INTRODUCTION
Medullary thyroid cancer (MTC) is a neuroendocrine tumor that originates from the parafollicular cells (or C cells) of the thyroid gland and accounts for 1% to 2% of thyroid cancers in the United States.1 The CNS is a rare site of metastasis in MTC, with < 15 cases reported in the medical literature; however, the prevalence may be underreported because of the infrequent use of CNS imaging in patients with MTC.1,2 The optimal treatment approach for patients with MTC with CNS involvement is currently unknown. Although surgical resection, radiation therapy, or stereotactic radiosurgery (SRS) may improve local disease control in some patients, most still succumb to their disease within months after the diagnosis of CNS metastases.1-4
The proto-oncogene RET (rearranged during transfection), located on chromosome 10q11.2, is central to development of a majority of cases of MTC.5 Germline gain-of-function RET point mutations result in multiple endocrine neoplasia 2, a hereditary syndrome associated with MTC and pheochromacytoma.6,7 Similar somatic mutations occur in approximately 50% of sporadic MTC, approximately 20% of sporadic pheochromocytomas, and rarely in other cancer types.8-10 Recent identification of highly potent and selective RET inhibitors holds great promise in the management of these RET-driven cancers as well as other RET-altered malignancies.11-14 In 2018, selpercatinib (LOXO-292) received US Food and Drug Administration Breakthrough Therapy designation for treatment of patients with previously treated metastatic RET-mutant MTC, RET fusion-positive non–small-cell lung cancer, and RET fusion–positive thyroid cancers. Durable intracranial responses with selpercatinib have been described in patients with brain metastases with RET fusion–positive lung and thyroid cancers.15,17 However, the activity of selpercatinib in patients with RET-mutant MTC with brain metastases and/or leptomeningeal disease (LMD) has not been characterized, to our knowledge. Here, we describe a patient with RET M918T–mutant MTC with extensive CNS metastases who had a clinically meaningful durable response to selective RET inhibition with selpercatinib.
Case Report
A 67-year-old woman presented to a local hospital with bilateral neck swelling. Neck ultrasound imaging revealed multinodular goiter and extensive bilateral neck adenopathy. Ultrasound-guided fine-needle aspiration of 1 of the enlarged lymph nodes showed malignant cells suspicious for thyroid carcinoma. Her blood calcitonin level was 338 pg/mL (normal, < 7.6 pg/mL). Her carcinoembryonic antigen (CEA) level was 186.5 ng/mL (normal, < 3.8 ng/mL). The patient underwent total thyroidectomy with bilateral central and lateral neck dissection. Surgical pathology revealed 2.3-cm MTC with extrathyroidal extension, lymphovascular invasion, and metastatic involvement of 54 of 70 removed lymph nodes. Consistent with the diagnosis of thyroid carcinoma, immunohistochemistry was diffusely positive for TTF-1, weakly positive for calcitonin, and negative for PAX-8. Her postoperative laboratory studies were notable for increasing calcitonin levels. Positron emission tomography imaging showed metabolically active, left-side, level V lymph nodes; a 1.1-cm, hypermetabolic, right lower lobe lung nodule; several hypermetabolic liver lesions; as well as lytic lesions in the C5 vertebral body, L4 left lamina, and left posterior iliac wing. Magnetic resonance imaging (MRI) of the brain revealed a 4-mm lesion in the right caudate nucleus.
The patient presented to the University of Texas MD Anderson Cancer Center for a second opinion. Germline genetic testing was negative for hereditary RET mutations. A next-generation sequencing–based analysis for the detection of somatic mutations in the coding sequence of 50 genes18 was performed on the DNA extracted from the surgical tumor sample in a Clinical Laboratory Improvement Amendments–certified molecular pathology laboratory and revealed a RET M918T missense mutation, a well-established gain-of-function alteration.1 In addition, the fluorescent in situ hybridization analysis using an Abbott Molecular CDKN2C/CKS1B dual-color probe (Abbott Laboratories, Chicago, IL) was positive for the deletion of the CDKN2C gene, which is associated with decreased overall survival in MTC.19 Repeated MRI of the brain and spine revealed 8 additional brain lesions and a progression of the multifocal spinal bone disease.
The patient received whole-brain radiation therapy and external beam radiation therapy to the spine lesions. She was then treated with standard-of-care cabozantinib but experienced multiple adverse effects, including hand-and-foot syndrome, mucositis, weight loss, nausea, and diarrhea, requiring several dose reductions ultimately to 40 mg daily. The patient achieved a partial response according to RECIST, version 1.120; at 8 months into treatment, disease progression developed (Fig 1).
FIG 1.
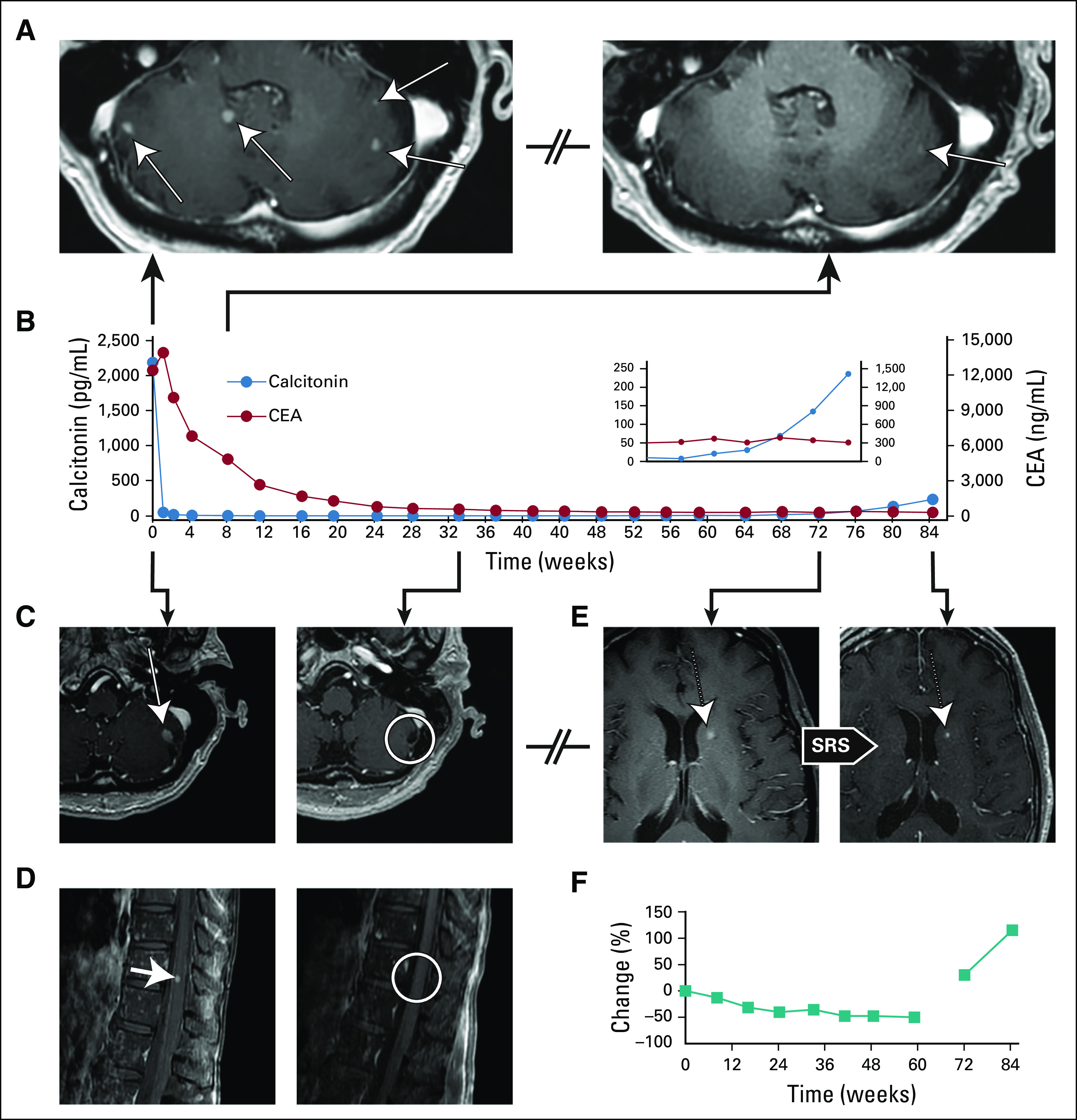
Clinical activity of selpercatinib (LOXO-292) in medullary thyroid cancer with extensive CNS metastases. (A) Magnetic resonance imaging (MRI) oblique axial cerebellar images of the patient’s metastatic brain disease (left panel) before and (right panel) 8 weeks after the patient initiated treatment with selpercatinib. (B) Serial monitoring of calcitonin (blue circles) and carcinoembryonic antigen (CEA; red circles) during selpercatinib treatment. (C) MRI axial cerebellar and (D) sagittal thoracic spine images of the patient’s metastatic disease (left panels) before and (right panels) 33 weeks after the patient initiated treatment with selpercatinib. (E) MRI axial brain images demonstrating a new caudate lesion after 17 months of therapy with selpercatinib (left panel) before and (right panel) after stereotactic radiosurgery (SRS). (F) RECIST plot indicating change in a target liver lesion according to RECIST, version 1.1. The first line indicates change from baseline. The second line indicates change from nadir.
Given the absence of any remaining standard therapies with established benefit, the patient enrolled in a phase I/II study of selpercatinib in patients with advanced solid tumors (LIBRETTO-001; ClinicalTrials.gov identifier: NCT03157128). The patient provided written informed consent before enrollment. Selpercatinib was administered at 160 mg twice daily. Imaging studies (MRI of the brain and spine; computed tomography of the neck, chest, abdomen, and pelvis) were performed every 8 weeks. Response was assessed according to RECIST, version 1.1. Baseline imaging at the time of the treatment initiation with selpercatinib revealed bilateral central and lateral neck disease; lytic lesions in the C5, T4, T8, L1 vertebrae and left posterior hilum;, nodular opacities in bilateral lungs; interlobular septal thickening suspicious for lymphangitic carcinomatosis; numerous hepatic lesions (largest, 7 cm in the dome of liver); ≥ 25 lesions in the brain and cerebellum (largest, 7 mm in the left cerebellar hemisphere; Fig 2A and 1C, left panels), and a 5-mm leptomeningeal nodule at the level of T11 (Fig 2D, left panel). Calcitonin and CEA levels measured 2,180 pg/mL and 12,312 ng/mL, respectively (Fig 2B).
FIG 2.
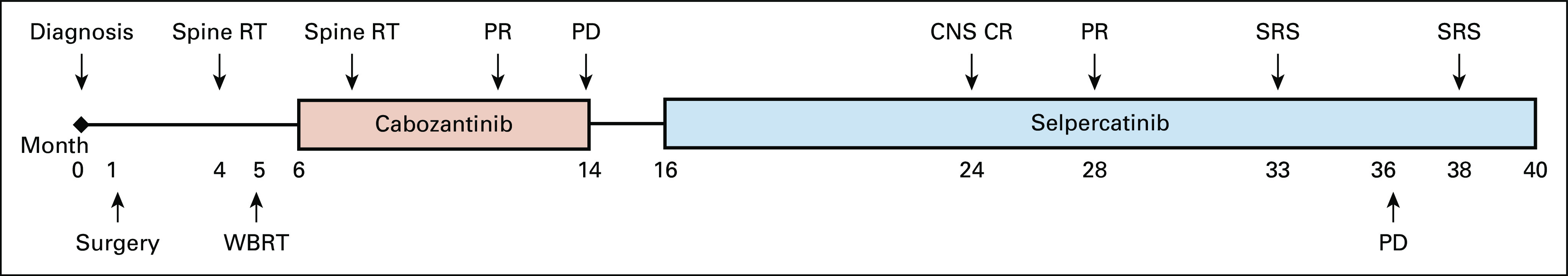
Patient treatment timeline. CR, complete response; PD, progressive disease; PR, partial response; RT, radiation therapy; SRS, stereotactic radiosurgery; WBRT, whole-brain radiation therapy.
The patient experienced clinical response within the first month of therapy, with resolution of baseline diarrhea and nausea, and with appetite improvement. Consistent with resolution of diarrhea, calcitonin levels decreased to < 2 pg/mL 3 months after therapy initiation (Fig 2B). The patient’s CEA level reached its nadir of 304.0 ng/mL after 16 months of therapy. MRI of the brain at 8 weeks demonstrated near-complete resolution of the previously identified brain metastases (Fig 2A, right panel), and MRI of the brain and spine at 33 weeks showed complete resolution of the brain metastases and the leptomeningeal nodule at the T11 level (Fig 2C and 2D, right panels).
At 1 year, the patient officially achieved a partial extracranial response by RECIST, version 1.1, criteria. At 17 months, an MRI of the brain showed an isolated, new, 5-mm left caudate lesion that was treated with SRS (Fig 2E, left and right panels) with continuation of selpercatinib. At 20 months, imaging revealed non-CNS multifocal progression with a new 6-mm bony lesion in the C7 vertebral body, as well as several new, right-side liver lobe lesions (the largest measured 2 cm). The patient’s calcitonin level rose to 235.9 pg/mL; her CEA level remained unchanged. Peripheral blood cell-free DNA analysis at the time of systemic progression revealed only the presence of the previously identified RET M918T missense mutation. The patient declined a liver biopsy.
CNS disease remained in response until 22 months, when several new brain lesions were found to have developed; these were managed with SRS (Fig 2). The patient continued treatment with selpercatinib beyond progressive disease because she was felt to derive residual clinical benefit from the therapy. Through her treatment, the patient tolerated selpercatinib well, reporting grade 1 fatigue and leukopenia attributed to study drug. Her treatment course has been complicated by several hospitalizations unrelated to selpercatinib.
DISCUSSION
Metastatic dissemination of MTC to the CNS is a rare but devastating complication associated with poor prognosis and no well-established treatment strategies.1 Although limited evidence from previous reports suggests that surgical resection and/or radiation therapy may be used in select cases, there remains a strong need for novel treatment modalities with durable therapeutic responses.1-3 CNS efficacy of multikinase inhibitors such as vandetanib or cabozantinib has not been reported in MTC.
Here, we present a report of successful treatment of the brain and leptomeningeal metastases with systemic therapy in a patient with RET-mutated MTC, using the purpose-built selective and CNS-penetrant RET inhibitor selpercatinib. Treatment with this agent resulted in complete and durable resolution of the measurable brain and leptomeningeal lesions in a patient with previous disease progression while she received cabozantinib and radiation therapy. Our findings are consistent with the previously reported selpercatinib activity in patients with RET fusion–positive lung and anaplastic thyroid cancers with CNS involvement, as well as in an orthotopic RET fusion–positive intracranial mouse tumor model.11,15-17 These observations, coupled with the favorable toxicity profile of selpercatinib and rapidity of typical therapeutic responses, suggest this agent may represent an important new therapeutic option for patients with RET-mutated cancers with brain metastases and/or LMD.
Notably, the patient's tumor harbored a heterozygous deletion in the CDKN2C gene linked to an aggressive disease phenotype in MTC.19 CDKN2C is a member of the INK4 family of cyclin-dependent kinase inhibitors that block the cell cycle progression in the G1 phase through interaction with cyclin-dependent kinases 4 and 6.19 CDKN2C is lost in approximately 40% to 50% MTCs and is associated with a significantly shorter time to distant metastasis and decreased overall survival in MTC, an effect further enhanced by concomitant RET M918T mutation.19,21 In addition, an earlier report showed that CDKN2C haploinsufficiency is associated with a strong trend toward reduced progression-free survival in RET-mutated MTC treated with systemic therapy.21 However, this relationship has not been studied in patients receiving selective RET inhibitors, to our knowledge, and the significance of CDKN2C loss in this setting remains to be determined.
The patient is continuing selpercatinib therapy, with gradual disease progression in the liver. Molecular mechanisms underlying resistance to selpercatinib remain poorly understood. A recent study demonstrated that RET solvent-front mutations (RET G810S/G810R/G810C) can cause on-target resistance to selective and multikinase RET inhibitors.22 A better understanding of mechanisms of acquired resistance to selective RET inhibitors and frequency of resistant alterations would guide the development of novel agents and combination therapies directed towards further improvement of treatment outcomes for patients with RET-altered malignancies.
In conclusion, we observed a radiographically confirmed durable response with selpercatinib in a patient with a heavily pretreated RET M918T–mutated MTC with extensive CNS metastases. This report further substantiates emerging data suggesting that selpercatinib has potential as a novel powerful treatment modality in patients with RET-driven malignancies with CNS involvement.
SUPPORT
Supported by the National Cancer Institute (Grants No.T32 CA009666 [A.A.-D.] and CA016672). V.S. is supported by the National Cancer Institute of the National Institutes of Health under Award Number R01CA242845-01A1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
AUTHOR CONTRIBUTIONS
Conception and design: Alexander Andreev-Drakhlin, Vivek Subbiah
Financial support: Vivek Subbiah
Administrative support: Vivek Subbiah
Provision of study material or patients: Behrang Amini, Vivek Subbiah
Collection and assembly of data: Alexander Andreev-Drakhlin, Behrang Amini, Vivek Subbiah
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Maria Cabanillas
Honoraria: Loxo
Consulting or Advisory Role: Loxo, Ignyta
Research Funding: Kura, Eisai, Roche, Exelixis
Vivek Subbiah
Consulting or Advisory Role: MedImmune, Helsinn Therapeutics, Loxo, R-Pharma-US, QED Pharma
Research Funding: Novartis (Inst), GlaxoSmithKline (Inst), NanoCarrier (Inst), Northwest Biotherapeutics (Inst), Roche (Inst), Berg Pharma (Inst), Bayer (Inst), Incyte (Inst), Fujifilm (Inst), PharmaMar (Inst), D3 Oncology Solutions (Inst), Pfizer (Inst), Amgen (Inst), AbbVie (Inst), Multivir (Inst), Blueprint Medicines (Inst), Loxo (Inst), Vegenics (Inst), Takeda (Inst), Alfasigma (Inst), Agensys (Inst), Idera (Inst), Boston Biomedical (Inst), Inhibrx (Inst), Exelixis (Inst), Amgen (Inst), Turningpoint Therapeutics (Inst)
Travel, Accommodations, Expenses: PharmaMar, Bayer, Novartis, Helsinn Therapeutics
Other Relationship: Medscape
No other potential conflicts of interest were reported.
REFERENCES
- 1. Wells SA Jr, Asa SL, Dralle H, et al: Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 25:567-610, 2015. [DOI] [PMC free article] [PubMed]
- 2.Börcek P, Asa SL, Gentili F, et al. Brain metastasis from medullary thyroid carcinoma. BMJ Case Rep. 2010;2010:bcr0920103301. doi: 10.1136/bcr.09.2010.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim I-Y, Kondziolka D, Niranjan A, et al. Gamma knife radiosurgery for metastatic brain tumors from thyroid cancer. J Neurooncol. 2009;93:355–359. doi: 10.1007/s11060-008-9783-2. [DOI] [PubMed] [Google Scholar]
- 4.McWilliams RR, Giannini C, Hay ID, et al. Management of brain metastases from thyroid carcinoma: A study of 16 pathologically confirmed cases over 25 years. Cancer. 2003;98:356–362. doi: 10.1002/cncr.11488. [DOI] [PubMed] [Google Scholar]
- 5. doi: 10.1038/nrclinonc.2017.175. Drilon A, Hu ZI, Lai GGY, et al: Targeting RET-driven cancers: Lessons from evolving preclinical and clinical landscapes. Nat Rev Clin Oncol 15:151-167, 2018 [Erratum: Nat Rev Clin Oncol15:150, 2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mulligan LM, Kwok JB, Healey CS, et al. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature. 1993;363:458–460. doi: 10.1038/363458a0. [DOI] [PubMed] [Google Scholar]
- 7.Hofstra RMW, Landsvater RM, Ceccherini I, et al. A mutation in the RET proto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature. 1994;367:375–376. doi: 10.1038/367375a0. [DOI] [PubMed] [Google Scholar]
- 8.Elisei R, Cosci B, Romei C, et al. Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: A 10-year follow-up study. J Clin Endocrinol Metab. 2008;93:682–687. doi: 10.1210/jc.2007-1714. [DOI] [PubMed] [Google Scholar]
- 9.Beldjord C, Desclaux-Arramond F, Raffin-Sanson M, et al. The RET protooncogene in sporadic pheochromocytomas: Frequent MEN 2-like mutations and new molecular defects. J Clin Endocrinol Metab. 1995;80:2063–2068. doi: 10.1210/jcem.80.7.7608256. [DOI] [PubMed] [Google Scholar]
- 10.Kato S, Subbiah V, Marchlik E, et al. RET aberrations in diverse cancers: Next-generation sequencing of 4,871 patients. Clin Cancer Res. 2017;23:1988–1997. doi: 10.1158/1078-0432.CCR-16-1679. [DOI] [PubMed] [Google Scholar]
- 11.Subbiah V, Velcheti V, Tuch BB, et al. Selective RET kinase inhibition for patients with RET-altered cancers. Ann Oncol. 2018;29:1869–1876. doi: 10.1093/annonc/mdy137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subbiah V, Gainor JF, Rahal R, et al. Precision targeted therapy with BLU-667 for RET-driven cancers. Cancer Discov. 2018;8:836–849. doi: 10.1158/2159-8290.CD-18-0338. [DOI] [PubMed] [Google Scholar]
- 13.Subbiah V, Cote GJ. Advances in targeting RET-dependent cancers. Cancer Discov. 2020;10:498–505. doi: 10.1158/2159-8290.CD-19-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Subbiah V, Yang D, Velcheti V, et al: State-of-the-art strategies for targeting RET-dependent cancers. J Clin Oncol 38:1209-1221, 2020. [DOI] [PMC free article] [PubMed]
- 15.Guo R, Schreyer M, Chang JC, et al. Response to Selective RET inhibition with LOXO-292 in a patient with RET fusion-positive lung cancer with leptomeningeal metastases. JCO Precis Oncol. 2019;3:1–6. doi: 10.1200/PO.19.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wirth L, Sherman E, Drilon A, et al: Registrational results of LOXO-292 in patients with RET-altered thyroid cancers. Ann Oncol 30: v851-v934, 2019 (suppl 5)
- 17.Drilon A, Oxnard GR, Wirth L, et al. A phase 1/2 trial of LOXO-292 in patients with RET fusion-positive lung cancers.Presented atIASLC 2019 World Conference on Lung Cancer hosted by the International Association for the Study of Lung Cancer September 7-10, 2019Barcelona, SpainAbstract PL02.08 [Google Scholar]
- 18.Meric-Bernstam F, Brusco L, Shaw K, et al. Feasibility of large-scale genomic testing to facilitate enrollment onto genomically matched clinical trials. J Clin Oncol. 2015;33:2753–2762. doi: 10.1200/JCO.2014.60.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grubbs EG, Williams MD, Scheet P, et al. Role of CDKN2C copy number in sporadic medullary thyroid carcinoma. Thyroid. 2016;26:1553–1562. doi: 10.1089/thy.2016.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Maxwell JE, Gule-Monroe MK, Subbiah V, et al. Novel use of a Clinical Laboratory Improvements Amendments (CLIA)-certified cyclin-dependent kinase N2C (CDKN2C) loss assay in sporadic medullary thyroid carcinoma. Surgery. 2020;167:80–86. doi: 10.1016/j.surg.2019.03.041. [DOI] [PubMed] [Google Scholar]
- 22.Solomon BJ, Tan L, Lin JJ, et al. RET solvent front mutations mediate acquired resistance to selective RET inhibition in RET-driven malignancies. J Thorac Oncol. 2020;15:541–549. doi: 10.1016/j.jtho.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


