an average follow-up of 33.4±7.9 months, have demonstrated that, in their cohort, the average Cobb angle of 49.4±11.0 preoperatively was corrected to 25.4±11.0 at two months, 17.0±12.4 at 16 months and 16.0±12.6 at last follow-up.
Disadvantages
Disadvantages and adverse events must be evaluated as these are reported as adverse events and complications, the rate of which is important. The most commonly demonstrated adverse events consist of those related to thoracic surgery (atelectasis, pneumothorax and pain), those related to growth (overcorrection) and mechanical and implant failures (screw pull-out, broken tethers), the rates for which will be discussed below (Fig. 2). Another disadvantage or concern regarding AVBT is the very limited nature of its published clinical results. As can be seen from the discussion above, clinical reports remain limited to a small number of teams, and remain to be substantiated by third parties.
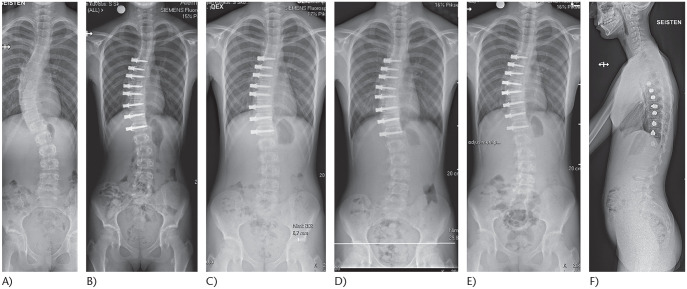
Fig. 2.
(A) An 11-year-old girl with juvenile idiopathic scoliosis Sanders 2. (B) Treated using anterior spinal tethering between T6 and L1. (C) Six-month postoperative radiograph demonstrates growth modulation: L1 is levelled. (D) One-year follow-up shows splaying between T9 and T10 screws. This suggests rupture of the tether. (E) Two-year follow-up shows a stable curve without further progression despite tether rupture. (F) Two-year lateral spinal radiograph demonstrates maintenance of the sagittal balance.
Theoretical disadvantages may be listed as concerns as to the long-term sustainability of the results both in terms of correction rates and the rates of adverse events or complications, the yet unproven claim of maintenance of motion in the instrumented segments, the inability to predict the behaviour and fate of the tethered intervertebral discs at longer follow-up, and, also, the potential effect of AVBT on the development and growth of the spinal canal in these patients. In short, we do not know the long-term outcomes of this technique as opposed to the results of fusion surgery and brace treatment. There is a chance that these patients may end up with stiff degenerated thoracic spines which may also be associated with spinal stenosis in these segments. This latter concern may be of utmost importance as a reduction in the enlargement of the spinal canal by growth has been demonstrated by Pekmezci et al67 on a porcine model of anterior fusion (not tethering). This phenomenon that has been described for fusion may or may not be a sequel following a non-fusion technology such as AVBT.
Complications
The reported nature of complications and their rates for AVBT appear promising. Samdani et al58 in their article discussed above, report their complications as two patients returning to the operating room at two years postoperatively for loosening of the tether to prevent overcorrection. Alanay et al65 report pulmonary complications (14%) as one atelectasis that resolved with physical therapy, and one pulmonary effusion that required re-admission (7%). Their mechanical complications were two overcorrections (14%) one of which was accompanied by a lower instrumented vertebra (LIV) screw loosening. On the other hand, Parent et al,66 focussing on the complications of this technique, report their complications as: revision surgery in six patients, one tether removal due to overcorrection, one lumbar tether added due to distal curve progression, one tether replaced due to breakage, one patient for screw re-positioning and two revised to a posterior spinal fusion due to progression. Sixteen (30%) of their patients had a suspected broken tether. Two patients had overcorrection that did not require revision. Two patients had pneumothorax, which developed after drain removal and resolved spontaneously. Two patients had a blood patch for dural tears recognized postoperatively. As can be seen, in addition to the scarcity of information, the available rates for complications also vary considerably between the reports.
In summary, AVBT appears to be a promising emerging technique for the surgical treatment of idiopathic scoliosis in immature patients. Its main premise is to achieve curve control and substantial correction in this group of patients without reverting to fusion surgery, thereby maintaining the mobility of the spinal column. As with any newly emerging technique, there are also concerns regarding AVBT which may be listed as a not-yet-proven outcome, unknown long-term results, and major discrepancies regarding the rates of complications and adverse effects in the available literature, which are at present a cause for concern.
Conclusions
Management of scoliosis is in young children is complex. There is no single recipe for every patient and treatment should be tailored based on the patient’s aetiology, curve pattern, maturity and co-morbidities. However, it is clear that surgical treatment, even growth-friendly surgical methods, should be postponed for as long as possible. Serial casting is a viable option in selected cases for this purpose. As a novel technique, vertebral body tethering allows correction of idiopathic deformity while preserving motion especially in juvenile cases with moderate curvature. However, long-term studies are needed to clarify complications and their prevention.
Footnotes
ICMJE Conflict of interest statement: AS reports payments as faculty in AOSpine Courses for AOSpine Foundation; is co-chair of the trauma module in the diploma programme of Eurospine; is supported by Eurospine for accommodation and travel expenses during courses; was co-chair of SRS Current Concepts in Deformity courses in 2019 with the Spine Society of Europe and Scoliosis Research paying for expenses during these activities, all outside the submitted work.
IH reports consultancy for and grants pending from Medtronic, K2M, outside the submitted work.
The other authors declare no conflict of interest relevant to this work.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Dobbs MB, Weinstein SL. Infantile and juvenile scoliosis. Orthop Clin North Am 1999;30:331–341, vii. [DOI] [PubMed] [Google Scholar]
- 2. El-Hawary R, Akbarnia BA. Letter to the editor, early onset scoliosis: time for consensus. Spine Deform 2015;3:105–106. [DOI] [PubMed] [Google Scholar]
- 3. Williams BA, Matsumoto H, McCalla DJ, et al. Development and initial validation a novel classification system for early-onset scoliosis: Classification of Early-Onset Scoliosis (C-EOS). J Bone Joint Surg Am 2014;96:1359–1367. [DOI] [PubMed] [Google Scholar]
- 4. Cyr M, Hilaire TS, Pan Z, Thompson GH, Vitale MG, Garg S; Childrenʼs Spine Study Group, Growing Spine Study Group. Classification of Early Onset Scoliosis has excellent interobserver and intraobserver reliability. J Pediatr Orthop 2017;37:e1–e3. [DOI] [PubMed] [Google Scholar]
- 5. Skaggs DL, Akbarnia BA, Flynn JM, Myung KS, Sponseller PD, Vitale MG; Chest Wall and Spine Deformity Study Group; Growing Spine Study Group; Pediatric Orthopaedic Society of North America; Scoliosis Research Society Growing Spine Study Committee. A classification of growth friendly spine implants. J Pediatr Orthop 2014;34:260–274. [DOI] [PubMed] [Google Scholar]
- 6. James JI. Two curve patterns in idiopathic structural scoliosis. J Bone Joint Surg Br 1951;33-B:399–406. [DOI] [PubMed] [Google Scholar]
- 7. Dede O, Sturm PF. A brief history and review of modern casting techniques in early onset scoliosis. J Child Orthop 2016;10:405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bradford EH, Brackett EG. Treatment of lateral curvature by means of pressure correction. Boston Med Surg J 1893;128:463–468. [DOI] [PubMed] [Google Scholar]
- 9. Cotrel Y, Morel G. [The elongation-derotation-flexion technic in the correction of scoliosis]. Rev Chir Orthop Reparatrice Appar Mot 1964;50:59–75. [PubMed] [Google Scholar]
- 10. Mehta M, Morel G. The non-operative treatment of infantile idiopathic scoliosis. In: Zorab P, Siegler D, eds. Sixth symposium on scoliosis September 17–18, 1979 London: Academic Press, 1979:71–84. [Google Scholar]
- 11. Cahill PJ, Marvil S, Cuddihy L, et al. Autofusion in the immature spine treated with growing rods. Spine (Phila Pa 1976) 2010;35:E1199–E1203. [DOI] [PubMed] [Google Scholar]
- 12. Sankar WN, Skaggs DL, Yazici M, et al. Lengthening of dual growing rods and the law of diminishing returns. Spine (Phila Pa 1976) 2011;36:806–809. [DOI] [PubMed] [Google Scholar]
- 13. Sanders JO, D’Astous J, Fitzgerald M, Khoury JG, Kishan S, Sturm PF. Derotational casting for progressive infantile scoliosis. J Pediatr Orthop 2009;29:581–587. [DOI] [PubMed] [Google Scholar]
- 14. Demirkiran HG, Bekmez S, Celilov R, Ayvaz M, Dede O, Yazici M. Serial derotational casting in congenital scoliosis as a time-buying strategy. J Pediatr Orthop 2015;35:43–49. [DOI] [PubMed] [Google Scholar]
- 15. Johnston CE, McClung AM, Thompson GH, Poe-Kochert C, Sanders JO; Growing Spine Study Group. Comparison of growing rod instrumentation versus serial cast treatment for early onset scoliosis. Spine Deform 2013;1:339–342. [DOI] [PubMed] [Google Scholar]
- 16. Gussous YM, Tarima S, Zhao S, et al. Serial derotational casting in idiopathic and non-idiopathic progressive early-onset scoliosis. Spine Deform 2015;3:233–238. [DOI] [PubMed] [Google Scholar]
- 17. Cao J, Zhang XJ, Sun N, et al. The therapeutic characteristics of serial casting on congenital scoliosis: a comparison with non-congenital cases from a single-center experience. J Orthop Surg Res 2017;12:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mehta MH. Growth as a corrective force in the early treatment of progressive infantile scoliosis. J Bone Joint Surg Br 2005;87:1237–1247. [DOI] [PubMed] [Google Scholar]
- 19. Fedorak GT, D’Astous JL, Nielson AN, MacWilliams BA, Heflin JA. Minimum 5-year follow-up of Mehta casting to treat idiopathic early-onset scoliosis. J Bone Joint Surg Am 2019;101:1530–1538. [DOI] [PubMed] [Google Scholar]
- 20. Hassanzadeh H, Nandyala SV, Puvanesarajah V, Manning BT, Jain A, Hammerberg KW. Serial Mehta cast utilization in infantile idiopathic scoliosis: evaluation of radiographic predictors. J Pediatr Orthop 2017;37:387–391. [DOI] [PubMed] [Google Scholar]
- 21. Welborn MC, D’Astous J, Bratton S, Heflin J. Infantile idiopathic scoliosis: factors affecting EDF casting success. Spine Deform 2018;6:614–620. [DOI] [PubMed] [Google Scholar]
- 22. Iorio J, Orlando G, Diefenbach C, et al. Serial casting for infantile idiopathic scoliosis: radiographic outcomes and factors associated with response to treatment. J Pediatr Orthop 2017;37:311–316. [DOI] [PubMed] [Google Scholar]
- 23. Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci 2003;23:876–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hu D, Flick RP, Zaccariello MJ, et al. Association between exposure of young children to procedures requiring general anesthesia and learning and behavioral outcomes in a population-based birth cohort. Anesthesiology 2017;127:227–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Canavese F, Botnari A, Dimeglio A, et al. Serial elongation, derotation and flexion (EDF) casting under general anesthesia and neuromuscular blocking drugs improve outcome in patients with juvenile scoliosis: preliminary results. Eur Spine J 2016;25:487–494. [DOI] [PubMed] [Google Scholar]
- 26. Badlani N, Korenblit A, Hammerberg K. Subclavian vein thrombosis after application of body cast. J Pediatr Orthop 2013;33:e1–e3. [DOI] [PubMed] [Google Scholar]
- 27. Dhawale AA, Shah SA, Reichard S, et al. Casting for infantile scoliosis: the pitfall of increased peak inspiratory pressure. J Pediatr Orthop 2013;33:63–67. [DOI] [PubMed] [Google Scholar]
- 28. Thorsness RJ, Faust JR, Behrend CJ, Sanders JO. Nonsurgical management of early-onset scoliosis. J Am Acad Orthop Surg 2015;23:519–528. [DOI] [PubMed] [Google Scholar]
- 29. Harrington PR. Treatment of scoliosis: correction and internal fixation by spine instrumentation. J Bone Joint Surg Am 1962;44-A:591–610. [PubMed] [Google Scholar]
- 30. Karol LA. Early definitive spinal fusion in young children: what we have learned. Clin Orthop Relat Res 2011;469:1323–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Karol LA, Johnston C, Mladenov K, Schochet P, Walters P, Browne RH. Pulmonary function following early thoracic fusion in non-neuromuscular scoliosis. J Bone Joint Surg Am 2008;90:1272–1281. [DOI] [PubMed] [Google Scholar]
- 32. Thompson GH, Akbarnia BA, Kostial P, et al. Comparison of single and dual growing rod techniques followed through definitive surgery: a preliminary study. Spine (Phila Pa 1976) 2005;30:2039–2044. [DOI] [PubMed] [Google Scholar]
- 33. Yang JS, McElroy MJ, Akbarnia BA, et al. Growing rods for spinal deformity: characterizing consensus and variation in current use. J Pediatr Orthop 2010;30:264–270. [DOI] [PubMed] [Google Scholar]
- 34. Sun ZJ, Qiu GX, Zhao Y, et al. Dual growing rod treatment in early onset scoliosis: the effect of repeated lengthening surgeries on thoracic growth and dimensions. Eur Spine J 2015;24:1434–1440. [DOI] [PubMed] [Google Scholar]
- 35. Shetty AP, Nikhil KV, Renjith KR, Vijayanand KSS, Kanna PRM, Rajasekaran S. Proximal anchor constructs and its influence on surgical outcome in growth rod technique: a comparison between rib hooks and pedicle screws. Spine Deform 2019;7:979–984. [DOI] [PubMed] [Google Scholar]
- 36. Thompson GH, Akbarnia BA, Campbell RM., Jr Growing rod techniques in early-onset scoliosis. J Pediatr Orthop 2007;27:354–361. [DOI] [PubMed] [Google Scholar]
- 37. Helenius IJ, Oksanen HM, McClung A, et al. Outcomes of growing rod surgery for severe compared with moderate early-onset scoliosis: a matched comparative study. Bone Joint J 2018;100-B:772–779. [DOI] [PubMed] [Google Scholar]
- 38. Cheung KM, Cheung JP, Samartzis D, et al. Magnetically controlled growing rods for severe spinal curvature in young children: a prospective case series. Lancet 2012;379:1967–1974.22520264 [Google Scholar]
- 39. Choi E, Yaszay B, Mundis G, et al. Implant complications after magnetically controlled growing rods for early onset scoliosis: a multicenter retrospective review. J Pediatr Orthop 2017;37:e588–e592. [DOI] [PubMed] [Google Scholar]
- 40. Yilgor C, Efendiyev A, Akbiyik F, et al. Metal ion release during growth-friendly instrumentation for early-onset scoliosis: a preliminary study. Spine Deform 2018;6:48–53. [DOI] [PubMed] [Google Scholar]
- 41. Lebon J, Batailler C, Wargny M, et al. Magnetically controlled growing rod in early onset scoliosis: a 30-case multicenter study. Eur Spine J 2017;26:1567–1576. [DOI] [PubMed] [Google Scholar]
- 42. Hosseini P, Pawelek J, Mundis GM, et al. Magnetically controlled growing rods for early-onset scoliosis: a multicenter study of 23 cases with minimum 2 years follow-up. Spine (Phila Pa 1976) 2016;41:1456–1462. [DOI] [PubMed] [Google Scholar]
- 43. Aslan C, Olgun ZD, Ayik G, et al. Does decreased surgical stress really improve the psychosocial health of early-onset scoliosis patients? A comparison of traditional growing rods and magnetically-controlled growing rods patients reveals disappointing results. Spine (Phila Pa 1976) 2019;44:E656–E663. [DOI] [PubMed] [Google Scholar]
- 44. Doany ME, Olgun ZD, Kinikli GI, et al. Health-related quality of life in early-onset scoliosis patients treated surgically: EOSQ scores in traditional growing rod versus magnetically controlled growing rods. Spine (Phila Pa 1976) 2018;43:148–153. [DOI] [PubMed] [Google Scholar]
- 45. Akbarnia BA. Management themes in early onset scoliosis. J Bone Joint Surg Am 2007;89:42–54. [DOI] [PubMed] [Google Scholar]
- 46. Sponseller PD, Jain A, Newton PO, et al. Posterior spinal fusion with pedicle screws in patients with idiopathic scoliosis and open triradiate cartilage: does deformity progression occur? J Pediatr Orthop 2016;36:695–700. [DOI] [PubMed] [Google Scholar]
- 47. Oksanen H, Lastikka M, Helenius L, Pajulo O, Helenius I. Posterior spinal fusion extended to stable vertebra provides similar outcome in juvenile idiopathic scoliosis patients compared with adolescents with fusion to the touched vertebra. Scand J Surg 2019;108:83–89. [DOI] [PubMed] [Google Scholar]
- 48. Spencer HT, Gold ME, Karlin LI, Hedequist DJ, Hresko MT. Gain in spinal height from surgical correction of idiopathic scoliosis. J Bone Joint Surg Am 2014;96:59–65. [DOI] [PubMed] [Google Scholar]
- 49. Helenius I, Remes V, Yrjönen T, et al. Harrington and Cotrel-Dubousset instrumentation in adolescent idiopathic scoliosis: long-term functional and radiographic outcomes. J Bone Joint Surg Am 2003;85:2303–2309. [DOI] [PubMed] [Google Scholar]
- 50. Marks MC, Bastrom TP, Petcharaporn M, et al. The effect of time and fusion length on motion of the unfused lumbar segments in adolescent idiopathic scoliosis. Spine Deform 2015;3:549–553. [DOI] [PubMed] [Google Scholar]
- 51. Parsch D, Gaertner V, Brocai DR, Carstens C. The effect of spinal fusion on the long-term outcome of idiopathic scoliosis: a case-control study. J Bone Joint Surg Br 2001;83:1133–1136. [DOI] [PubMed] [Google Scholar]
- 52. Roaf R. Vertebral growth and its mechanical control. J Bone Joint Surg Br 1960;42-B:40–59. [DOI] [PubMed] [Google Scholar]
- 53. Newton PO, Upasani VV, Farnsworth CL, et al. Spinal growth modulation with use of a tether in an immature porcine model. J Bone Joint Surg Am 2008;90:2695–2706. [DOI] [PubMed] [Google Scholar]
- 54. Bouchard M. Guided growth: novel applications in the hip, knee, and ankle. J Pediatr Orthop 2017;37:S32–S36. [DOI] [PubMed] [Google Scholar]
- 55. Roaf R. The treatment of progressive scoliosis by unilateral growth-arrest. J Bone Joint Surg Br 1963;45:637–651. [PubMed] [Google Scholar]
- 56. Betz RR, Ranade A, Samdani AF, et al. Vertebral body stapling: a fusionless treatment option for a growing child with moderate idiopathic scoliosis. Spine (Phila Pa 1976) 2010;35:169–176. [DOI] [PubMed] [Google Scholar]
- 57. Crawford CH, III, Lenke LG. Growth modulation by means of anterior tethering resulting in progressive correction of juvenile idiopathic scoliosis: a case report. J Bone Joint Surg Am 2010;92:202–209. [DOI] [PubMed] [Google Scholar]
- 58. Samdani AF, Ames RJ, Kimball JS, et al. Anterior vertebral body tethering for idiopathic scoliosis: two-year results. Spine (Phila Pa 1976) 2014;39:1688–1693. [DOI] [PubMed] [Google Scholar]
- 59. Newton PO, Kluck DG, Saito W, Yaszay B, Bartley CE, Bastrom TP. Anterior spinal growth tethering for skeletally immature patients with scoliosis: a retrospective look two to four years postoperatively. J Bone Joint Surg Am 2018;100:1691–1697. [DOI] [PubMed] [Google Scholar]
- 60. Sanders JO, Khoury JG, Kishan S, et al. Predicting scoliosis progression from skeletal maturity: a simplified classification during adolescence. J Bone Joint Surg Am 2008;90:540–553. [DOI] [PubMed] [Google Scholar]
- 61. Jain V, Lykissas M, Trobisch P, et al. Surgical aspects of spinal growth modulation in scoliosis correction. Instr Course Lect 2014;63:335–344. [PubMed] [Google Scholar]
- 62. Aubin CÉ, Clin J, Rawlinson J. Biomechanical simulations of costo-vertebral and anterior vertebral body tethers for the fusionless treatment of pediatric scoliosis. J Orthop Res 2018; 36:254–264. [DOI] [PubMed] [Google Scholar]
- 63. Cobetto N, Parent S, Aubin CE. 3D correction over 2 years with anterior vertebral body growth modulation: a finite element analysis of screw positioning, cable tensioning and postoperative functional activities. Clin Biomech (Bristol, Avon) 2018;51:26–33. [DOI] [PubMed] [Google Scholar]
- 64. Samdani AF, Ames RJ, Kimball JS, et al. Anterior vertebral body tethering for immature adolescent idiopathic scoliosis: one-year results on the first 32 patients. Eur Spine J 2015;24:1533–1539. [DOI] [PubMed] [Google Scholar]
- 65. Alanay A, Yucekul A, Kindan P, Tanriover HH, Zulemyan T, Ergene G, Senay S, Ay B, Yavuz Y, Yilgor C. Thoracoscopic vertebral body tethering for adolescent idiopathic scoliosis: minimum 2 years results of patients reaching skeletal maturity. Presented at the Annual Meeting of Eurospine, Helsinki, 2019. [Google Scholar]
- 66. Parent S, Alzakri A, Roy-Beaudry M, Turgeon I, Beauséjour M, Turcot O. Surgical complications of anterior vertebral body growth modulation for skeletally immature patients with idiopathic scoliosis. Presented at the Annual Meeting of Eurospine, Helsinki, 2019. [Google Scholar]
- 67. Pekmezci M, Yilmaz G, Daglioglu K, et al. The effect of anterior spinal fusion on spinal canal development in an immature porcine model. Spine (Phila Pa 1976) 2009;34:E501–E506. [DOI] [PubMed] [Google Scholar]