Abstract
PURPOSE
To identify factors that may influence physician participation in tumor profiling studies and to assess the routine use of tumor profiling in clinical practice.
METHODS
Physicians in the National Cancer Institute–Molecular Analysis for Therapy Choice (NCI-MATCH) were invited to participate in an electronic survey consisting of 73 questions related to participation in genomic profiling studies, tumor profiling practices and education during usual patient care, and physician background and practice characteristics.
RESULTS
The survey response rate was 8.9% (171 surveys returned of 1,931 sent). A majority of respondents practiced in academic medical centers (AMCs). Participation in NCI-MATCH increased workload and cost but resulted in increased professional satisfaction, confidence in treatment recommendation, and subsequent use of tumor profiling. Barriers to patient participation included length of wait time for results and lack of a therapeutic option from the testing. Physicians who worked in AMCs reported a higher use of tumor profiling than did those who worked in non-AMC settings (43% v 18%; P = .0009). Access to a molecular tumor board was perceived as valuable by 56%. The study identified a need for educational materials to guide both physicians and patients in the field of genomic profiling.
CONCLUSION
Physicians who participate in NCI-MATCH perceive value to patient treatment that outweighs the additional effort required; survey results help identify barriers that may limit participation. The current findings have implications for the design of future genomic and other profiling studies.
INTRODUCTION
The National Cancer Institute (NCI) launched the Molecular Analysis for Therapy Choice (NCI-MATCH) clinical trial initiative to test the hypothesis that targeting a molecular variant can identify responders independent of cancer type. This initiative was led by the Eastern Cooperative Oncology Group and the American College of Radiology Imaging Network Cancer Research Group (ECOG-ACRIN), with participation from all National Clinical Trials Network (NCTN) groups as well as the NCI Community Oncology Research Program.1 In the NCI-MATCH trial, patients with metastatic cancers underwent a screening step (step 0) in which tumor biopsy specimens were screened for alterations in approximately 143 cancer-related genes, as well as immunohistochemical assays for expression of MLH1, MSH2, and PTEN.2,3 Participants found to harbor actionable mutations were assigned to therapeutic subprotocols of agents targeting the specific molecular variant (and which excluded those cancer types for which an agent had known efficacy or lack of efficacy). Rescreening at the time of tumor progression, with the possibility of assignment to another therapy with NCI-MATCH, was offered.1 The trial opened in August 2015 and completed its screening portion in May 2017. Accrual was held for a planned interim analysis in November 2015. A preplanned enrollment pause occurred from November 2015 to May 2016 and was designed to permit an interim feasibility and systems analysis.4
CONTEXT
Key Objective
The National Cancer Institute launched the Molecular Analysis for Therapy Choice (NCI-MATCH) clinical trial initiative to test the hypothesis that targeting a molecular variant can identify responders agnostic of histology. This survey of physicians participating in NCI-MATCH was designed to identify factors that may influence participation in tumor profiling studies and to assess the routine use of tumor profiling in clinical practice.
Knowledge Generated
Physicians indicated that participation in NCI-MATCH increased workload and cost but led to increased confidence in treatment recommendations and subsequent use of tumor profiling; some findings differed on the basis of whether physicians worked in academic medical centers (AMCs) or non-AMC settings. Barriers to patient participation included length of wait time for results and a lack of a therapeutic option from the testing.
Relevance
These survey results help identify barriers that may limit participation in genomic and profiling studies and have implications for future study design.
For large tumor profiling clinical trials such as NCI-MATCH to succeed, their design and methods must be broadly acceptable to physicians and patients across the enrollment sites, so that sufficient numbers of participants can be identified in a reasonable timeframe. We conducted a survey of physicians at clinical practices participating in NCI-MATCH to identify factors that may influence participation in tumor profiling studies and to assess the routine use of tumor profiling in clinical practice. This survey targeted the initial sites that had enrolled patients by time of the interim analysis.
The range of oncology practices that participate in the NCTN allowed us to examine whether the use of tumor profiling varies by practice setting. A 2017 survey of 1,281 oncologists in the United States (National Survey of Precision Medicine in Cancer Treatment) did not find differences in the use of next-generation sequencing (NGS) tests on the basis of whether oncologists’ primary practices were affiliated with academic institutions, although those who had faculty appointments showed higher use of NGS tests than did those who did not.5 The current survey explored in more detail the influence of practice setting to help understand tumor profiling use patterns among oncologists.
METHODS
The survey used in this study was developed by the authors. The initial draft survey was tested with four physicians who had registered patients in the NCI-MATCH trial and was edited on the basis of their input. Questions from the initial survey were modified and administered in qualitative interviews with 12 physicians and 12 patients who had participated in the NCI-MATCH trial, to ensure the inclusion of relevant topics and terminology. The four principal investigators of the NCI-MATCH trial also provided input into the survey and approved the survey in its final form.
The final, electronic survey consisted of 65 questions for which respondents were required to click an answer on a 4-point or 5-point Likert-scale (eg, from “not at all important” to “extremely important”) or to click all answers that applied (full survey available in Appendix 1). The survey also included eight optional, open-ended questions for which physicians could document their experiences or opinions.
The first part of the survey focused on learning from physicians’ experiences with the NCI-MATCH trial to aid in the development of future tumor profiling clinical trials. Questions were related to participation in the NCI-MATCH trial and future genomic profiling trials (n = 37 + n = 7 open ended), tumor profiling practices and education during usual patient care (n = 14), and physician background and practice characteristics (n = 14). The final open-ended question sought additional comments or any issues not covered by the survey. Tumor profiling was defined in the survey as multiparameter molecular testing intended to identify targeted treatments and was accompanied by the following additional text: “Consider testing for more than 5 genes, such as tumor profiling or circulating tumor DNA testing (e.g., FoundationOne, Cambridge, MA; Caris, Phoenix, AZ).” The entire survey was designed to take approximately 10 minutes to complete.
An e-mail inviting participation in the survey was sent on October 16, 2017, by the central offices of ECOG-ACRIN to all physicians participating in the NCI-MATCH trial who had registered at least one participant to step 0 (screening) as of October 12, 2017; the physicians were at 553 sites and not all were from ECOG-ACRIN. The e-mail contained a hyperlink to the survey, which was accessible until November 14, 2017. Reminders were sent to physicians on October 30 and November 7, 2017.
The invitation e-mail specified that responses would be confidential and would be submitted directly to an independent research firm (CBWhite, Evanston, IL). Each respondent’s answers were saved using a unique de-identified numeric ID, maintained by the independent research firm, and merged with the respondents’ practice information (ie, the number of patients enrolled in each step in the NCI-MATCH trial and the respondent’s practice setting, information provided by ECOG-ACRIN).
Results were summarized descriptively with frequencies (as percentages), medians, and interquartile ranges where appropriate. Responses regarding tumor profiling practices were tabulated by practice setting; respondents were considered to work in academic medical centers (AMCs) if they selected the options of AMCs, medical school-main campus, and/or satellite clinic of an AMC. All others were considered non-AMCs. Differences in categorical data between practice settings were evaluated using Fisher’s exact test. All P values were two sided, with a value of < .05 considered statistically significant. All analyses were performed using SAS 9.4.
RESULTS
Survey Respondents
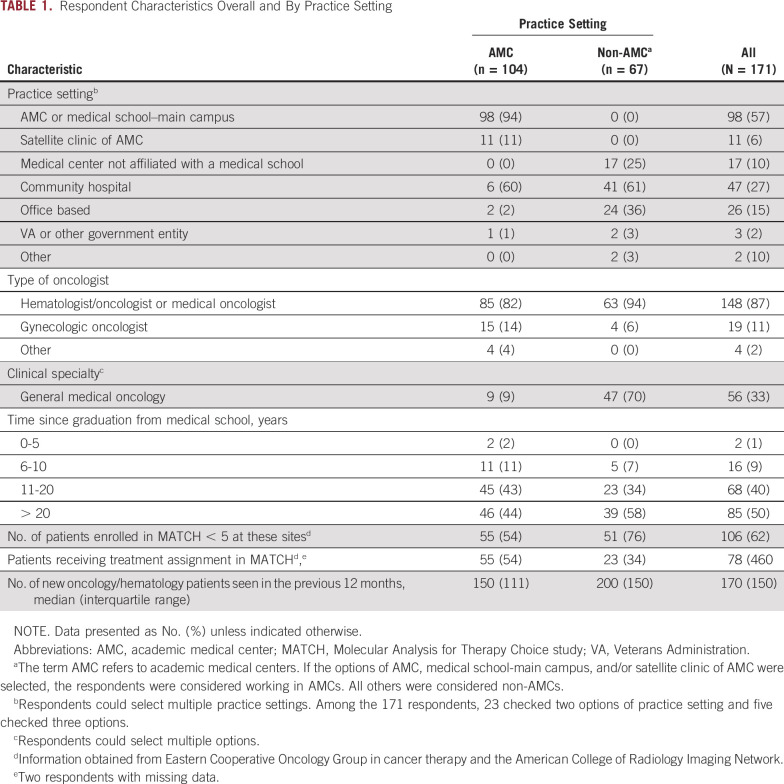
Of 1,931 surveys sent, 171 were returned, for a response rate of 8.9%. Table 1 summarizes the respondents’ basic information by practice setting (AMC v non-AMC) and overall. Most respondents were combined hematologists/oncologists or medical oncologists (87%). One half of the respondents had been out of medical school for more than 20 years, and 40% had been out for 11-20 years. Most (82%) indicated that they had seen ≥ 100 new oncology/hematology patients within the previous 12 months. On average, respondents estimated that they spent 65% of their professional time providing patient care, 15% on research, 10% on administration, and 5% on teaching.
TABLE 1.
Respondent Characteristics Overall and By Practice Setting
The most common practice setting was AMC or main campus of medical school (57%), followed by community hospital (27%), office-based practice (15%), medical center not affiliated with medical school (10%), satellite clinic of AMC (6%), Veterans Administration or other government entity (2%), and other (1%). When dichotomized by practice setting, 104 respondents (61%) were classified as AMC, whereas the remainder (39%) did not indicate an AMC setting and were classified as non-AMC. More AMC physicians than non-AMC physicians enrolled more than four patients into the MATCH trial (46% v 24%; P = .004), which could reflect the size of the site.
Participation in Tumor Genomic Profiling Trials
For most respondents, participating in the NCI-MATCH trial increased workloads and costs: 61%-69% and 10%-15% of respondents indicated a somewhat or greatly increased workload, respectively. Just over one half (52%) reported that their personal workload was somewhat increased, and 5% reported that it was greatly increased; 57% reported somewhat increased and 8% reported greatly increased costs to the site. Trial participation also increased the time required to discuss treatment options with patients (56% reported somewhat increased and 18% reported greatly increased time).
Participating in NCI-MATCH somewhat or greatly increased physician satisfaction with care provided for 57% and increased confidence in recommending a treatment for 48%. Seventy percent reported that participating in NCI-MATCH somewhat (56%) or greatly (14%) increased the number of patients for whom they recommend tumor profiling. A majority (62%) said participation in NCI-MATCH did not affect prognostic information for their patients.
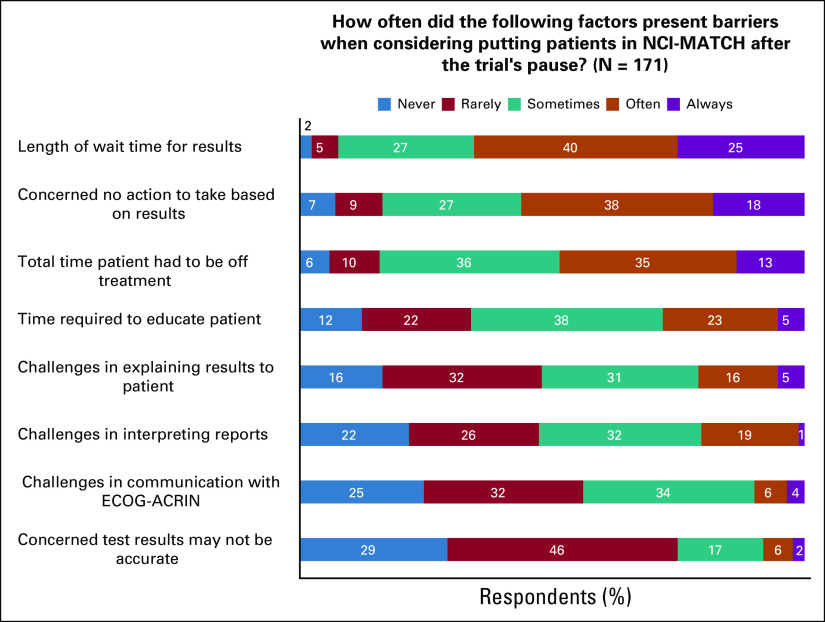
Of the barriers to placing patients in NCI-MATCH, the length of wait time for results and no target were chosen as “often” or “always” barriers most frequently (Fig 1). The time patients had to be off treatment was also “often” or “always” a barrier for 48% of respondents in placing patients in NCI-MATCH. In contrast, concern over the accuracy of the test results was not a barrier (Fig 1). Note that the question on barriers referred only to those encountered after the NCI-MATCH trial’s preplanned interim analysis.4
FIG 1.
Barriers to enrolling patients in the National Cancer Institute–Molecular Analysis for Therapy Choice (NCI-MATCH) trial. ECOG-ACRIN, Eastern Cooperative Oncology Group and American College of Radiology Imaging Network.
Current Tumor Profiling Practices
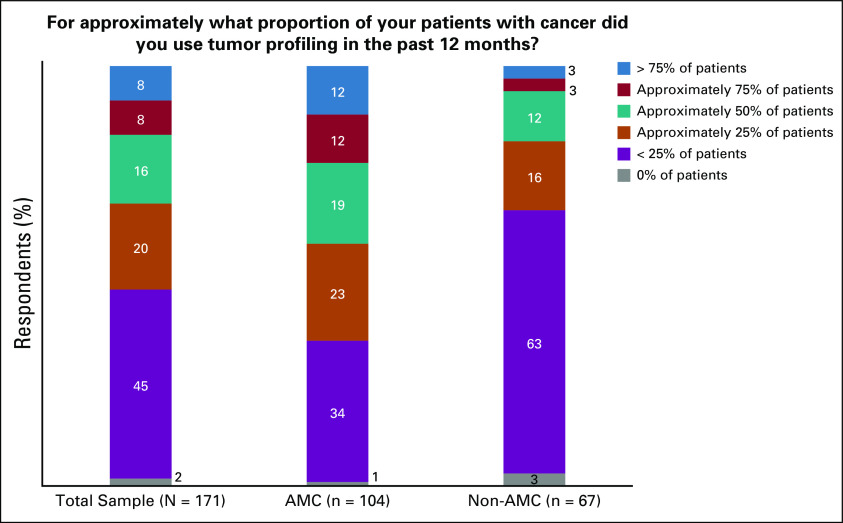
Approximately one half of the respondents reported using tumor profiling in less than a quarter of their patients over the previous 12 months (Fig 2). However, the proportion using tumor profiling tests in 50% or more of their patients differed between respondents practicing in AMCs (43%) and those practicing in non-AMCs (18%; P = .0009). Overall, 74% of respondents reported that when they used tumor profiling, it was “somewhat useful” (65%) or “very useful” (9%); no differences were observed between AMCs and non-AMCs regarding this question (76% AMC v 70% non-AMC; P = .58)
FIG 2.
Estimated use of tumor profiling. AMC, academic medical center.
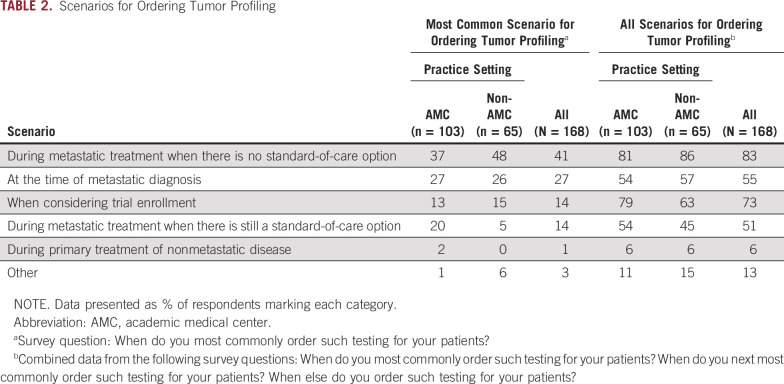
Table 2 lists respondents’ most common scenarios for ordering tumor profiling for their patients. A statistically significant difference between AMC and non-AMC physicians was noted in the responses to this question (P = .014). When answers from all relevant questions were combined to determine when respondents ordered tumor profiling for their patients (ie, not limited to the most common scenario), 83% indicated that they ordered testing during metastatic treatment when there were no standard-of-care (SOC) options (Table 2). More AMC physicians than non-AMC physicians indicated that they ordered tumor profiling tests when considering trial enrollment (79% v 63%; P = .034). When considering patients for whom tumor profiling was used to determine treatment in the previous 12 months, 85% of respondents indicated that one half or fewer were part of a clinical trial. The distribution of responses did not significantly differ between AMC and non-AMC respondents regarding patients who received tumor profiling within a clinical trial.
TABLE 2.
Scenarios for Ordering Tumor Profiling
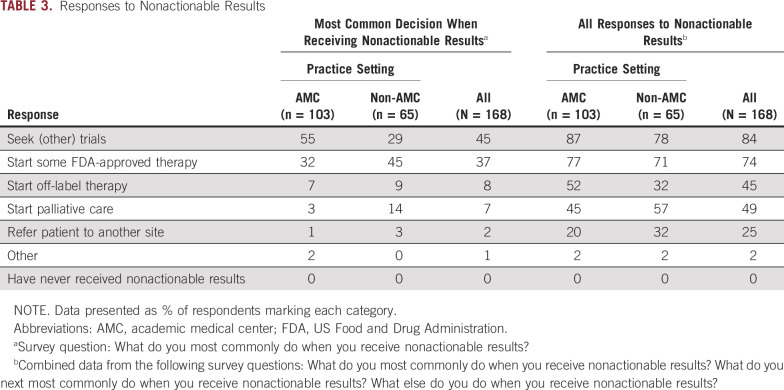
Table 3 summarizes physicians’ most common decisions after receiving nonactionable results. The distribution of responses to this question differed by practice setting (P = .002).
TABLE 3.
Responses to Nonactionable Results
When responses to all questions regarding the response to nonactionable results were considered (ie, any response to nonactionable results; Table 3), similar response ranking across institution type was observed. Significantly more AMC respondents indicated that they would start off-label therapy (52% AMC v 32% non-AMC; P = .011).
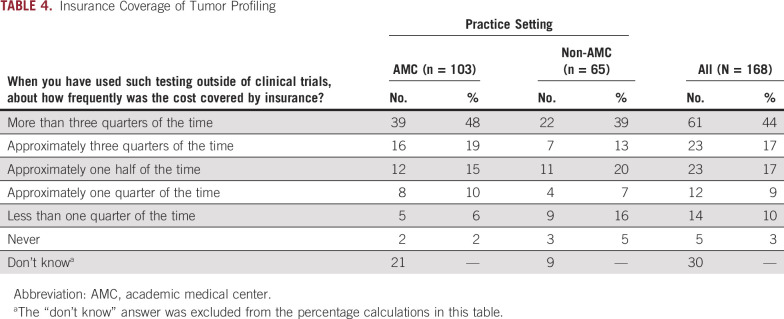
Table 4 lists the frequency and percentage of physicians’ responses regarding insurance coverage of patients’ tumor profiling outside of clinical trials. Approximately one half of respondents indicated that testing was covered by insurance more than 75% of the time. Nearly one fifth of respondents did not know whether their patients’ tumor profiling was covered outside of clinical trials. There was no significant difference between respondents from AMC and non-AMC sites regarding whether they knew if insurance covered tumor profiling (20% AMC v 14% non-AMC responding with “Don't know;” P = .31). Excluding respondents who did not know about insurance coverage, more non-AMC physicians indicated that insurance covered their patients’ tumor profiling less than a quarter of the time or never (21% v 8%; P = .043).
TABLE 4.
Insurance Coverage of Tumor Profiling
Access to Services Related to Tumor Profiling
Approximately one half of the respondents (52%) had access to a pathology laboratory that performs tumor profiling within their institution or system. However, 94% had access to outside testing laboratories to perform tests not available within their institution or system, and 93% had genetic counselors within their institutions. Approximately one half (49%-50%) had access to internal policies/protocols for genomic/biomarker testing and molecular tumor boards. Only 12% of respondents had access to medical records that alert them when a tumor profiling test is recommended before ordering a drug. AMC respondents were more likely to have tumor profiling laboratories within their institutions (69% v 25% non-AMC; P < .0001), internal policies and protocols for genomic/biomarker testing (59% v 36% non-AMC; P = .013), and molecular tumor boards (63% v 27% non-AMC; P < .0001).
Among the 84 respondents who had access to a molecular tumor board, 25% indicated that they use it often, 36% use it sometimes, and 24% use it rarely. When these 84 respondents were asked how valuable it is to have access to the molecular tumor board, 30% indicated extremely, 26% indicated very, 23% indicated somewhat, and 20% indicated a little. No statistical differences were noted in the frequency distribution (P = .381) or value distribution (P = .905) between respondents from AMCs and respondents from non-AMCs. It is important to note that NCI-MATCH used a rule-based algorithm instead of a molecular tumor board to determine treatment.
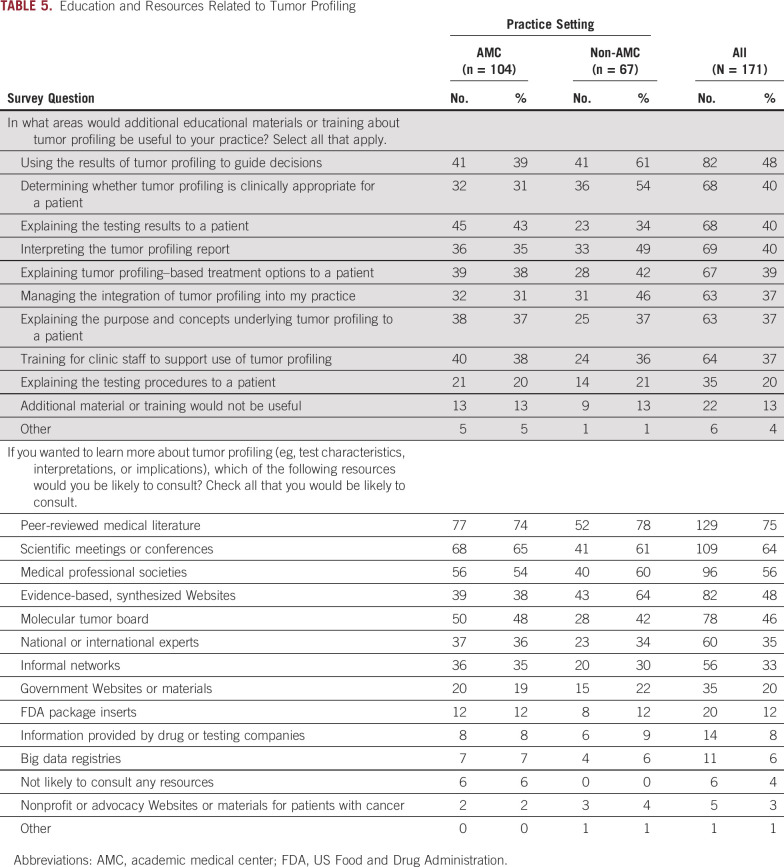
Education Related to Tumor Profiling
The top one half of Table 5 lists areas for which additional educational materials or training would be useful. Nearly one half of the respondents thought that education on how to use tumor profiling results to guide patient treatment decisions would be useful. Non-AMC respondents were more interested than AMC respondents in educational materials/training on using results to guide patient treatment decisions (61% non-AMC v 39% AMC; P = .008) and to determine the clinical appropriateness of profiling (54% non-AMC v 31% AMC; P = .004).
TABLE 5.
Education and Resources Related to Tumor Profiling
The second half of Table 5 presents resources respondents would consult to learn more about tumor profiling. Among all listed options, respondents most frequently indicated peer-reviewed medical literature (75%), followed by scientific meetings or conferences (64%), and medical professional societies (56%). Responses to all these options did not significantly differ by institution type, with the exception that non-AMC respondents were more likely to consult evidence-based Websites (64% v 38% AMC; P = .001).
Approximately two thirds of respondents (63%) indicated that the most useful time for patient education is when considering tumor profiling tests. No statistically significant differences were observed between practice settings regarding this question. When asked which Websites or other resources respondents recommend to their patients to help them understand tumor profiling, 50% marked the NCI Website option and 24% marked the Cancer.net option; 42% indicated that they do not recommend any resources to their patients. No statistically significant differences were observed between practice settings regarding any of these recommendations.
Participation in Future Tumor Genomic Profiling Trials
In considering participation in future genomic profiling trials, more than one half the respondents indicated that all the factors presented in the survey were very or extremely important: relevant patient inclusion criteria (98%), a simple administrative process (89%), a feasible testing process for all sites (87%), designed as a “basket” trial (78%), research study covers cost of test (77%), every patient in trial gets treatment (63%), and patient allowed to receive some therapy during the wait (53%). For 95% of respondents, the maximum feasible time off treatment to perform molecular profiling for eligibility in future clinical trials was 2-4 weeks, with approximately one third of respondents each indicating 2, 3, or 4 weeks.
Among the most important motivations for participating in future trials were access to targeted drugs, generating evidence that leads to treatment options, and identifying treatment options on the basis of profiling; these were very or extremely important for at least 90% of respondents. Other important motivations were help getting reimbursement for off-label drugs and increasing confidence in treatment recommendations (very or extremely important for 82% and 71% of respondents, respectively). Treatments that would positively affect respondents’ interest in participating in future tumor profiling clinical trials were new regimens with both experimental and approved drugs (71%), experimental drugs (68%), and new use of approved drugs (62%). At least one half of the respondents indicated that the following types of patients would positively affect their interest in participating in tumor profiling clinical trials: those with metastatic disease where there is no SOC (76%), rare solid tumors (64%), common tumors (colon, lung, prostate, breast; 53%), and metastatic disease for which there is an SOC (50%). Respondents from AMCs more frequently indicated an interest in studying patients with metastatic disease where there is a SOC (59% AMC v 36% non-AMC; P = .005), but indicated less interest in studying patients with hematologic tumors (12% AMC v 37% non-AMC; P = .0001).
DISCUSSION
The goal of the NCI-MATCH at the time of conception was to bring precision medicine closer to patients. The results of our survey provide insight into the acceptability of the NCI-MATCH trial to investigators and identified areas for improvement for future tumor profiling studies. Given the potential for a change in the landscape and the complexity of the trial, features were built in from the outset to allow for maximal adaptability: an interim analysis after 500 patients,4 constant monitoring by a steering committee, and this survey to evaluate the acceptance of this trial by both the treating physician investigator and patients.
The top three barriers to participation in NCI-MATCH were the length of wait time for the results of tumor profiling, a finding of nonactionable results, and being off treatment during the testing period. The NCI-MATCH built-in interim analysis also identified wait time as an important issue; in response, increasing manpower, among other changes, to streamline the process was implemented before reopening the trial in May 2016. In addition, the number of actionable mutations was increased when new arms were added to the study. Last, patients were allowed to continue to receive treatment during the testing period. These concerns all relate to the study design: removing reliance on a qualifying biopsy and a uniform test, as is true in the current NCI-MATCH trial iteration and future planned trials, will alleviate them. However, for oncology community interest to be sustained, there must be a reasonable chance that a patient will qualify for a treatment. Studies that fail to incorporate sufficient treatment options from the outset will be at high risk of failure.
One other concern raised in this survey is the increased workload at the participating sites. Although the majority of respondents noted an increased workload (> 70% of respondents) and cost (65% of respondents) at their institutions caused by participation in NCI-MATCH, the majority also reported increased satisfaction with care (57%), increased confidence in recommending a treatment (48%), and greater subsequent use of tumor profiling (70%). Placing patients in a trial such as NCI-MATCH definitely takes more time than referring them, but it also provides benefits such as allowing patients access to more treatment options on the basis of their tumor profile, continuity of care with their home oncologist, and the ability to receive treatment closer to home, potentially leading to greater satisfaction for both patients and oncologists.
Respondents who worked in AMCs more frequently reported the use of tumor profiling in 50% or more of their patients over the previous 12 months than did those who worked in non-AMC settings (43% v 18%). This difference may be a result of the total number of available clinical trials at a site. More trials would translate into greater use of testing. Physicians at AMCs likely see a higher proportion of patients who have had standard therapies in the community and now present for alternatives. It seems unlikely to reflect a more prevalent belief among AMCs that tumor testing is useful, because no significant differences were found between AMCs and non-AMCs in their views of the value of tumor profiling. It is possible that insurance coverage contributed to the difference, given that nearly twice as many non-AMC respondents indicated that the tests were covered by insurance less than a quarter of the time or never (21% v 8%). This difference may also reflect a greater tendency of physicians in non-AMC settings to check insurance coverage before conducting the tests, although this variable was not assessed. However, the most frequent response for both groups was that insurance covered tumor profiling more than three quarters of the time (38% AMC and 34% non-AMC). This has changed since this survey, because Medicare now provides coverage for tumor profiling. The differences in tumor profiling practices between AMCs and non-AMCs identified here are worthy of additional consideration and may suggest underuse of tumor profiling in certain practice settings, although it is recognized that an “optimal” level remains to be defined. This interpretation is in line with findings from a recent survey of 105 cancer genetic counselors, in which 87% indicated their institutions used tumor profiling, but only 7% did so routinely.6
Respondents are likely to order tumor profiling tests during metastatic treatment when there is no SOC (76%), regardless of practice setting. This practice reflects the limited number of cancer types for which genomically guided targeted therapy is a first-line standard. The most common scenario for ordering tumor profiling tests differed among respondents practicing in AMC versus non-AMC settings, but it is unclear whether this reflects differences in the most common tumor types seen or true differences in practice.
The NCI-MATCH trial did not have a molecular tumor board as a resource for investigators. The survey found that approximately one half of the respondents, irrespective of practice setting, relied on such boards to help guide therapy. This finding is consistent with the appetite for educational materials that has been indicated in responses to other questions. Given that genomic trials and their design constitute a rapidly evolving area of science, these findings support the consideration of more intensive educational resources for the future.
After receiving nonactionable results from tumor profiling, respondents who practice in AMC settings are clearly more likely to seek clinical trials (55% v 29%) and less likely to start US Food and Drug Administration–approved therapy (32% v 45%) than are those in non-AMC settings, whereas the latter are more likely to begin palliative care (14% v 3%). This finding may simply reflect the larger portfolio of available trials in AMCs and the patients who seek care in the AMC setting.
The limitations of this analysis include the fact that the respondents, all of whom had activated the NCI-MATCH trial, were clearly committed to a positive view of genomic profiling. Nonetheless, because these will be the oncologists who are most likely to participate in future studies, an understanding of the elements of success is immediately relevant. Another limitation of this survey was the response rate of 8.9%. This rate is within the range of some electronic physician surveys7 but is lower than others.8 Low survey response rates do not necessarily introduce bias; this depends on the similarity of respondents and nonrespondents on the variables being measured.9 However, the potential for bias in the current analysis is mitigated by the intended use of the data, which is primarily to assess the experience of NCI-MATCH investigators and to inform future trial design.
Since this survey, NCI-MATCH has addressed some of the concerns to make the trial even more patient/physician friendly. First, NCI-MATCH has had a total of 39 arms, although not all have been available at the same time. In response to the wider use of tumor profiling because of insurance coverage, NCI-MATCH now uses designated outside laboratories to identify patients so that biopsies are no longer necessary, and this does decrease the workload of the investigators. The other advantage is that the time to initiate treatment has been shortened because only confirmation of the results is needed. The disadvantage is not knowing if there are genomic changes because of interim therapy from the genomic testing. These data provide the first (to our knowledge) broad analysis of physician and patient perspectives on the experience of conducting genomic trials, inform a needs assessment to optimize processes in the clinic, and are a benchmark against which the results of future surveys may be compared.
ACKNOWLEDGMENT
We acknowledge the professional writing assistance of Mary Ann Chapman, PhD, in the preparation of this manuscript.
SUPPORT
Supported by the National Cancer Institute of the National Institutes of Health under the Research Advocacy Network (Mary Lou Smith, PI) research sub-award agreement U10CA180820-01-RAN1 and conducted under an ECOG-ACRIN Leadership Service Agreement.
AUTHOR CONTRIBUTIONS
Conception and design: Alice Chen, Keith Flaherty, Peter J. O'Dwyer, Donna M. Marinucci, Elda Railey, Mary Lou Smith, Carol White, Barbara Conley
Administrative support: Alice Chen, Elda Railey
Provision of study material or patients: Alice Chen
Collection and assembly of data: Alice Chen, Keith Flaherty, Peter J. O'Dwyer, Donna M. Marinucci, Elda Railey, Mary Lou Smith, Carol White
Data analysis and interpretation: Alice Chen, Keith Flaherty, Peter J. O'Dwyer, Bruce Giantonio, Donna M. Marinucci, Ju-Whei Lee, Mary Lou Smith, Carol White, Barbara Conley
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Keith Flaherty
Stock and Other Ownership Interests: Clovis Oncology, Loxo, X4 Pharma, Strata Oncology, PIC Therapeutics, Fount Therapeutics, Shattuck Labs, Apricity Health, Oncoceutics, Fog Pharma, Tvardi, Checkmate Pharmaceuticals, Kinnate
Consulting or Advisory Role: Novartis, Genentech, Merck, Lilly, Amgen, Sanofi, Oncoceutics, Bristol Myers Squibb, Adaptimmune, Aeglea Biotherapeutics, Loxo, Roche, Asana Biosciences, Incyte, Shattuck Labs, Tolero Pharmaceuticals, Array BioPharma, FOG Pharma, Neon Therapeutics, Tvardi, Takeda, Verastem, Boston Biomedical, Pierre Fabre, Cell Medica, Debiopharm Group
Research Funding: Novartis, Sanofi
Travel, Accommodations, Expenses: Pierre Fabre, Debiopharm Group
Peter J. O'Dwyer
Consulting or Advisory Role: Genentech, Array
Research Funding: Bristol Myers Squibb (Inst), Pfizer (Inst), Novartis (Inst), Genentech (Inst), Mirati Therapeutics (Inst), Celgene (Inst), GlaxoSmithKline (Inst), BBI Healthcare (Inst), Pharmacyclics (Inst), Five Prime Therapeutics (Inst), Forty Seven (Inst), Amgen (Inst), H3 Biomedicine (Inst), Taiho (Inst), Array BioPharma (Inst), Eli Lilly/ImClone (Inst), Syndax (Inst), Minneamrata (Inst)
Expert Testimony: Eli Lilly
Elda Railey
Employment: Advanced Pharmacy (I)
Research Funding: Genomic Health (Inst), Genentech/Roche (Inst), Novartis (Inst), Celgene (Inst), Eli Lilly (Inst), Seattle Genetics (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Genentech
Mary Lou Smith
Consulting or Advisory Role: Novartis, Pfizer
Research Funding: Genentech (Inst), Celgene (Inst), Genomic Health (Inst), Novartis (Inst), Foundation Medicine (Inst)
Travel, Accommodations, Expenses: Genentech/Roche
Carol White
Research Funding: Genentech (Inst), Genomic Health (Inst), Novartis (Inst), Celgene (Inst), Foundation Medicine (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Conley BA, Doroshow JH. Molecular analysis for therapy choice: NCI MATCH. Semin Oncol. 2014;41:297–299. doi: 10.1053/j.seminoncol.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Khoury JD, Wang WL, Prieto VG, et al. Validation of immunohistochemical assays for integral biomarkers in the NCI-MATCH EAY131 clinical trial. Clin Cancer Res. 2018;24:521–531. doi: 10.1158/1078-0432.CCR-17-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lih CJ, Harrington RD, Sims DJ, et al. Analytical validation of the next-generation sequencing assay for a nationwide signal-finding clinical trial: Molecular analysis for therapy choice clinical trial. J Mol Diagn. 2017;19:313–327. doi: 10.1016/j.jmoldx.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flaherty KT, Gray R, Chen A, et al. The Molecular Analysis for Therapy Choice (NCI-MATCH) trial: Lessons for genomic trial design J Natl Cancer Inst[epub ahead of print on January 10, 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freedman AN, Klabunde CN, Wiant K, et al. Use of next-generation sequencing tests to guide cancer treatment: Results from a nationally representative survey of oncologists in the United States JCO Precis Oncol 10.1200/PO.18.00169 [epub ahead of print on November 13, 2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goedde LN, Stupiansky NW, Lah M, et al. Cancer genetic counselors’ current practices and attitudes related to the use of tumor profiling. J Genet Couns. 2017;26:878–886. doi: 10.1007/s10897-017-0065-z. [DOI] [PubMed] [Google Scholar]
- 7.Cook DA, Wittich CM, Daniels WL, et al. Incentive and reminder strategies to improve response rate for Internet-based physician surveys: A randomized experiment. J Med Internet Res. 2016;18:e244. doi: 10.2196/jmir.6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunningham CT, Quan H, Hemmelgarn B, et al. Exploring physician specialist response rates to Web-based surveys. BMC Med Res Methodol. 2015;15:32. doi: 10.1186/s12874-015-0016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halpern SD, Asch DA. Commentary: Improving response rates to mailed surveys: What do we learn from randomized controlled trials? Int J Epidemiol. 2003;32:637–638. doi: 10.1093/ije/dyg184. [DOI] [PubMed] [Google Scholar]