Abstract
Objective
Reactive oxygen species reduce the male sex hormone levels and disrupt the hormonal balance that regulates male reproductive functions. They disrupt spermatozoa and other testicular cells. This study aimed at evaluating the effect of antioxidant treatment on serum gonadal hormones and sperm parameters in men with idiopathic infertility.
Material and methods
A total of 100 idiopathic infertile men aged 23–46 years were included in the study. Control group and antioxidant treatment group consisted of 50 men each. Patients in the treatment group received oral antioxidant supplement once a day. The antioxidant supplement content included L-carnitine, L-arginine, vitamin E, vitamin C, coenzyme Q, glutathione, beta-carotene, magnesium, vitamin B12, zinc, vitamin A, vitamin B6, vitamin D3, folic acid, and selenium. Reproductive hormones and sperm parameters were compared between the groups at 6 months after starting the antioxidant therapy.
Results
No significant differences were observed in the patient age (p=0.861), partner age (p=0.081), total motile sperm count (TMSC) (p=0.324), and follicle-stimulating hormone (FSH) (p=0.557), luteinizing hormone (LH) (p=0.235), and total testosterone levels (p=0.851) at baseline between the treatment and control groups. Although the mean TMSC did not increase significantly, the mean FSH (p=0.008), LH (p=0.008), and total testosterone (p=0.006) levels significantly increased from baseline to post-treatment in the treatment group. However, no significant differences from baseline to post-treatment were observed in TMSC (p=0.486), FSH (p=0.712), LH (p=0.696), and total testosterone levels (p=0.546) in the control group.
Conclusion
The research draws attention to the alternate treatment approaches in infertile men. Antioxidant treatment can increase the serum sex hormone levels.
Keywords: Antioxidants, gonadotropins, male infertility, sperm parameters, testosterone
Introduction
Infertility is the inability to have a child despite an unprotected relationship for 1 year. In 50% of cases, male factor is the cause. Whereas 60%–75% of couples can get pregnant in 6 months, 90% couples can get pregnant in 1 year. Idiopathic infertility is the absence of a reason to explain the abnormal semen analysis of a patient, and it occurs at a frequency of 31%.[1] No medical treatment has been yet approved by the Food and Drug Administration or the European Medicines Agency for idiopathic male infertility because there is no correctable cause.[2] However, in the literature, empirical medical treatment or assisted reproductive methods are recommended for these patients.[3]
Although the etiology of idiopathic infertility has not been fully elucidated, it is believed that it has a multifactorial etiology, including genetic, environmental, and hormonal parameters, and especially, DNA fragmentation and oxidative stress are considered to be responsible.[4] Reactive oxygen species (ROS), such as hydrogen peroxide, nitric oxide (NO), and peroxynitrite, were reported to be associated with male infertility.[5] It is emphasized that ROS-related sperm damage is a significant cause in 30%–80% of infertile men.[6] Oxidative stress is the result of decreased antioxidant defense system and/or overproduction of ROS, targeting the lipids, proteins, and DNA, reducing mitochondrial activity and sperm motility, and consequently causing damage to the paternal genomes.[7] Diseases, such as varicocele and testicular torsion, are common conditions associated with oxidative stress leading to DNA damage and low motility in the sperm.[8] In addition to lifestyle modification, it has been suggested that dietary measures, such as vitamin C, vitamin E, and beta-carotene, may improve male reproductive potential by reducing the intensity of oxidative stress.[9] High ROS levels can increase the possibility of infertility not only directly by inducing oxidative stress but also indirectly by acting through the hypothalamic axes of hormone release.[10]
Although many studies have demonstrated the positive effects of antioxidants on sperm parameters [11,12], various in vivo and randomized controlled trials have failed to report positive outcomes on semen parameters, and some have even reported negative outcomes in terms of increased sperm DNA fragmentation or chromatin decondensation.[13] Therefore, there are many conflicting remarks about the use of antioxidants in male infertility in the literature, and a consensus has not been reached yet. Several studies suggested that increased ROS levels may reduce male sex hormone levels and disrupt the hormonal balance, leading to infertility.[14] However, no clinical study has investigated the effect of antioxidants on male serum gonadal hormone levels. In this study, the effect of antioxidants on sperm parameters and serum gonadal hormone levels in men with idiopathic infertility was investigated.
Material and Methods
The study included 100 infertile men who had presented to male infertility outpatient clinics. The study was approved by the institutional ethical committee (approval no.: 2018/409). Written informed consent was obtained from patients who participated in this study. The study was conducted between March 2018 and March 2020. Only patients with idiopathic infertility were included in the study. All the patients lived in the same geography. Patients with varicocele, undescended testes, previous testicular surgery, previous orchitis, obesity, endocrine disorders, such as diabetes, thyroid disease, or other systemic disease, or who received chemotherapy and radiotherapy for cancer were excluded from the study. Patients who had ever smoked in the past 6 months and those with fever during follow-up were excluded.
All the patients were oligospermic and normospermic. Azoospermic patients were excluded from the study because they were considered to have obstruction in the male genital tract and genetic and/or hormonal abnormalities. Sperm and blood tests were performed for the patients before and after 6 months of starting antioxidant supplement. The study participants were subjected to sperm test after 3–5 days of sexual abstinence, and their blood samples were collected between 8 and 10 a.m. At baseline, a minimum of 3 specimens were collected at an interval of 2–4 weeks. All semen analyses were performed in the andrology laboratory according to the World Health Organization (WHO) criteria. Pretreatment and post-treatment total motile sperm count (TMSC=ejaculate volume x concentration×motile fraction) were calculated on all sperm analyses.[15] For each patient, the greatest TMSC value from pretreatment to post-treatment was used and compared.
Of the 100 infertile men, 50 received oral antioxidant supplements once a day for 6 months and were considered as the treatment group, and 50 received no treatment and were considered as the control group. The antioxidant supplement (Promenk ACT, Neupharma, İzmit-Kocaeli, Turkey; produced in La Rioja, Spain) content included L-carnitine (1 g), L-arginine (0.3 g), vitamin E (100 mg), vitamin C (250 mg), coenzyme Q (100 mg), glutathione (75 mg), beta-carotene (2.5 mg), magnesium (60 mg), vitamin B12 (10 g), zinc (7.5 mg), vitamin A (500 μg, vitamin B6 (5 mg), vitamin D3 (5 u μg), folic acid (400 ugg μg), and selenium (400 ugg μg). The data of the patients were analyzed prospectively. There was no industry sponsorship and financial conflict of interest.
Statistical analyses
Sperm parameters and serum gonadal hormone levels were evaluated before and after 6 months of the study. Statistical analyses were performed using paired sample t-tests to compare the sperm parameters and serum hormone levels from baseline to post-treatment. Independent samples t-test was used to compare the baseline values of the treatment and control groups. The chi-square test was performed to compare the spontaneous pregnancy rates between the two groups. The two-way repeated measures analysis of variance (ANOVA) test was used to compare the group×time interaction of TMSC, follicle-stimulating hormone (FSH), luteinizing hormone (LH), and total testosterone values of the groups. Values were presented as mean±standard error, and probability values of <0.05 were considered to be significant. Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS Inc.; Chicago, IL, USA) for Windows v11.0 package.
Results
Table 1 presents the mean ages of the patients and partners and TMSC and serum hormone values of the patients at baseline. No significant differences were observed in the patient age (p=0.861) and partner age (p=0.081), TMSC (p=0.324), FSH (p=0.557), LH (p=0.235), and total testosterone levels (p=0.851) at baseline between the treatment and control groups.
Table 1.
Comparison of baseline values of the treatment and control groups
| Treatment group (n=50) | Control group (n=50) | p | |
|---|---|---|---|
| Patient age (years) | 32.45±5.90 | 31.42±5.84 | 0.861 |
| Partner age (years) | 28.52±5.41 | 26.37±4.38 | 0.081 |
| TMSC (million) | 27.98±26.86 | 29.21±19.56 | 0.324 |
| FSH (mIU/mL) | 5.07±3.35 | 5.27±2.74 | 0.557 |
| LH (mIU/mL) | 5.55±3.55 | 5.18±2.40 | 0.235 |
| Total testosterone (ng/mL) | 3.79±1.90 | 4.29±1.94 | 0.851 |
TMSC: total motile sperm count; FSH: follicle-stimulating hormone; LH: luteinizing hormone
Table 2 presents pre- and post-treatment TMSC and serum hormone values of the groups. In the treatment group, the mean TMSC increased from 27.98±26.86 to 34.3±35.39 million, but the difference was not statistically significant (p=0.09). The mean FSH (p=0.008), LH (p=0.008), and total testosterone (p=0.006) levels significantly increased from baseline to post-treatment. No significant differences were observed from baseline to post-treatment in TMSC (p=0.486), FSH (p=0.712), LH (p=0.696), and total testosterone (p=0.546) values in the control group.
Table 2.
Comparison of baseline and post-treatment values of the groups
| Treatment group(n=50) | Baseline | At 6 months | p |
|---|---|---|---|
| TMSC (million) | 27.98±26.86 | 34.30±35.39 | 0.090 |
| FSH (mIU/mL) | 5.07±3.35 | 6.59±3.94 | 0.008 |
| LH (mIU/mL) | 5.55±3.55 | 7.80±6.38 | 0.008 |
| Total testosterone (ng/mL) | 3.79±1.90 | 5.32±2.12 | 0.006 |
| Control group (n=50) | Baseline | At 6 months | p |
| TMSC (million) | 29.21±19.56 | 31.17±23.79 | 0.486 |
| FSH (mIU/mL) | 5.27±2.74 | 5.22±2.73 | 0.712 |
| LH (mIU/mL) | 5.18±2.40 | 5.03±2.65 | 0.696 |
| Total testosterone (ng/mL) | 4.29±1.94 | 4.19±2.02 | 0.546 |
TMSC: total motile sperm count; FSH: follicle-stimulating hormone; LH: luteinizing hormone
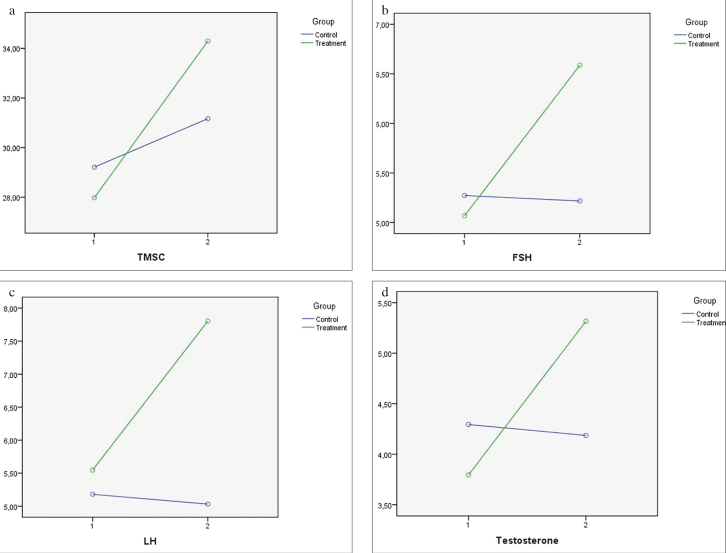
In the two-way repeated measures ANOVA test, FSH, LH, and total testosterone values were detected in the group x time interaction (Figures 1a–d). Although FSH, LH, and total testosterone levels did not change significantly in terms of statistical analyses in the control group, they increased in the treatment group (p=0.003 for FSH, p=0.011 for LH, and p=0.001 for total testosterone). TMSC, however, did not change significantly in both the groups.
Figure 1. a–d.
(a) Estimated marginal means of total motile sperm count (group×time interaction was detected, p=0.351; two-way repeated measures analysis of variance). (b) Estimated marginal means of follicle-stimulating hormone (group×time interaction was detected, p=0.003; two-way repeated measures analysis of variance). (c) Estimated marginal means of luteinizing hormone (group×time interaction was detected, p=0.011; two-way repeated measures analysis of variance). (d) Estimated marginal means of total testosterone (group×time interaction was detected, p=0.001; two-way repeated measures analysis of variance)
Discussion
This is the first study to investigate the effects of antioxidants on reproductive hormones. The study investigated the effect of antioxidant use not only on sperm parameters but also on serum hormone levels. It is reported that sperm cells are very susceptible to damage induced by ROS because their plasma membranes are composed of highly polyunsaturated fatty acids.[16]. This peroxidative damage to the sperm cell plasma membrane plays an important role in the pathophysiological mechanism of human male infertility. Oxidative damage can cause sperm membrane dysfunction and abnormal morphological sperm formation.[17] In normal semen samples, ROS, which are necessary for cell activity and controlled by antioxidant mechanisms, work beneficially by preventing cell damage, whereas oxidative stress and damage start when ROS increase is high, antioxidant capacity is low, and balance is deteriorated. Semen sperm cells and leukocytes are the two basic free radical sources. Oxidative stress plays a role in impairing spermatogenesis, leading to male infertility. Several studies have reported that antioxidant treatment may improve sperm DNA damage and other sperm parameters.[18] The parameter for assessing the quality of sperm is TMSC. TMSC has a better predictive value for spontaneous pregnancy than the WHO classification system. Sperm morphology parameter is not used in this calculation.[19] TMSC has been found to have a higher correlation with both blastocyst development and expansion as well as ongoing pregnancy rate in both intrauterine insemination and in vitro fertilization cycles.[20] Therefore, we used TMSC in this study.
In a previous meta-analysis, Omar et al.[12] demonstrated a significant improvement in the sperm concentration, morphology, and motility using antioxidant supplements (pentoxifylline, coenzyme Q10, and L-carnitine) compared with placebo. The duration of therapy was 6 months as it was in our study. They concluded that antioxidants may protect against free radical injury, with infertile men having higher ROS levels. It has been presented that antioxidants improve spermatogenic function and sperm DNA integrity.[21] Reducing the oxidative stress with antioxidant supplements has the potential to improve semen parameters similar to the TMSC increase observed in the treatment group of this study. Several clinical trials and systemic reviews involving the use of various combinations of antioxidants (L-carnitine, selenium, N-acetylcysteine, coenzyme Q10, ubiquinol, vitamin E, vitamin C, and lycopene) in infertile men have reported beneficial effects of antioxidants on sperm concentration, motility, and DNA integrity.[22] Another study, which involved 148 idiopathic infertile men, indicated that the intake of oral antioxidants for 3 months significantly increased sperm concentration and motility after treatment. These beneficial changes in semen quality have been reported to improve the chance of natural conception in several but not all studies.[23] However, the improving effect of antioxidant treatment is not clearly known. This study investigated the effect of antioxidant treatment on reproductive hormones.
Being the primary male sex organs, testes are involved in spermatogenesis and secretion of many hormones. These hormones are effective in the regulation of gonadotropin secretion, spermatogenesis, sexual differentiation, male phenotype formation, and normal sexual behavior.[24] ROS affect endocrine pathways and disrupt normal hormonal secretion and reproductive functions. Many studies have reported that ROS disrupt sperm parameters in men with idiopathic infertility. Oxidative stress has been reported to increase the blood norepinephrine and cortisol levels.[25] These hormones significantly increase the intracellular levels of ROS and reactive nitrogen species. They damage cellular microstructures and activate the immune and inflammatory systems. Uncontrolled ROS production directly damages the reproductive tissues. ROS directly inhibit the male reproductive function by increasing the effect of glucocorticoids on Leydig cells.[26] As a result, the circulating testosterone levels decrease because of the suppression of androgen synthesis and induction of apoptosis in the Leydig cells.[27] LH receptors are located on the membrane of Leydig cells, whereas FSH receptors are located on the Sertoli cells.[28] They coordinate to synthesize testosterone and maintain normal spermatogenesis and sperm health and density. Stress-induced elevation of glucocorticoid levels can directly reduce the testosterone levels.[29] There is also a significant decrease in LH and gonadotropin releasing hormone levels in case of chronic stress.[30] Reduced LH levels cannot sufficiently stimulate the Leydig cells for testosterone production. Decreasing the FSH levels reduces the release of androgen binding protein from the Sertoli cells. As a result, oxidative stress decreases the amount of circulating testosterone. At the same time, during oxidative stress, testicular inhibition and E2 secretion increase and inhibit the testosterone release. Decreasing testosterone cannot adequately regulate proper spermatogenesis to produce mature spermatozoa.
In our study, FSH, LH, and total testosterone levels significantly increased in the treatment group. It can be concluded that antioxidant use improves the serum sex hormone levels and increases an individual’s fertility chances. Increase in the spontaneous pregnancy rates along with improvement in sperm concentration and motility was reported by the studies evaluating the effect of oral use of antioxidants on male infertility.[11] The limitations of this study are that there was no placebo group and sperm DNA damage could not be examined. Randomized placebo-controlled trials are needed to determine the improved effect of antioxidant treatment on male reproductive hormones and the effectiveness of antioxidant support in the treatment of idiopathic male infertility. Another limitation of the study is that the molecules in the antioxidant supplement can affect the hormones in another way or directly, apart from the antioxidant effects. For example, vitamin D acts as a steroid hormone with a progesterone effect.[31] Vitamin A metabolite affects thehypothalamic–pituitary–adrenal axis through the retinoid[32], zinc mediates the effect of androgens[33], and L-arginine affects hormones through NO.[34]
Some of the infertile men with no correctable pathology need medical/supportive therapy. Among the supportive treatments, the use of antioxidant supplements improves the sperm parameters in infertile men and increases the possibility of pregnancy. Antioxidant support therapy is also useful in couples who want a chance of spontaneous pregnancy or who want to increase the probability of pregnancy with intracytoplasmic sperm injection. We have shown that antioxidant supplements have a positive effect on serum sex hormone levels in men with idiopathic infertility. The promising but unproven clinical status of fertility- and pregnancy-promoting support products should be overcome in the placebo-controlled trials.
Main Points.
Idiopathic infertility occurs in men at a frequency of 31%. No medical treatment has yet received approval for idiopathic male infertility because there is no correctable cause.
Idiopathic infertility has a multifactorial etiology, including genetic, environmental, and hormonal parameters, and especially, DNA fragmentation and oxidative stress are considered responsible. Oxidative stress contributes to impaired spermatogenesis, leading to male infertility.
In this study, the effects of antioxidants on reproductive hormones and sperm parameters were investigated. Written informed consent was obtained from patients who participated in this study. FSH, LH, and testosterone levels significantly increased in the treatment group.
Our results suggest that antioxidant use not only increases the sperm parameters but also improves serum hormone levels and increases an individual’s fertility chances.
Randomized placebo-controlled trials are needed to determine the improved effect of antioxidant treatment on male reproductive hormones.
Acknowledgments
The authors thank Mr. Ozan Efesoy, Mersin City Research and Training Hospital, Department of Urology, for his assistance with statistical analysis.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Mersin University (approval No: 2018/409).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – B.S.; Design – S.Ç.; Supervision – B.S..; Materials – B.S.; Data Collection and/or Processing – B.S.; Analysis and/or Interpretation – S.Ç.; Literature Search – S.Ç.; Writing Manuscript – B.S.; Critical Review – S.Ç.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Jungwirth A, Diemer T, Kopa Z. Male Infertility. EAU guidelines EAU. 2018 [Google Scholar]
- 2.Ko EY, Siddiqi K, Brannigan RE, Sabanegh ES., Jr Empirical medical therapy for idiopathic male infertility: a survey of the American Urological Association. J Urol. 2012;187:973–8. doi: 10.1016/j.juro.2011.10.137. [DOI] [PubMed] [Google Scholar]
- 3.Schiff JD, Ramirez ML, Bar-Chama N. Medical and surgical management male infertility. Endocrinol Metab Clin North Am. 2007;36:313–31. doi: 10.1016/j.ecl.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Tremellen K. Oxidative stress and male infertility-a clinical perspective. Hum Reprod Update. 2008;14:243–58. doi: 10.1093/humupd/dmn004. [DOI] [PubMed] [Google Scholar]
- 5.Aitken RJ, Clarkson JS. Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. J Reprod Fertil. 1987;81:459–69. doi: 10.1530/jrf.0.0810459. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal A, Nallella KP, Allamaneni SS, Said TM. Role of antioxidants in treatment of male infertility: an overview of the literature. Reprod Biomed Online. 2004;8:616–27. doi: 10.1016/S1472-6483(10)61641-0. [DOI] [PubMed] [Google Scholar]
- 7.Bennetts LE, Aitken RJ. A comparative study of oxidative DNA damage in mammalian spermatozoa. Mol Reprod Dev. 2005;71:77–87. doi: 10.1002/mrd.20285. [DOI] [PubMed] [Google Scholar]
- 8.Anderson JB, Williamson RC. Fertility after torsion of the spermatic cord. Br J Urol. 1990;65:225–30. doi: 10.1111/j.1464-410X.1990.tb14715.x. [DOI] [PubMed] [Google Scholar]
- 9.Silver EW, Eskenazi B, Evenson DP, Block G, Young S, Wyrobek AJ. Effect of antioxidant intake on sperm chromatin stability in healthy nonsmoking men. J Androl. 2005;26:550–6. doi: 10.2164/jandrol.04165. [DOI] [PubMed] [Google Scholar]
- 10.Spiers JG, Chen HJ, Sernia C, Lavidis NA. Activation of the hypothalamic-pituitary-adrenal stress axis induces cellular oxidative stress. Front Neurosci. 2014;8:456. doi: 10.3389/fnins.2014.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Majzoub A, Agarwal A. Systematic review of antioxidant types and doses in male infertility: Benefits on semen parameters, advanced sperm function, assisted reproduction and live-birth rate. Arab J Urol. 2018;16:113–24. doi: 10.1016/j.aju.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Omar MI, Pal RP, Kelly BD, Bruins HM, Yuan Y, Diemer T, et al. Benefits of Empiric Nutritional and Medical Therapy for Semen Parameters and Pregnancy and Live Birth Rates in Couples with Idiopathic Infertility: A Systematic Review and Meta-analysis. Eur Urol. 2019;75:615–25. doi: 10.1016/j.eururo.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal A, Rana M, Qiu E, AlBunni H, Bui AD, Henkel R. Role of oxidative stress, infection and inflammation in male infertility. Andrologia. 2018;50:e13126. doi: 10.1111/and.13126. [DOI] [PubMed] [Google Scholar]
- 14.Appasamy M, Muttukrishna S, Pizzey AR, Ozturk O, Groome NP, Serhal P, et al. Relationship between male reproductive hormones, sperm DNA damage and markers of oxidative stress in infertility. Reprod Biomed Online. 2007;14:159–65. doi: 10.1016/S1472-6483(10)60783-3. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: World Health Organization; 2010. [Google Scholar]
- 16.Sharma RK, Agarwal A. Role of reactive oxygen species in male infertility. Urology. 1996;48:835–50. doi: 10.1016/S0090-4295(96)00313-5. [DOI] [PubMed] [Google Scholar]
- 17.Christova Y, James PS, Jones R. Lipid diffusion in sperm plasma membranes exposed to peroxidative injury from oxygen free radicals. Mol Reprod Dev. 2004;68:365–72. doi: 10.1002/mrd.20084. [DOI] [PubMed] [Google Scholar]
- 18.Majzoub A, Agarwal A, Esteves SC. Antioxidants for elevated sperm DNA fragmentation: a mini review. Transl Androl Urol. 2017;6:649–53. doi: 10.21037/tau.2017.07.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hajder M, Hajder E, Husic A. The effects of total motile sperm count on spontaneous pregnancy rate and pregnancy after IUI treatment in couples with male factor and unexplained infertility. Med Arch. 2016;70:39–43. doi: 10.5455/medarh.2016.70.39-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tiegs AW, Landis J, Garrido N, Scott RT, Jr, Hotaling JM. Total motile sperm count trend over time: Evaluation of semen analyses from 119,972 men from subfertile couples. Urology. 2019;132:109–16. doi: 10.1016/j.urology.2019.06.038. [DOI] [PubMed] [Google Scholar]
- 21.Elumalai P, Krishnamoorthy G, Selvakumar K, Arunkumar R, Venkataraman P, Arunakaran JJR. Studies on the protective role of lycopene against polychlorinated biphenyls (Aroclor 1254)-induced changes in StAR protein and cytochrome P450 scc enzyme expression on Leydig cells of adult rats. Reprod Toxicol. 2009;27:41–5. doi: 10.1016/j.reprotox.2008.11.053. [DOI] [PubMed] [Google Scholar]
- 22.Agarwal A, Parekh N, Panner Selvam MK, Henkel R, Shah R, et al. Male oxidative stress infertility (MOSI): proposed terminology and clinical practice guidelines for management of idiopathic male infertility. World J Mens Health. 2019;37:296–312. doi: 10.5534/wjmh.190055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henkel R, Sandhu IS, Agarwal AJA. The excessive use of antioxidant therapy: A possible cause of male infertility? Andrologia. 2019;51:e13162. doi: 10.1111/and.13162. [DOI] [PubMed] [Google Scholar]
- 24.Kavoussi P, Costabile RA, Salonia A. Clinical urologic endocrinology: principles for Men’s health. Springer Science & Business Media; 2012. [DOI] [Google Scholar]
- 25.Flaherty RL, Owen M, Fagan-Murphy A, Intabli H, Healy D, Patel A, et al. Glucocorticoids induce production of reactive oxygen species/reactive nitrogen species and DNA damage through an iNOS mediated pathway in breast cancer. Breast Cancer Res. 2017;19:35. doi: 10.1186/s13058-017-0823-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hardy MP, Gao H-B, Dong Q, Ge R, Wang Q, Chai WR, et al. Stress hormone and male reproductive function. Cell Tissue Res. 2005;322:147–53. doi: 10.1007/s00441-005-0006-2. [DOI] [PubMed] [Google Scholar]
- 27.O’Hara L, McInnes K, Simitsidellis I, Morgan S, Atanassova N, Slowikowska-Hilczer J, et al. Autocrine androgen action is essential for Leydig cell maturation and function, and protects against late-onset Leydig cell apoptosis in both mice and men. FASEB J. 2015;29:894–910. doi: 10.1096/fj.14-255729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Darbandi M, Darbandi S, Agarwal A, Sengupta P, Durairajanayagam D, Henkel R, et al. Reactive oxygen species and male reproductive hormones. Reprod Biol Endocrinol. 2018;16:1–14. doi: 10.1186/s12958-018-0406-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao HB, Tong MH, Hu YQ, You HY, Guo QS, Ge RS, et al. Mechanisms of glucocorticoid-induced Leydig cell apoptosis. Mol Cell Endocrinol. 2003;199:153–63. doi: 10.1016/S0303-7207(02)00290-3. [DOI] [PubMed] [Google Scholar]
- 30.Wagenmaker ER, Breen KM, Oakley AE, Tilbrook AJ, Karsch FJ. Psychosocial stress inhibits amplitude of gonadotropin-releasing hormone pulses independent of cortisol action on the type II glucocorticoid receptor. Endocrinology. 2009;150:762–9. doi: 10.1210/en.2008-0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monastra G, De Grazia S, De Luca L, Vittorio S, Unfer V. Vitamin D: a steroid hormone with progesterone-like activity. Eur Rev Med Pharmacol Sci. 2018;22:2502–12. doi: 10.26355/eurrev_201804_14845. [DOI] [PubMed] [Google Scholar]
- 32.Marissal-Arvy N, Hamiani R, Richard E, Moisan MP, Pallet V. Vitamin A regulates hypothalamic-pituitary-adrenal axis status in LOU/C rats. J Endocrinol. 2013;19:21–7. doi: 10.1530/JOE-13-0062. [DOI] [PubMed] [Google Scholar]
- 33.Koehler K, Parr MK, Geyer H, Mester J, Schänzer W. Serum testosterone and urinary excretion of steroid hormone metabolites after administration of a high-dose zinc supplement. Eur J Clin Nutr. 2009;63:65–70. doi: 10.1038/sj.ejcn.1602899. [DOI] [PubMed] [Google Scholar]
- 34.Battaglia C, Regnani G, Marsella T, Facchinetti F, Volpe A, Venturoli S, et al. Adjuvant L-arginine treatment in controlled ovarian hyperstimulation: a double-blind, randomized study. Hum Reprod. 2002;17:659–65. doi: 10.1093/humrep/17.3.659. [DOI] [PubMed] [Google Scholar]