Abstract
Prostate cancer (PCa) is a disease with high morbidity and mortality rates, which requires finding new lines of approach. The significant advances in and interest of molecular biology in this condition have led to the discovery of elements profiled as an essential research target. Accordingly, we consider the importance of studying the role that monocarboxylate transporters (MCTs) play in PCa. These transporters might have a functional characterization, possible diagnostic and therapeutic implications, and influence on the progression and prognosis of this cancer. We reviewed literature published from January 2010 to June 2020 in different databases and search engines to find studies that respond to our question. MCTs have a close correlation with PCa, contributing to their phenotype of glycolytic and acid-resistant metabolism. They determine the maintenance and progression of the disease depending on the expression of different molecular types of the transporter. Thus, MCT2 highlights as a biomarker in early diagnosis and MCT4 in poor prognosis and resistance. Finally, MCT1 and MCT4 profile as a potential therapeutic target by decreasing cell proliferation. In conclusion, MCTs play an essential role in PCa; therefore, they should be taken into account in subsequent studies for finding tools with clinical applicability and contributing to the reduction of the disease burden.
Keywords: Prostate cancer, monocarboxylate transport, Warburg effect
Introduction
Prostate cancer (PCa) is a significant oncological disease that generates high global disease and mortality burden. PCa is the second leading cause of death in the world, and it is an essential research topic both clinically and in public health.[1] According to the global impact of PCa, some authors investigated several molecular aspects that contribute to influencing this behavior. One of those potential aspects at the molecular level is the microenvironment and metabolism of cancer cells.[2] The interest in this research topic lies in the study of the Warburg effect. It consists of the shift from aerobic mitochondrial metabolism to glycolytic metabolism despite adequate concentrations of tissue oxygen derived from glucose metabolism in cancer cells. This factor is critical in understanding the proliferation of these cells.[3,4] Characterization of the carriers of monocarboxylate transporters (MCTs) involved in glycolytic metabolism through the transport of substances such as lactic acid in the cell membrane highlights a useful and novel perspective to outline their implementation as a goal in the clinical and experimental field.[2,5] This article describes the role that MCTs play in PCa, characterizing the overall function and possible diagnostic, therapeutic, and interference implications in the progression and prognosis of this cancer.
Methods
We performed a search strategy using Medline (PubMed), EMBASE, and Google scholar from January 2010 to June 2020. We included reviews, clinical trials, and studies with experimental models that describe relevant elements related to PCa, glucose metabolism, and MCT carriers. The issues were characterization of the receptor and diagnostic, therapeutic, and progression implications. We used the following keywords in the strategy: prostate cancer, monocarboxylate transporter, and Warburg effect. Finally, we included 28 articles that accomplished the inclusion criteria.
Functional aspects of MCTs
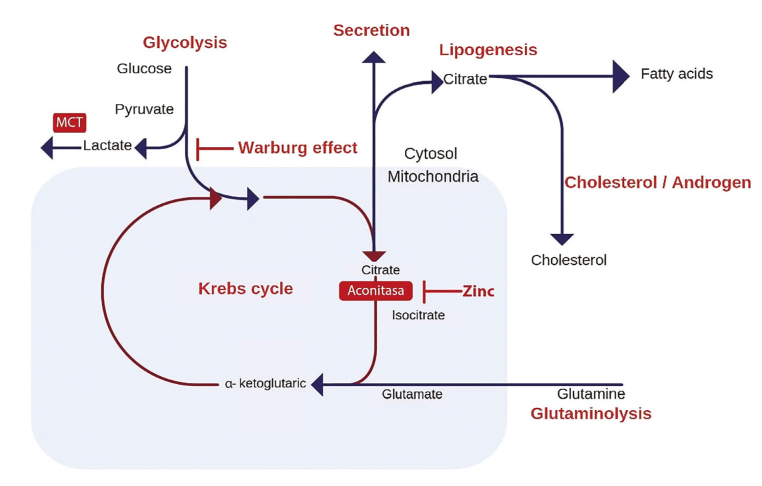
Cancer cells can produce adenosine triphosphate (ATP) through the anerobic pathway (anerobic glycolysis) instead of through the tricarboxylic acid cycle (Krebs cycle) even in the absence of oxygen (Figure 1). A subpopulation of these cells can consume high levels of glucose and secrete high levels of lactate, whereas the others use the lactate produced as their primary energy source. The high production of lactic acid promotes an acidic microenvironment, and the competition for substrates favors the tumor cells.[6] Therefore, the expression of MCTs as part of the acid-resistant and glycolytic metabolism phenotype plays an essential role in the metabolism of lactate, making them the subject of the study.
Figure 1.
Regulated metabolic pathways involved in energy production and maintenance of prostate cells[4]
MCTs catalyze the proton-bound transport of L-lactate, pyruvate, and ketones through the plasma membrane and cell organelles.[5] There are 4 isoforms, MCT 1 to 4, that are known to perform this function in mammals, each with affinities for different inhibitors and substrates. They play a vital role in the metabolism of carbohydrates, lipids, and amino acids because lactic acid is used as a substrate for oxidation, gluconeogenesis, or lipogenesis.[7,8] Therefore, MCTs play a dual role in maintaining the PCa acid-resistant phenotype,[6] leading to the maintenance of a high glycolytic rate by allowing lactate flow and regulation of pH by joint transport of protons (Figure 2).[2]
Figure 2.
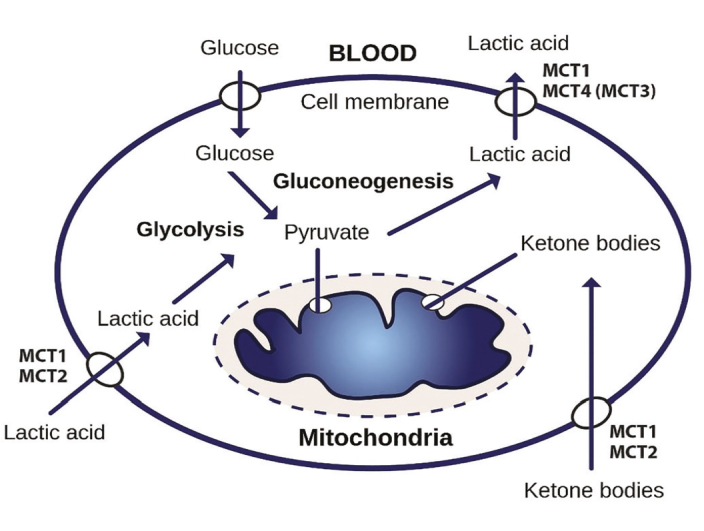
General scheme of the operation of monocarboxylate transporters (MCTs). Depending on the tissue and species, MCT1 or MCT2 are used to absorb lactic acid and ketone bodies for oxidation or lactic acid for gluconeogenesis, whereas MCT4 is used for export[7]
Virtually all the tissues express MCT1, sometimes along with other isoforms of MCTs whose distribution within and between the cells may be quite different from that of MCT1. The primary physiological function of MCT1 is to facilitate the entry or exit of lactic acid (more affine substrate) from the cells depending on their metabolic state. During hypoxia, anoxia, or in the presence of tumor cells, all the cells become more dependent on glycolysis for their ATP production. Therefore, they require the export of lactic acid, which will usually be mediated by MCT1 because this is the predominant isoform in most tissues. Its first inhibitor is α-cyano-4-hydroxycinnamate.[4,7,8]
MCT2 has a higher affinity for pyruvate and lactate than MCT1. Tissues that absorb lactic acid in significant amounts as an energy source (e.g., neurons) or perform gluconeogenesis (liver parenchyma and kidney) express MCT2.[7,8] Recently, some authors described overexpression of this carrier in PCa cell peroxisomes according to an epigenetic control over the SLC16A7 gene.[9–11]
The expression of MCT3 is limited to the basal membrane of the pigmented retinal and choroid plexus epithelium. Along with MCT1, it plays a central role in facilitating the transport of lactic acid derived from the glycolytic retina.[7,8]
In contrast, MCT4 distributes in virtually all the tissues, especially in those depending on glycolysis, such as muscle fibers, astrocytes, white blood cells, and chondrocytes. There is a critical correlation between the expression of MCT4 and MCT1 in muscle fibers in exporting lactic acid.[7,8] In PCa cells, androgen influx modulates the expression of MCT4 by increasing the external transmission of lactic acid by balancing the cell pH.[12]
Relationship of MCTs with PCa
Advanced PCa cells increase anerobic glycolysis. This process leads to a high concentration of intracellular lactic acid. Prostate tumor cells compensate by expressing MCTs to reduce the intracellular lactate levels. An avidly MCT-producing phenotype correlates with more aggressive cancer and worse prognosis. By blocking the activity of MCTs, cells can accumulate toxic metabolic products at a faster rate. Therefore, the expression of lactate carriers within PCa cells represents a possible future therapeutic target as well as a possible indicator of diagnosis and prognosis.[2,4]
The most expressed MCTs in PCa cells are MCT1 and MCT4 for their mutual spatial correlation and broad distribution in tissues in 90% of the cancer cells.[2,8] Different physiological and metabolic phenomena occur when there is experimental silencing, modification, or overexpression of these carriers.[5,13] Silencing MCT4 increases the intracellular lactate levels under hypoxic conditions. The modification of MCT4 increases the sensitivity of the cancer cell to docetaxel, and the overexpression of MCT1 stops the cell cycle in G2/M phase (more susceptible to radiation therapy). In addition, MCT4 overexpression aids the removal of toxic components.[13] The changes in the MCTs, as mentioned earlier, demonstrate the great importance of these carriers in the metabolism of prostate tumor cells and the critical relationship with cell proliferation.
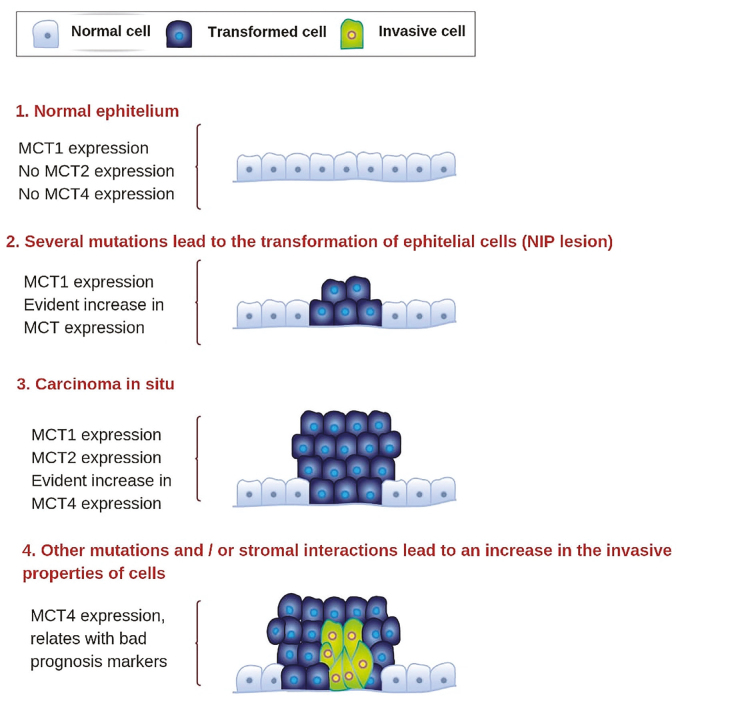
Complementary, MCT1 is mostly expressed in noncancer epithelial cells, MCT2 in intraepithelial neoplastic lesions, and MCT4 in localized PCa (cell invasion) cells. This pattern indicates the role of detection at different stages of PCa (Figure 3).[6,14]
Figure 3.
Schematic representation of the expression of different monocarboxylate transporters depending on the stage of the disease[5]
Diagnostic implications of monocarboxylate carrier in PCa
As mentioned earlier, the research describes lactic acid metabolism through MCTs as a critical factor in PCa, which directs the experimental studies toward finding new biomarkers. The expression of MCT2, measured by immunohistochemistry, is a possible positive biomarker associated with the enzyme alpha-methylacyl-CoA-racemase (AMACR) and negative biomarkers such as p63 and 34-E12. The combination of 2 positive (MCT2/AMACR) and 1 negative (p63 or 34-E12) biomarker might have better sensitivity and negative predictive value than that of 1 positive and 2 negative biomarkers.[5,9] This comparison supports the possibility of the viability of MCT2 as a possible biomarker.
Implications in progression and prognosis of monocarboxylate carrier in PCa
The expression patterns, location, stages of cancer, and degree of expression with which MCTs occur in PCa tumor cells sets the route to try to establish its progression, aggressiveness, and prognosis. From this point of view, overexpression of MCT4 and CD147 could correlate to poor prognosis in PCa. In addition, there is an association with higher prostate-specific antigen levels, Gleason tumor stage (pT3) and invasion, and biochemical recurrence.[5,14] Similarly, the transport system between the tumor and stromal cells correlates with pT3 and poor clinical outcomes based on the increase in MCT4 and MCT1 expression in the cancer-associated fibroblasts (stromal cells).[15] Besides, the expression of MCT1 and MCT4 in tumor cells and tumor-associated stroma, respectively, could contribute to the progression and be a key in the prognosis of biochemical failure.[16,17]
MCT2 and AMACR are also present in the prostate intraepithelial neoplasia. Therefore, they are not only associated as potential biomarkers but may also be involved in malignant transformation and disease progression.[5,9] In contrast, the presence of MCT1 in localized tumors and MCT4 in more aggressive tumors suggests that there are metabolic adaptations in the evolution of cancer.[5]
Finally, this finding highlights the clinical-pathological importance of the lactate transport system that appears generally linked to the parameters of poor prognosis, progression, and the presence of biochemical recurrence after surgery. Immunohistochemical detection of the proteins involved in the system may be useful as prognostic markers.[5]
Therapeutic implications of monocarboxylate carrier in PCa
The particular complexity of the cellular metabolism of PCa, especially that of lactic acid, does the search for a therapeutic target, which is a difficult task.[18] Therefore, it is still controversial to choose the metabolic pathway that could represent the most appropriate target for inhibition in PCa.[5,19] Experimental studies attempt to establish the effects of silencing and modification of MCT1 and MCT4, the results of which suggest that in both normoxic or hypoxic conditions, there is a condition of the viability of the PCa cell lines.[18]
Studies that have used in vivo models showed that there were no significant differences in the tumor volumes after treatment with an MCT1 inhibitor.[5,20] In contrast, pharmacological inhibition of MCT1 activity significantly affected the cell survival of prostate carcinoma and tumor growth.[5,21] A xenographic model-specific inhibition of MCT4 expression led to a reduction in lactic acid secretion, glycolytic metabolism, and proliferation of neuroendocrine PCa.[22] There are also similar results in castration-resistant PCa cells.[23] Therefore, the actual value of the orientation of MCTs in PCa is still mostly unknown. However, most experimental studies indicate a trend toward the beneficial effect of MCTs as a target.
MCTs are a promising target because of the decisive role that they play in the phenotype of glycolytic metabolism of cancer cells. Haloderivatives, such as 3-bromopyruvate, dichloroacetate, and iodoacetate have antiglycolytic effects and serve as substrates for MCTs. The experimental approach in vivo or in vitro establishes that not only the inhibition of MCTs can be useful, but that MCTs could also serve as vehicles for new anticancer drugs.[24]
Another critical aspect to highlight is the implication of MCTs in the resistance. MCT1 and MCT4 overexpress in the androgen-resistant cell lines. Silencing and modification have opposite effects and eliminate the viability of resistant cells. There is also overexpression of MCT4 in castration-resistant cancer cells[25] and docetaxel-resistant cells.[26,27] The modification of MCT4 by short interfering ribonucleic acid inhibits the proliferation and induces apoptosis of castration-resistant PCa cells.[28] All the information presented in this article supports the fact that MCTs represent as not only therapeutic targets but also targets for the reduction of the resistant histological types.
Conclusions
MCTs play an essential role as potential determinants of diagnosis, prognosis, progression, and treatment of PCa. This study presented the current state of the relevant literature of the subject and the importance of the underlying molecular study for further clinical applicability. First, we highlighted the importance of MCT2 as a possible adoptive biomarker, which could assist in diagnosing early PCa by giving a way to the possibility of delving into research work on this subject. Second, we found that the progression and prognosis of PCa are closely related to the expression of MCT1 and MCT4, highlighting the importance of MCT4 as a possible biomarker of PCa progression and aggressiveness. Finally, the findings on the inhibition of MCT1 and MCT4 that could contribute to being therapeutic targets open a new research opportunity. These carriers are the basis for molecular research for PCa, so their clinical applicability has not yet been tested, and their clinical measurement is still far from being implemented.
Main Points.
Monocarboxylate transporters (MCTs) have a close correlation with prostate cancer (PCa), contributing to their phenotype of glycolytic and acid-resistant metabolism.
MCT2 highlights as a biomarker in early diagnosis and MCT4 in poor prognosis and resistance.
MCT1 and MCT4 profile as a potential therapeutic target by decreasing cell proliferation.
MCTs play an essential role as potential determinants of diagnosis, prognosis, progression, and treatment of PCa.
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – H.G.; Design – J.R., H.G.; Supervision – H.G.; Resources - J.R., H.G.; Materials – J.R., H.G.; Data Collection and/or Processing – J.R.; Analysis and/or Interpretation – J.R., H.G.; Literature Search – J.R., H.G.; Writing Manuscript – J.R., H.G.; Critical Review – J.R., H.G.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study Global Burden. JAMA Oncol. 2017;3:524–48. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinheiro C, Longatto-Filho A, Azevedo-Silva J, Casal M, Schmitt FC, Baltazar F. Role of monocarboxylate transporters in human cancers: State of the art. J Bioenerg Biomembr. 2012;44:127–39. doi: 10.1007/s10863-012-9428-1. [DOI] [PubMed] [Google Scholar]
- 3.Liberti MV, Locasale JW. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem Sci. 2016;41:211–8. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eidelman E, Twum-Ampofo J, Ansari J, Siddiqui MM. The Metabolic Phenotype of Prostate Cancer. Front Oncol. 2017;7:1–6. doi: 10.3389/fonc.2017.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pértega-Gomes N, Baltazar F. Lactate transporters in the context of prostate cancer metabolism: What do we know? Int J Mol Sci. 2014;15:18333–48. doi: 10.3390/ijms151018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Perdomo H. An understanding of the prostate cancer pathophysiology for the identification of biomarkers that support an early diagnosis. 2018 [Google Scholar]
- 7.Halestrap AP. The monocarboxylate transporter family-Structure and functional characterization. IUBMB Life. 2012;64:1–9. doi: 10.1002/iub.573. [DOI] [PubMed] [Google Scholar]
- 8.Halestrap AP, Wilson MC. The monocarboxylate transporter family-Role and regulation. IUBMB Life. 2012;64:109–19. doi: 10.1002/iub.572. [DOI] [PubMed] [Google Scholar]
- 9.Pértega-Gomes N, Vizcaíno JR, Gouveia C, Jerõnimo C, Henrique RM, Lopes C, et al. Monocarboxylate transporter 2 (MCT2) as putative biomarker in prostate cancer. Prostate. 2013;73:763–9. doi: 10.1002/pros.22620. [DOI] [PubMed] [Google Scholar]
- 10.Pertega-Gomes N, Vizcaino JR, Felisbino S, Warren AY, Shaw G, Kay J, et al. Epigenetic and oncogenic regulation of SLC16A7 (MCT2) results in protein over-expression, impacting on signalling and cellular phenotypes in prostate cancer. Oncotarget. 2015;6:21675–84. doi: 10.18632/oncotarget.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valença I, Pértega-Gomes N, Vizcaino JR, Henrique RM, Lopes C, Baltazar F, et al. Localization of MCT2 at peroxisomes is associated with malignant transformation in prostate cancer. J Cell Mol Med. 2015;19:723–33. doi: 10.1111/jcmm.12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaz CV, Marques R, Alves MG, Oliveira PF, Cavaco JE, Maia CJ, et al. Androgens enhance the glycolytic metabolism and lactate export in prostate cancer cells by modulating the expression of GLUT1, GLUT3, PFK, LDH and MCT4 genes. J Cancer Res Clin Oncol. 2016;142:5–16. doi: 10.1007/s00432-015-1992-4. [DOI] [PubMed] [Google Scholar]
- 13.Hutchinson L, Boyers A, Chadwick A, Stratford I. The impact of monocarboxylate transporter expression on metabolic function in prostate cancer cells. Eur J Cancer. 2016;61:S79–80. doi: 10.1016/S0959-8049(16)61276-5. [DOI] [Google Scholar]
- 14.Pértega-Gomes N, Vizcaíno JR, Miranda-Gonçalves V, Pinheiro C, Silva J, Pereira H, et al. Monocarboxylate transporter 4 (MCT4) and CD147 overexpression is associated with poor prognosis in prostate cancer. BMC Cancer. 2011;11:312. doi: 10.1186/1471-2407-11-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pértega-Gomes N, Vizcaíno JR, Attig J, Jurmeister S, Lopes C, Baltazar F. A lactate shuttle system between tumour and stromal cells is associated with poor prognosis in prostate cancer. BMC Cancer. 2014;14:1–8. doi: 10.1186/1471-2407-14-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanità P, Capulli M, Teti A, Galatioto GP, Vicentini C, Chiarugi P, et al. Tumor-stroma metabolic relationship based on lactate shuttle can sustain prostate cancer progression. BMC Cancer. 2014;14:1–14. doi: 10.1186/1471-2407-14-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersen S, Solstad Ø, Moi L, Donnem T, Eilertsen M, Nordby Y, et al. Organized metabolic crime in prostate cancer: The coexpression of MCT1 in tumor and MCT4 in stroma is an independent prognosticator for biochemical failure. Urol Oncol Semin Orig Investig. 2015;33:338.e9–338.e17. doi: 10.1016/j.urolonc.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Pertega-Gomes N, Felisbino S, Massie CE, Vizcaino JR, Coelho R, Sandi C, et al. A glycolytic phenotype is associated with prostate cancer progression and aggressiveness: A role for monocarboxylate transporters as metabolic targets for therapy. J Pathol. 2015;236:517–30. doi: 10.1002/path.4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flavin R, Zadra G, Loda M. Metabolic alterations and targeted therapies in prostate cancer. J Pathol. 2011;223:283–94. doi: 10.1002/path.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim HS, Masko EM, Poulton SL, Kennedy KM, Pizzo SV, Dewhirst MW, et al. Carbohydrate restriction and lactate transporter inhibition in a mouse xenograft model of human prostate cancer. BJU Int. 2012;110:1062–9. doi: 10.1111/j.1464-410X.2012.10971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiaschi T, Marini A, Giannoni E, Taddei ML, Gandellini P, De Donatis A, et al. Reciprocal metabolic reprogramming through lactate shuttle coordinately influences tumor-stroma interplay. Cancer Res. 2012;72:5130–40. doi: 10.1158/0008-5472.CAN-12-1949. [DOI] [PubMed] [Google Scholar]
- 22.Choi SYC, Ettinger SL, Lin D, Xue H, Ci X, Nabavi N, et al. Targeting MCT4 to reduce lactic acid secretion and glycolysis for treatment of neuroendocrine prostate cancer. Cancer Med. 2018;7:3385–92. doi: 10.1002/cam4.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi SYC, Xue H, Wu R, Fazli L, Lin D, Collins CC, et al. The MCT4 gene: A novel, potential target for therapy of advanced prostate cancer. Clin Cancer Res. 2016;22:2721–33. doi: 10.1158/1078-0432.CCR-15-1624. [DOI] [PubMed] [Google Scholar]
- 24.Paulo S, Oncology M, Hospital BC, Paulo S, Biology E, De Gualtar C. Monocarboxylate transporters as targets and mediators in cancer therapy response. Histol Histopathol. 2014;29:1511–24. doi: 10.14670/HH-29.1511. [DOI] [PubMed] [Google Scholar]
- 25.Bedaj M, Rao K, Robson C, McCracken S. 39 Targeting cell metabolism to improve prostate cancer therapeutics. Eur Urol Suppl. 2016;15:e39. doi: 10.1016/S1569-9056(16)60041-6. [DOI] [Google Scholar]
- 26.Hao J, Chen H, Madigan MC, Cozzi PJ, Beretov J, Xiao W, et al. Co-expression of CD147 (EMMPRIN), CD44v3–10, MDR1 and monocarboxylate transporters is associated with prostate cancer drug resistance and progression. Br J Cancer. 2010;103:1008–18. doi: 10.1038/sj.bjc.6605839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hao J, Madigan MC, Khatri A, Power CA, Hung TT, Beretov J, et al. In vitro and in vivo prostate cancer metastasis and chemoresistance can be modulated by expression of either CD44 or CD147. PLoS One. 2012;7:1–14. doi: 10.1371/journal.pone.0040716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun Q, Hu L, Fu Q. Mct4 Promotes Cell Proliferation and Invasion of Castration-Resistant Prostate Cancer Pc-3 Cell Line. EXCLI J. 2019;18:187–94. doi: 10.17179/excli2018-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]