Abstract
PURPOSE
Androgen receptor splice variant 7 (AR-V7) detection in circulating tumor cells (CTCs) is associated with a low probability of response and short progression-free (PFS) and overall survival (OS) in men with metastatic castration-resistant prostate cancer (mCRPC) treated with enzalutamide or abiraterone. However, it is unclear whether such men benefit from taxane chemotherapy.
PATIENTS AND METHODS
PROPHECY is a multicenter prospective blinded study of patients with poor-risk mCRPC starting abiraterone or enzalutamide and observed through subsequent progression and taxane chemotherapy. We assessed AR-V7 status using the Johns Hopkins modified AdnaTest CTC AR-V7 messenger RNA assay and the Epic Sciences CTC nuclear-localized AR-V7 protein assay before treatment. The primary objective was to validate the independent prognostic value of CTC AR-V7 status based on radiographic/clinical PFS. OS, confirmed prostate-specific antigen (PSA), and objective radiologic responses were secondary end points.
RESULTS
We enrolled 118 men with mCRPC treated with abiraterone or enzalutamide, 51 of whom received subsequent docetaxel or cabazitaxel. Pretreatment CTC AR-V7 status by the Johns Hopkins and Epic Sciences assays was independently associated with worse PFS (hazard ratio [HR], 1.7; 95% CI, 1.0 to 2.9 and HR, 2.1; 95% CI, 1.0 to 4.4, respectively) and OS (HR, 3.3; 95% CI, 1.7 to 6.3 and HR, 3.0; 95% CI, 1.4 to 6.3, respectively) and a low probability of confirmed PSA responses, ranging from 0% to 11%, during treatment with abiraterone or enzalutamide. At progression, subsequent CTC AR-V7 detection was not associated with an inferior PSA or radiographic response or worse PFS or OS with subsequent taxane chemotherapy after adjusting for CellSearch CTC enumeration and clinical prognostic factors.
CONCLUSION
Detection of AR-V7 in CTCs by two different blood-based assays is independently associated with shorter PFS and OS with abiraterone or enzalutamide, but such men with AR-V7–positive disease still experience clinical benefits from taxane chemotherapy.
INTRODUCTION
Men with metastatic castration-resistant prostate cancer (mCRPC) have improved survival when treated with androgen receptor (AR) signaling inhibitor enzalutamide or abiraterone, as well as with taxane chemotherapy, including docetaxel and cabazitaxel.1-4 After progression during treatment with an AR inhibitor and docetaxel, cabazitaxel improves survival in men with mCRPC when compared with a second AR inhibitor.5 In men with poor-risk clinical features or prior exposure to an AR inhibitor, response rates to a second AR inhibitor are low, and progression-free (PFS) and overall survival (OS) times are short because of cross-resistance.5-8 Although some men may derive clinical benefit from a second-line AR inhibitor, clinical features alone are unable to predict cross-resistance.6 Therefore, predictive biomarkers are needed to optimize treatment selection.
CONTEXT
Key Objective
We sought to prospectively validate the association between circulating tumor cell (CTC) androgen receptor splice variant 7 (AR-V7) detection using two analytically validated assays with poor clinical outcomes with abiraterone or enzalutamide in men with metastatic castration-resistant prostate cancer (mCRPC) and determine whether such men with AR-V7–positive disease can still benefit from taxane chemotherapy as an alternative approach.
Knowledge Generated
CTC AR-V7 status by either the Johns Hopkins or Epic Sciences assay was prospectively validated in this multicenter trial of outcomes with abiraterone or enzalutamide, including short progression-free and overall survival and low response rates, but such men still benefited from subsequent taxane chemotherapy, similar to men with AR-V7–negative mCRPC.
Relevance
CTC AR-V7 testing, in the context of other clinical parameters, can help inform treatment decisions for men with poor-risk mCRPC. CTC AR-V7 status explains some of the resistance to AR therapy in men with mCRPC, but AR-V7 heterogeneity within patients and over time suggests broad and novel approaches are needed to overcome resistance in such patients.
We recently demonstrated in a multicenter prospective study (PROPHECY) that circulating tumor cell (CTC) AR splice variant 7 (AR-V7) expression at the RNA and protein levels, as determined by either of two analytically and clinically validated RNA- or nuclear protein–based assays, is strongly associated with short PFS and OS with AR therapy in poor-risk patients with mCRPC and patients with mCRPC receiving second-line treatment,9 confirming prior single-institution and retrospective studies.10-12 Prior work has suggested that AR-V7–positive PCs may also be resistant to taxane chemotherapy, but clinical data suggest that AR-V7–positive disease may still be compatible with responsiveness to taxane chemotherapy.11-13
Here we report the final results of the prospective double-blinded multicenter PROPHECY trial, in which men with mCRPC were observed longitudinally, with CTC AR-V7 testing performed before AR inhibitor treatment and again at AR therapy progression before taxane chemotherapy, and observed for long-term clinical outcomes.
PATIENTS AND METHODS
Patients
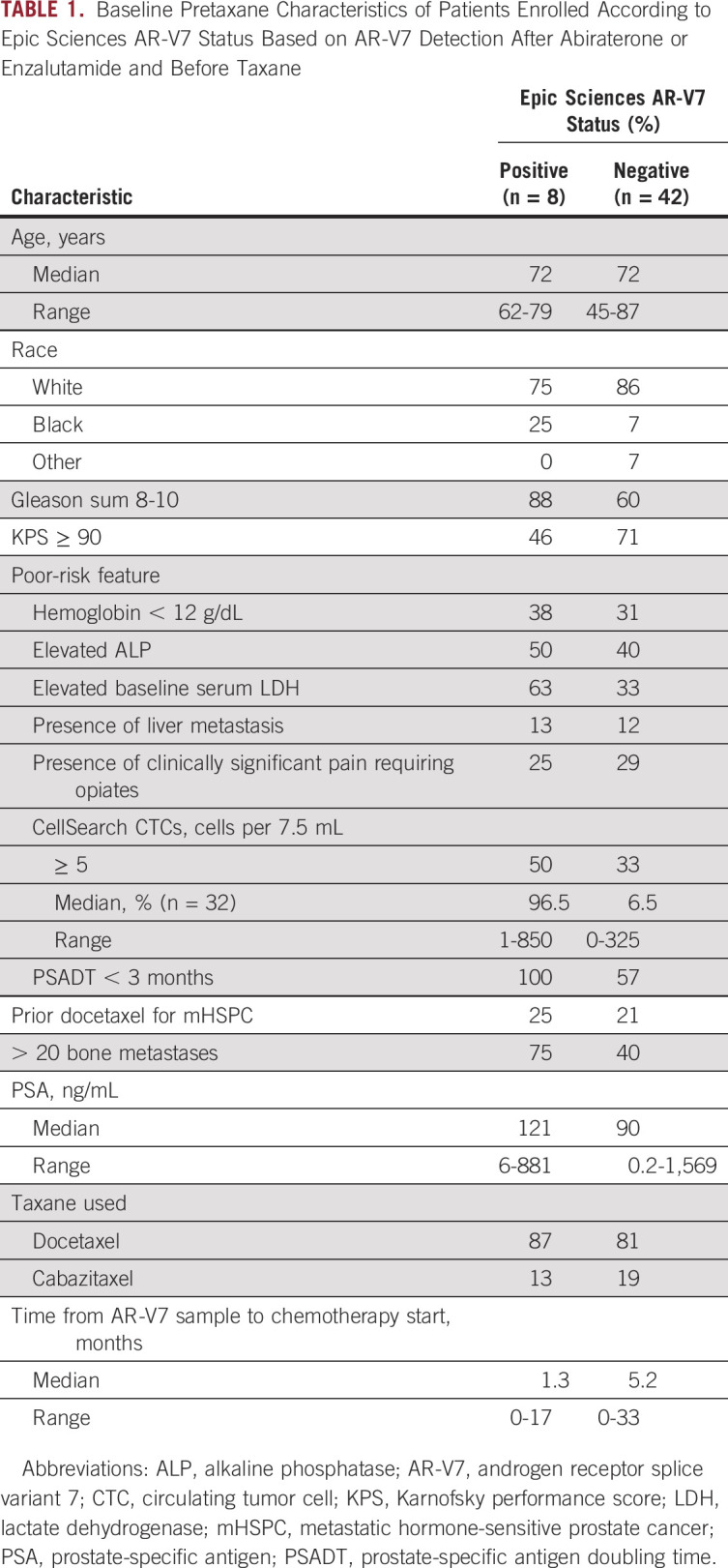
At five clinical sites, we prospectively enrolled men with progressive high-risk mCRPC initiating standard-of-care treatment with enzalutamide or abiraterone. Prior exposure to enzalutamide or abiraterone was permitted for men who were planning to receive the alternative agent. Eligibility information was published previously; at least two poor-prognosis clinical factors were required9,14,15 (Table 1). All patients provided written informed consent under institutional review board approval at all participating centers within the Department of Defense–funded Prostate Cancer Clinical Trial Consortium.16
TABLE 1.
Baseline Pretaxane Characteristics of Patients Enrolled According to Epic Sciences AR-V7 Status Based on AR-V7 Detection After Abiraterone or Enzalutamide and Before Taxane
Study Design and Assessments
PROPHECY is a prospective multicenter study evaluating the ability of baseline (pretreatment) AR-V7 status in CTCs to predict treatment outcomes with abiraterone or enzalutamide as well as subsequent taxane chemotherapy upon disease progression. Patients treated with prior enzalutamide were offered abiraterone and vice versa, and patients without prior abiraterone or enzalutamide exposure were treated according to physician choice. Upon disease progression, patients were offered standard-of-care taxane chemotherapy with either docetaxel or cabazitaxel. All authors vouch for the completeness of data and data integrity and for the fidelity of the study to the clinical protocol (Data Supplement). Peripheral blood samples for analysis of CTC number and CTC AR-V7 status were obtained from eligible patients at prespecified time points: baseline before abiraterone or enzalutamide initiation; at clinical, radiographic, or biochemical progression during treatment with abiraterone or enzalutamide; and at progression during taxane-based chemotherapy. CellSearch (Menarini Silicon Biosystems, Huntington Valley, PA) CTC enumeration was performed at these time points for all patients and processed in a College of American Physicians/Clinical Laboratory Improvement Amendments–approved central laboratory at Memorial Sloan Kettering Cancer Center.17,18
Treatment selection was at the discretion of the treating physician without knowledge of AR-V7 status. Laboratory investigators were blinded to clinical outcomes. All data sets were separately sent to the study statistician (S.H.), who unblinded the data after database lock.
Analysis of CTCs
CTCs were analyzed in two central laboratories, each blinded to the results of the other. CTC identification by the Epic Sciences CTC nuclear AR-V7 assay and CTC heterogeneity evaluations were performed as described previously.9,11,12 The Johns Hopkins AR-V7 modified AdnaTest was performed as previously described using validated methods.10,13,19-21 Established standard operating procedures for sample collection, overnight shipping, processing, and analysis were followed by study sites and the central laboratory at the Johns Hopkins University.10,13,19-21
Clinical Outcomes
The primary efficacy end point in the trial was PFS during abiraterone or enzalutamide therapy, defined from date of registration to clinical or radiographic progression or death, whichever occurred first. Radiographic progression was assessed at each center using PCWG3-modified RECIST (version 1.1) soft tissue and bone scan criteria.22 Clinical progression was defined as a composite end point including death, escalating pain or other symptomatic progression, initiation of new systemic therapy, or a skeletal-related event. Secondary clinical end points included confirmed ≥ 50% prostate-specific antigen (PSA) decline, radiographic response per RECIST (verson 1.1),23 and OS, and these same outcomes for subsequent taxane chemotherapy.
Data Analysis
The primary objective of PROPHECY was to validate that patients with pretreatment AR-V7–negative CTCs have prolonged PFS with abiraterone or enzalutamide compared with AR-V7–positive patients. The prespecified secondary analysis reported here focused on assessing the association between CTC AR-V7 status after abiraterone or enzalutamide and subsequent pretaxane therapy by two independent assays and outcomes with taxane chemotherapy. With longer follow-up, analyses of the time-to-event end points (PFS and OS) were also updated, and patients who received taxane chemotherapy were observed for response, radiographic progression, and death. Details regarding study design have been published elsewhere.9
Patients with no evaluable CTCs were considered AR-V7 negative, and all patients with sufficient blood collection were analyzed regardless of their evaluable CTCs. In secondary analyses, the proportional hazards model was used for assessing the prognostic value of AR-V7 status for PFS and OS after adjusting for validated prognostic factors (risk score).14,15,24
For taxane outcomes, PFS was defined from the date of starting chemotherapy to clinical or radiographic progression or death, whichever occurred first. The Kaplan-Meier product-limit approach was used to estimate median PFS and OS distributions by AR-V7 status. No power or sample size analysis is provided for this secondary descriptive analysis, because PROPHECY was powered around the primary objective and previously reported.
RESULTS
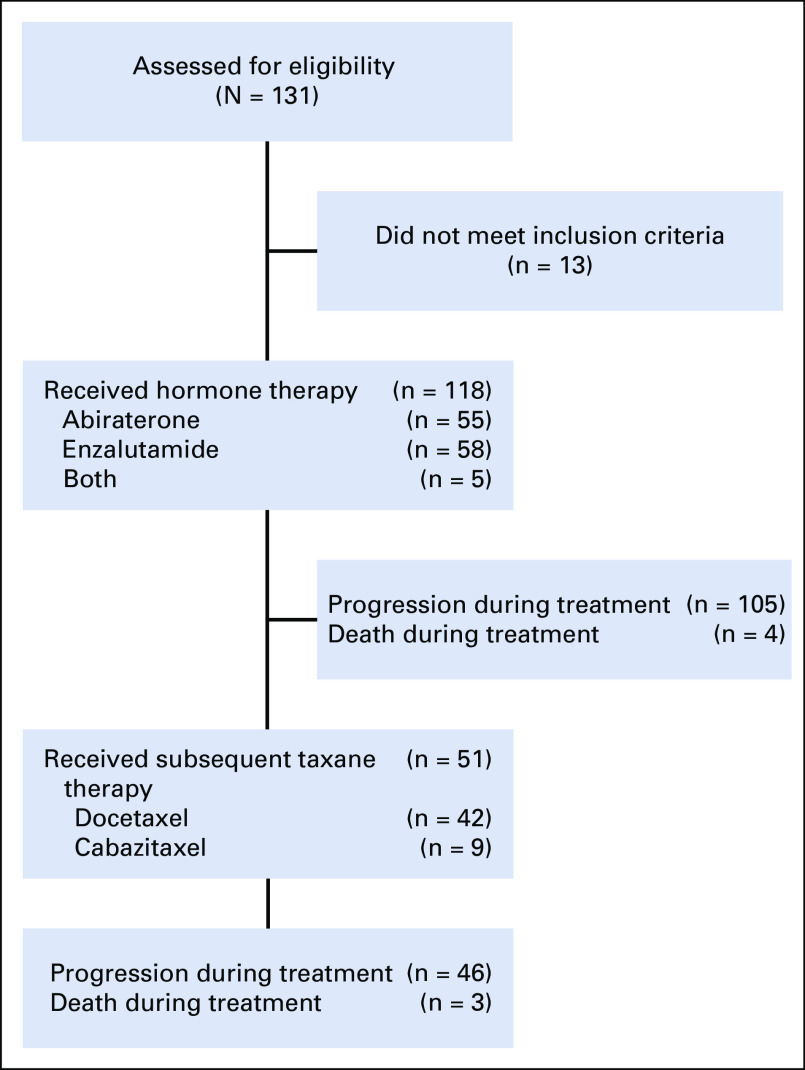
Between May 2015 and January 2017, we prospectively enrolled 118 men with high-risk mCRPC who were initiating treatment with abiraterone, enzalutamide, or both at one of five academic medical centers. Baseline characteristics of the cohort were previously published and included 36 men who had received prior enzalutamide or abiraterone therapy.9 Of these 118 men treated with subsequent AR inhibitor therapy, 51 experienced progression and were treated with taxane chemotherapy, including docetaxel (n = 42) or cabazitaxel (n = 9; CONSORT diagram provided in Appendix Fig A1). Baseline characteristics of these patients at the time of taxane chemotherapy are listed in Table 1 based on Epic Sciences AR-V7 status and Appendix Table A1 based on Johns Hopkins AR-V7 status. As of the final database lock on October 15, 2019, median follow-up times from study registration (before abiraterone or enzalutamide treatment) and taxane initiation were 35 and 23 months, respectively. Of the PROPHECY cohort of 118 men, 105 experienced progression during abiraterone or enzalutamide treatment, in whom 101 of the progression events were radiographic or clinical progression events and four were based on rapid PSA rises that led to a change in systemic therapy; 92 men (78%) died. Of the 51 men treated with taxane chemotherapy, 50 experienced progression, based on radiographic or clinical progression in 48, and 41 died.
AR-V7 Status and Efficacy of AR Signaling Inhibition
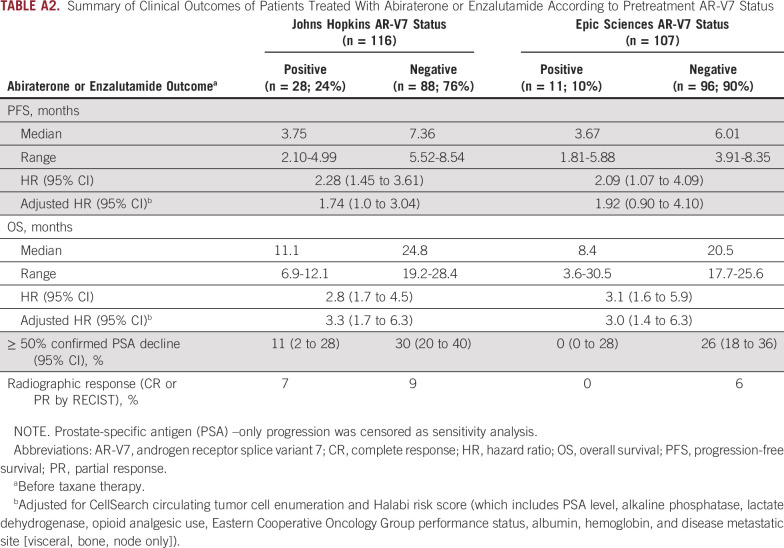
The primary end point of PFS during AR inhibitor therapy with abiraterone or enzalutamide was different in men with AR-V7–positive as compared with AR-V7–negative disease for both AR-V7 assays when assessed before treatment, after adjusting for CellSearch CTC enumeration and clinical Halabi prognostic risk score.14 For the Johns Hopkins AR-V7 assay (n = 116, with 28 patients [24%] AR-V7 positive), median PFS with AR therapy for patients with AR-V7–positive versus AR-V7–negative disease was 3.7 versus 7.2 months, respectively (adjusted hazard ratio [HR], 1.7; 95% CI, 1.0 to 2.9). For the Epic Sciences AR-V7 protein assay (n = 107, with 11 patients [10%] AR-V7 positive), median PFS for those with AR-V7–positive versus AR-V7–negative disease was 3.7 versus 6.0 months, respectively (adjusted HR, 2.1; 95% CI, 1.0 to 4.4). In a sensitivity analysis, PFS results were largely unchanged when four patients with PSA-only progression events were considered censored for both Johns Hopkins and Epic Sciences AR-V7 positivity (Appendix Table A2).
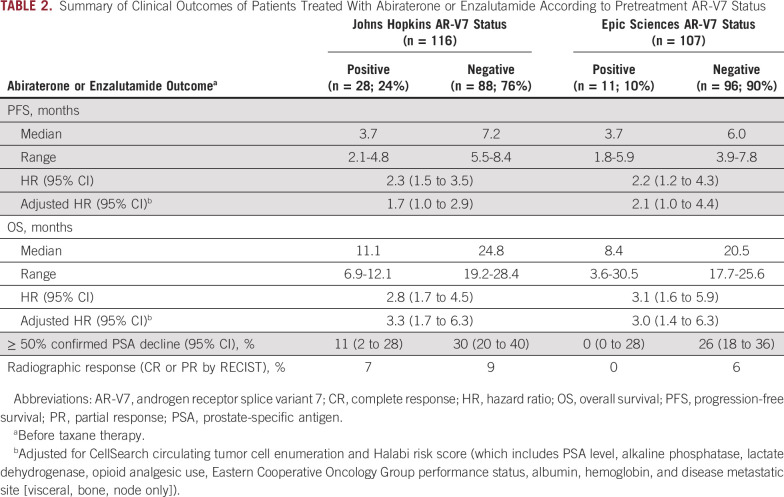
OS with AR-targeted therapy from the time of study registration substantially differed according to CTC AR-V7 status. For the Johns Hopkins AR-V7 RNA assay, median OS for those with AR-V7–positive versus AR-V7–negative disease was 11.1 versus 24.8 months, respectively (adjusted HR, 3.3; 95% CI, 1.7 to 6.3). For the Epic Sciences AR-V7 protein assay, median OS for those with AR-V7–positive versus AR-V7–negative disease was 8.4 versus 20.5 months, respectively (adjusted HR, 3.0; 95% CI, 1.4 to 6.3). Table 2 and Figure 1 summarize the results of PFS and OS by baseline AR-V7 status for each CTC assay.
TABLE 2.
Summary of Clinical Outcomes of Patients Treated With Abiraterone or Enzalutamide According to Pretreatment AR-V7 Status
FIG 1.
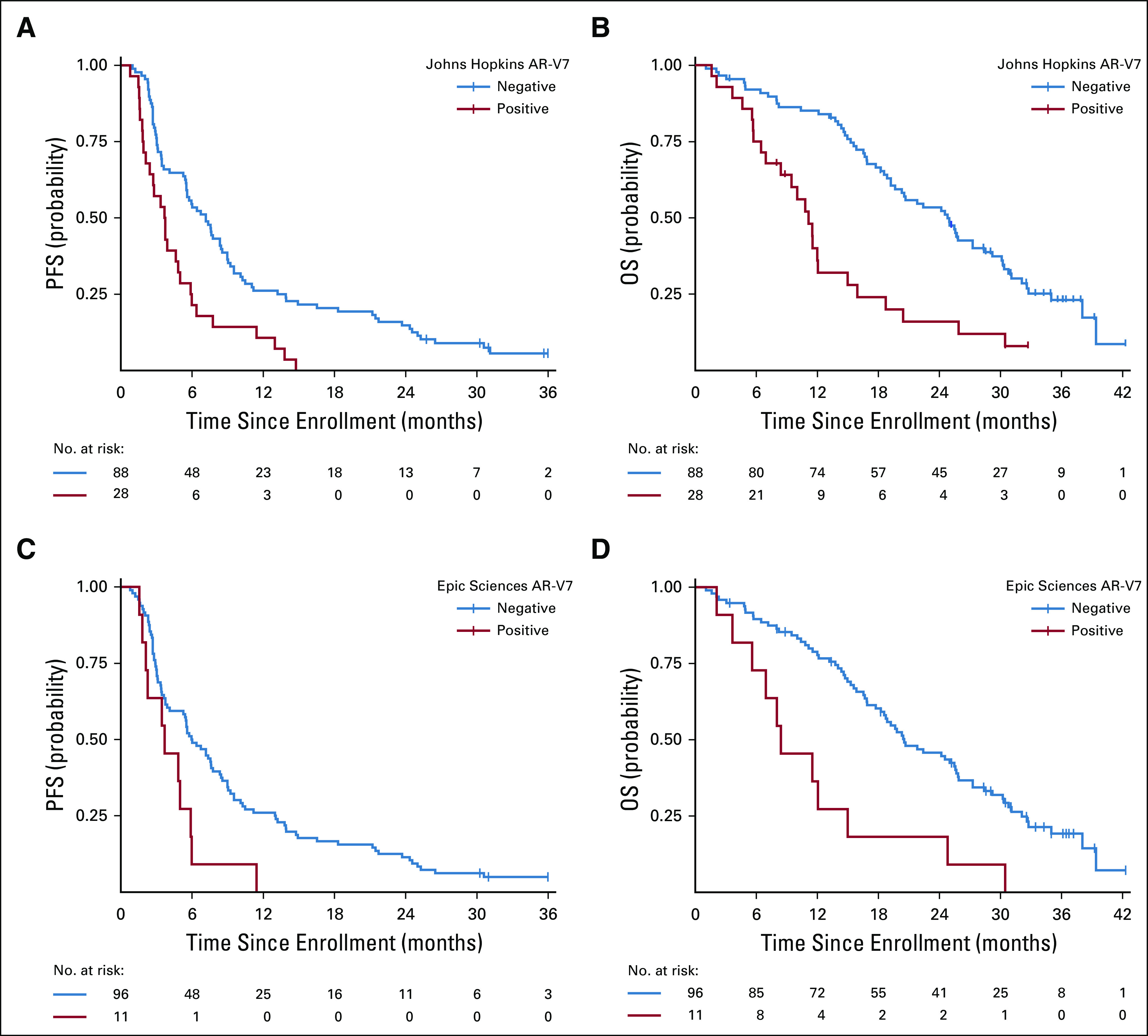
Kaplan-Meier plot of outcomes in men with metastatic castration-resistant prostate cancer treated with abiraterone or enzalutamide in PROPHECY: (A) progression-free survival (PFS) and (B) overall survival (OS) by pretreatment Johns Hopkins circulating tumor cell (CTC) androgen receptor splice variant 7 (AR-V7) detection criteria and (C) PFS and (D) OS by pretreatment Epic Sciences CTC AR-V7 detection criteria. Time (months) is defined from date of registration to event of interest (PFS or OS).
Confirmed ≥ 50% PSA declines were observed in 11% and 30% of patients with AR-V7–positive and AR-V7–negative disease by Johns Hopkins assay, respectively, and radiographic responses (complete or partial responses by RECIST [version 1.1] criteria) were seen in 7% and 9% of patients, respectively. Confirmed ≥ 50% PSA declines were observed in 0% and 26% of those with AR-V7–positive and AR-V7–negative disease by Epic Sciences nuclear assay, respectively, and radiographic responses (complete or partial responses by RECIST [version 1.1] criteria) were observed in 0% and 6% of patients, respectively (Table 2).
Repeat AR-V7 and Subsequent Taxane Chemotherapy Efficacy
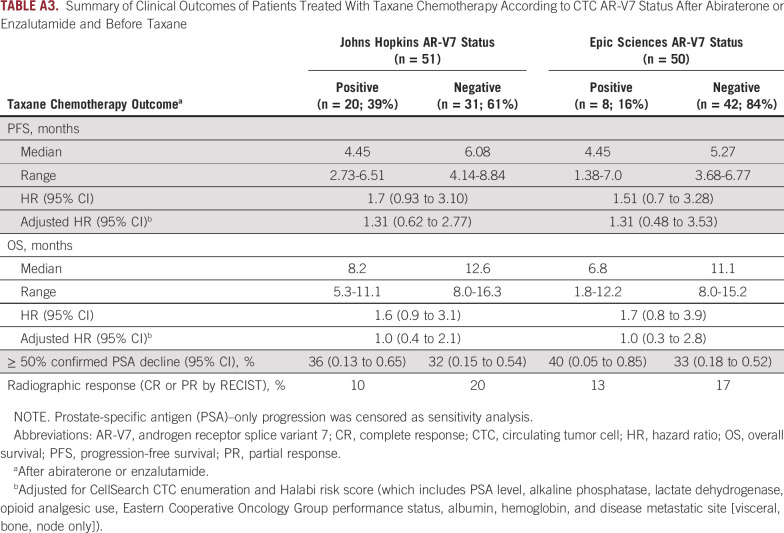
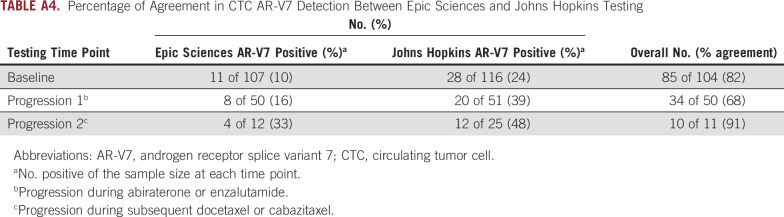
After progressive disease with abiraterone or enzalutamide, 51 men received subsequent taxane-based chemotherapy (docetaxel, n = 42; cabazitaxel, n = 9), before which CTC AR-V7 status was assessed a second time by the same two independent blinded central laboratories using the two different methods. Using the Epic Sciences nuclear AR-V7 protein assay, eight (16%) of 50 evaluable men had AR-V7–positive disease, and 20 (39%) of 51 evaluable men had AR-V7–positive disease according to the Johns Hopkins modified AdnaTest AR-V7 messenger RNA (mRNA) assay. The percentages of agreement for positivity and negativity were 75% and 67%, respectively.
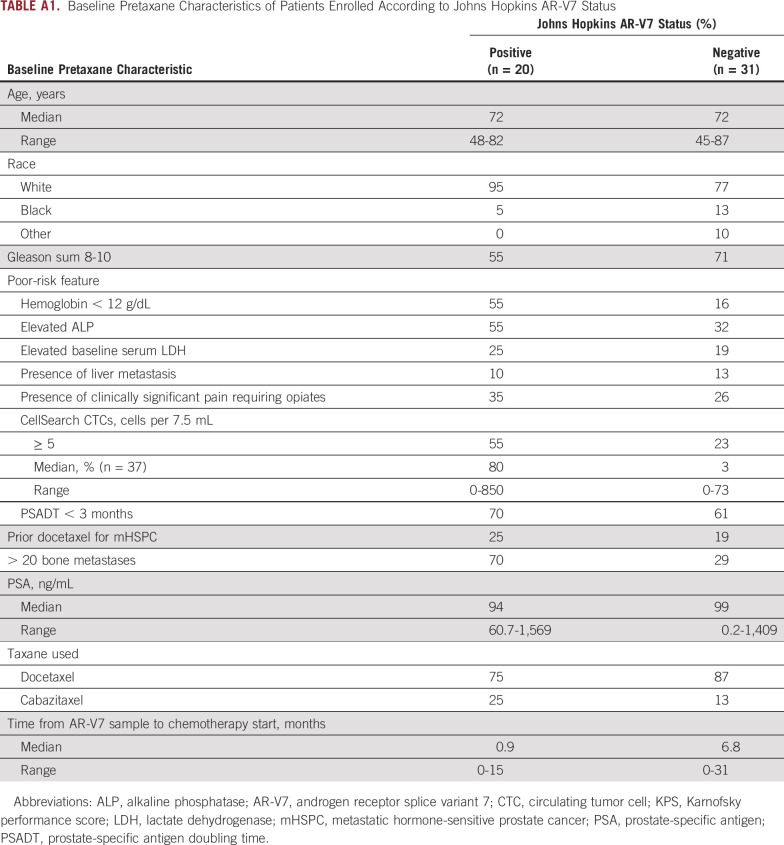
AR-V7 detection after abiraterone or enzalutamide and before taxane therapy by either assay was associated with poor prognostic features at the time of taxane initiation. Men who had a positive Epic Sciences nuclear detect AR-V7 test result were more likely to have Gleason 8 to 10 disease (88% v 60%), poor functional status (Karnofsky performance score < 90 in 54% v 29%), high lactate dehydrogenase (LDH; 63% v 33%), high CellSearch CTC count (median, 96.5 v 6.5 cells per 7.5 mL of whole blood; 50% v 33% with ≥ five CTCs), and higher number of bone metastases (> 20 in 75% v 40%) compared with men without AR-V7 CTCs (Table 1). AR-V7 detection was also associated with a shorter time from sample collection to taxane chemotherapy initiation (1.3 v 5.2 months). Similar results were seen in patients with AR-V7–positive disease relative to those with AR-V7–negative disease by Johns Hopkins assay, a greater proportion of whom had multiple adverse prognostic factors, including high CellSearch CTCs, high LDH, anemia, and high burden of bone metastases. Among men with zero CTCs by the CellSearch assay before taxane therapy (n = 5), one and zero men had AR-V7 detection by the John Hopkins and Epic Sciences assays, respectively.
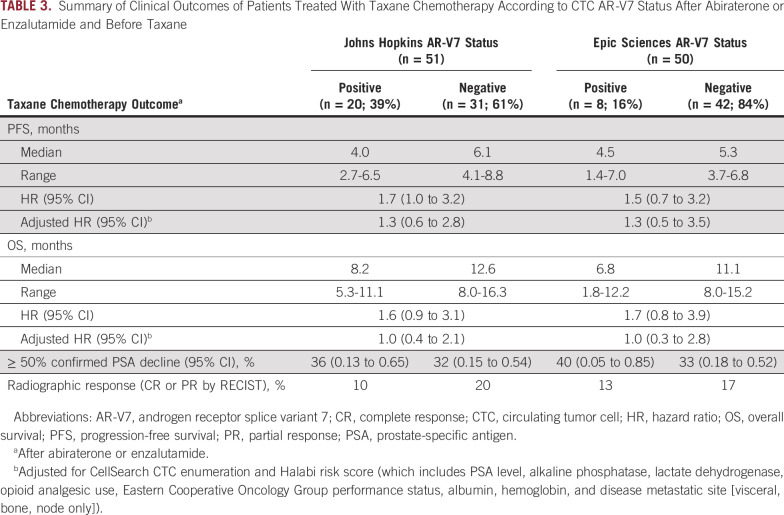
The primary end point of PFS during taxane chemotherapy was not different in men with AR-V7–positive disease pretreatment compared with men with AR-V7–negative disease pretreatment for both AR-V7 assays, after adjusting for CellSearch CTC enumeration and Halabi clinical risk score (Table 3; Fig 2). For the Johns Hopkins AR-V7 assay (n = 51, with 20 patients [39%] AR-V7 positive), median PFS for those with AR-V7–positive versus AR-V7–negative disease receiving taxane treatment was 4.0 months (95% CI, 2.7 to 6.5 months) versus 6.1 months (95% CI, 4.1 to 8.8 months), respectively (adjusted HR, 1.3; 95% CI, 0.6 to 2.8). For the Epic Sciences AR-V7 protein assay (n = 50, with eight patients (16%) AR-V7 positive), median PFS for those with AR-V7–positive versus AR-V7–negative disease was 4.5 months (95% CI, 1.4 to 7.0 months) versus 5.3 months (95% CI, 3.7 to 6.8 months), respectively (adjusted HR, 1.3; 95% CI, 0.5 to 3.5). Results were unchanged when two patients with PSA-only progression were considered censored (Appendix Table A3).
TABLE 3.
Summary of Clinical Outcomes of Patients Treated With Taxane Chemotherapy According to CTC AR-V7 Status After Abiraterone or Enzalutamide and Before Taxane
FIG 2.
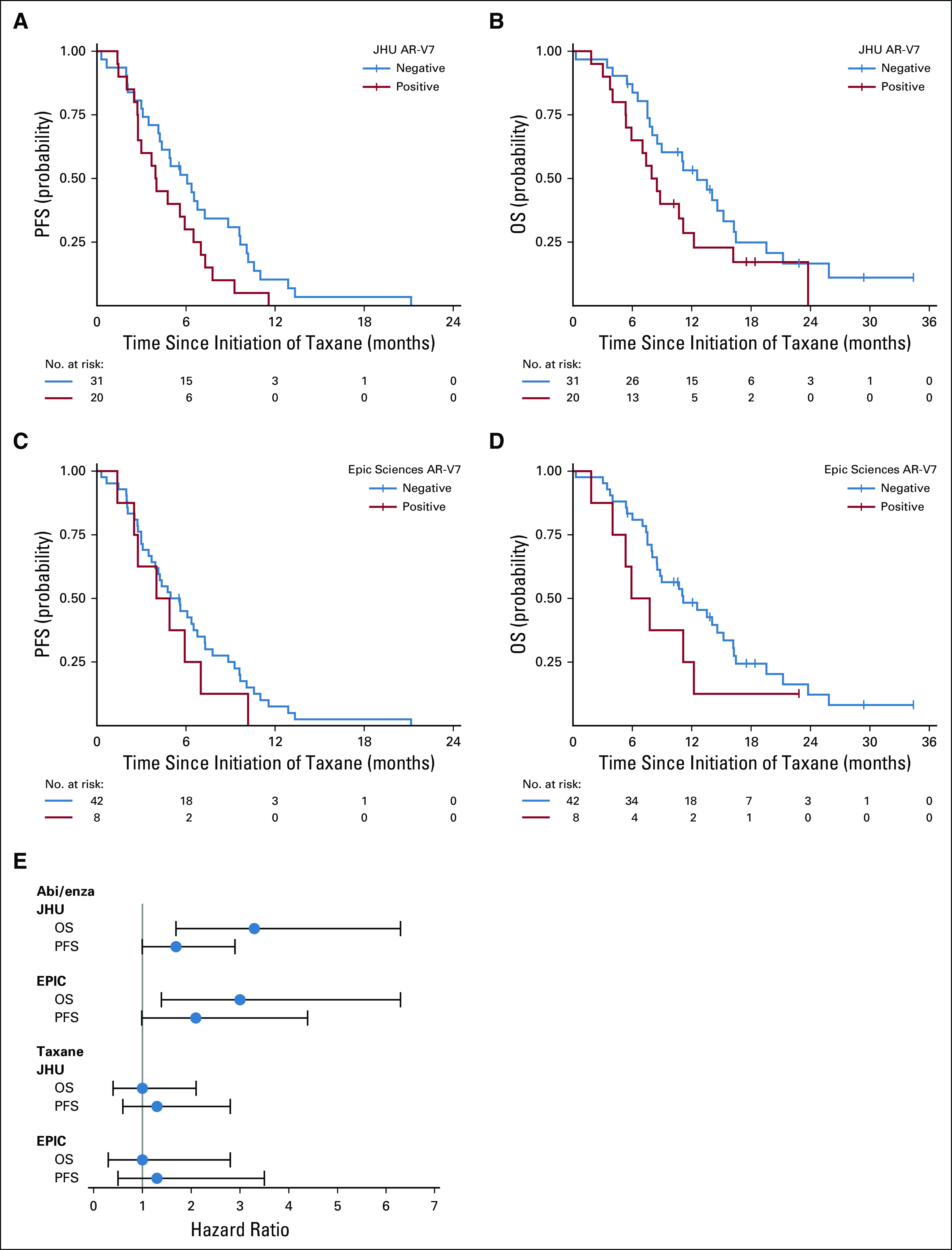
Kaplan-Meier plot of outcomes in men with metastatic castration-resistant prostate cancer treated with taxane chemotherapy after progression with abiraterone (abi) or enzalutamide (Enza) in PROPHECY: (A) progression-free survival (PFS) and (B) overall survival (OS) by Johns Hopkins (JHU) circulating tumor cell (CTC) androgen receptor splice variant 7 (AR-V7) detection criteria after abiraterone or enzalutamide but before taxane therapy and (C) PFS and (D) OS by Epic Sciences CTC AR-V7 detection criteria after abiraterone or enzalutamide but before taxane therapy. Time (months) is defined from the date of taxane chemotherapy to event of interest (PFS or OS). (E) Forest plot of hazard ratios for PFS and OS according to treatment and pretreatment AR-V7 status. NOTE. The abiraterone or enzalutamide–treated and taxane-treated men are the same patients who received sequential therapies, and AR-V7 status for taxane prediction was updated and assessed at progression during abiraterone or enzalutamide and pretaxane treatment.
OS did not differ from the start of taxane chemotherapy by AR-V7 status. For the Johns Hopkins AR-V7 RNA assay, median OS for those with AR-V7–positive versus AR-V7–negative disease was 8.2 months (95% CI, 5.3 to 11.1 months) versus 12.6 months (95% CI, 8.0 to 16.3 months), respectively (adjusted HR, 1.0; 95% CI, 0.4 to 2.1). For the Epic Sciences AR-V7 protein assay, median OS for those with AR-V7–positive versus AR-V7–negative disease was 6.8 months (95% CI, 1.8 to 12.2 months) versus 11.1 months (95% CI, 8.0 to 15.2 months), respectively (adjusted HR, 1.0; 95% CI, 0.3 to 2.8). Table 3 and Figure 2 summarize the results of PFS and OS by baseline AR-V7 status for each CTC assay.
Confirmed ≥ 50% PSA responses with taxane therapy were observed in 36% and 32% of patients with AR-V7–positive versus AR-V7–negative disease by Johns Hopkins assay, respectively, and radiographic responses (complete or partial responses by RECIST [version 1.1]) were observed in 10% and 20% of patients, respectively. Confirmed ≥ 50% PSA responses were observed in 40% and 33% of those with AR-V7–positive versus AR-V7–negative disease by Epic Sciences nuclear assay, respectively, and radiographic responses (complete or partial responses by RECIST [version 1.1]) were observed in 13% and 17% of patients, respectively (Appendix Fig A2). Thus, CTC AR-V7 detection by either assay was not associated with differential outcomes with taxane chemotherapy in men with mCRPC after progression during treatment with abiraterone or enzalutamide. Figure 2E summarizes the overall differences in PFS and OS efficacy outcomes after treatment with a taxane for men with AR-V7–positive versus AR-V7–negative disease pretreatment who received abiraterone or enzalutamide and pretaxane therapy.
Finally, we examined how AR-V7 changes during the treatment course from before abiraterone or enzalutamide to progression and then after progression during taxane chemotherapy. At baseline, AR-V7 positivity was 10% versus 24% by Epic Sciences and Johns Hopkins assays, respectively, with a majority of men with AR-V7–positive disease by Epic Sciences (nine of 11; 82% positive agreement) also testing positive by Johns Hopkins assay. Johns Hopkins–positive, Epic Sciences–negative results were observed, particularly in those men with low Epic Sciences CTCs or nonnuclear AR-V7 protein expression. Although a majority of CTCs in men with mCRPC were AR-V7 negative, even in those with AR-V7–positive disease, the proportion of AR-V7–positive cells ranged from 1% to 100% (median, 20%; Fig 3). At progression with abiraterone or enzalutamide before taxane, eight (16%) of 51 evaluable men had AR-V7 detection by Epic Sciences criteria and 20 (39%) of 51 had detection by Johns Hopkins criteria, suggesting the induction or selection of CTC AR-V7 expression. At progression during taxane chemotherapy, 33% (four of 12) and 48% (12 of 25) of evaluable men had AR-V7 detected by Epic Sciences and Johns Hopkins criteria, respectively (Appendix Table A4). The percentage of agreement between Johns Hopkins and Epic Sciences CTC AR-V7 assays at abiraterone or enzalutamide progression was 68%; at progression during taxane chemotherapy, it was 91%. CTC AR-V7 detection by either assay was clearly associated with CTC detection by the CellSearch assay; however, many men with high CellSearch CTC numbers lacked CTC AR-V7 detection by either assay at all time-points (Fig 3).
FIG 3.
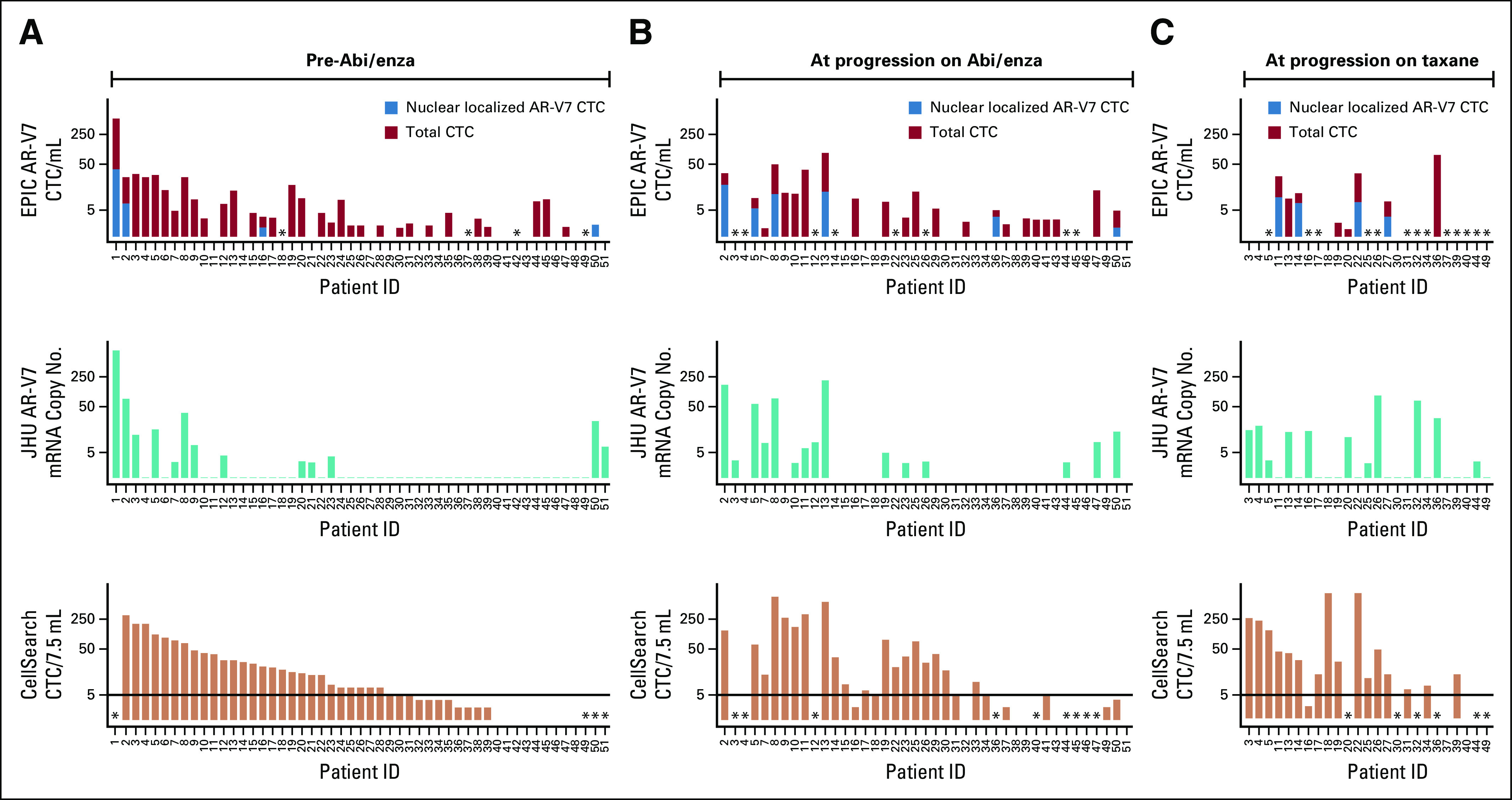
Bar plot demonstrating heterogeneity of androgen receptor splice variant 7 (AR-V7) expression in circulating tumor cells (CTCs) by either the Johns Hopkins (JHU) messenger RNA assay or the Epic Sciences nuclear protein assay collected from men in the PROPHECY study at one of three time points: (A) before abiraterone (abi) or enzalutamide (Enza) therapy, (B) at progression during abiraterone or enzalutamide treatment, and (C) at progression during subsequent taxane chemotherapy. CTC enumeration is provided by CellSearch criteria. For the Epic Sciences nuclear protein assay, approximately 1 mL of blood is analyzed, and total and nuclear-localized AR-V7–positive CTCs are expressed per 1 mL of blood. CTC enumeration is provided by CellSearch criteria and expressed as the total count detected per 7.5 mL of blood. (*) No data.
DISCUSSION
A critical aspect of clinical utility for any biomarker is whether clinical management and patient outcomes are improved by the results of testing. A majority of biomarkers in oncology fail to undergo independent, prospective testing for their predictive or prognostic significance. Here we show that pretreatment CTC AR-V7 status, independently determined by two analytically and clinically validated blood-based assays, reliably distinguishes the clinically meaningful outcomes of response, PFS, and OS in men with mCRPC who are treated with AR pathway inhibitors. Importantly, these same men with CTC AR-V7–positive disease who experience progression with abiraterone or enzalutamide still derive similar benefits from subsequent taxane chemotherapy, suggesting that AR signaling inhibitor therapy should not be offered to men who test positive for AR-V7 by either assay. These data further validate previously reported single-institution studies and retrospective multicenter studies, particularly for men with poor-prognosis mCRPC,10-13,19,25 for whom the timely selection of an effective treatment is critical to maximizing quality of life and survival.
In this prospective multicenter blinded study of AR-V7 detection in CTCs, we demonstrate that men with poor-risk mCRPC who have CTC AR-V7–positive status by mRNA or nuclear protein assay and do not experience benefit from abiraterone or enzalutamide may still benefit from docetaxel or cabazitaxel treatment. These results confirm previously published data showing AR-V7–positive disease remains sensitive to taxane chemotherapy.11-13 In the second- or third-line mCRPC setting, cabazitaxel improves OS and should be considered a reasonable third-line treatment option,5 and docetaxel or cabazitaxel are effective treatments after progression with abiraterone or enzalutamide, with PFS estimates of 4 to 6 months in these poor-risk men.14,24,26,27 The results are particularly important with regard to current practice, given the recent proven benefit and use of these same AR inhibitors in the metastatic hormone-sensitive PC and nonmetastatic CRPC settings, where AR therapy cross-resistance concerns remain when a patient experiences progression to mCRPC during therapy.28-32
A majority of men with AR-V7–positive disease by Epic Sciences assay (nine [82%] of 11) were also positive by Johns Hopkins assay; however, 17 (60%) of 28 patients with positive disease by Johns Hopkins assay were negative by Epic Sciences assay at baseline. These differences largely reflect the differential sensitivities of the assays for AR-V7 detection in CTC-based RNA expression versus protein and the distinction between nuclear versus cytoplasmic localization. The percentage of agreement between these assays over three time points ranged from 68% to 91%, and AR-V7 detection was associated with CTC burden. Some patients developed AR-V7–positive disease at progression during abiraterone or enzalutamide treatment, despite being AR-V7 negative at baseline, whereas some men with AR-V7–positive disease converted to AR-V7–negative status at progression, illustrating the need to assess CTC biomarker status at each management decision point (Fig 3). A limitation of our study includes the lack of random assignment to taxane or AR therapy based on AR-V7 test results, and as such, our results show a prognostic rather than predictive utility. A second limitation is the small sample size, limiting our power to distinguish modest effect sizes for CTC AR-V7 status with taxane outcomes. A key strength of the study is the design, which ensured that clinicians and laboratories were double blinded to the results of CTC AR-V7 assays, and treatment decisions by physicians were independent of the assay results and unbiased. Taken together, knowledge of CTC AR-V7 status combined with standard measures used to assess prognosis can inform the likelihood of benefit from abiraterone or enzalutamide relative to taxane chemotherapy.13,14,28
Although a positive pretreatment AR-V7 test was associated strongly with primary and cross-resistance to novel hormonal agents, AR-V7–negative status does not completely explain sensitivity to subsequent AR inhibition, despite a greater probability of response. Recent prospective treatment sequencing studies suggest common cross-resistance of sequential AR-targeted therapies in men with mCRPC.6 Other resistance mechanisms independent of AR-V7 include lineage plasticity and AR indifference leading to small-cell or neuroendocrine PC, such as CTC-discordant alterations that may promote divergent clonal evoluation,33-36 glucocorticoid receptor activation,37 AR gain or ligand-binding domain mutations,38-40 alternative AR variants and genomic structural rearrangements,41-43 and additional oncogenic pathways like loss of DNA damage repair pathways and TP53 or RB1 tumor suppressors.44-47 Critical to the development of a precision medicine algorithm for men with mCRPC will be the standardization and clinical validation of assays that capture the range of potential resistance mechanisms in a timely manner to inform treatment decisions.
The PROPHECY data further prospectively support CTC AR-V7 status as one such important clinically useful biomarker for men with poor-prognosis mCRPC who are facing a decision on further AR-targeted or taxane therapy. Larger controlled prospective studies that more comprehensively assess CRPC genotypes, phenotypes, and AR splice variants are needed to confirm the predictive utility of CTC AR-V7 in the context of patient and tumor genomic factors.
ACKNOWLEDGMENT
We thank the study coordinators at Weill Cornell, Duke University, Johns Hopkins, University of Chicago, and Memorial Sloan Kettering Cancer Center and acknowledge the dedication of our patients in providing blood samples at no clear benefit to them but for the benefit of all patients with prostate cancer.
APPENDIX
FIG A1.
CONSORT diagram.
FIG A2.
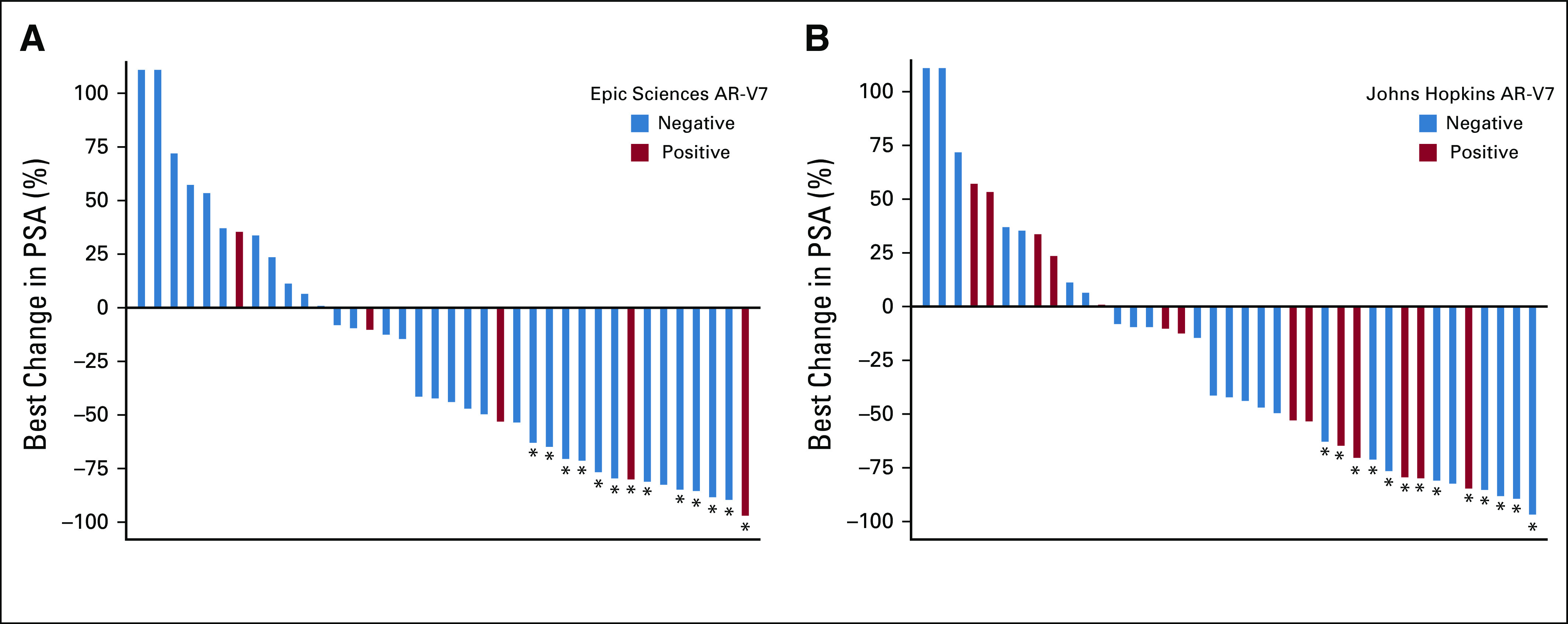
Waterfall plots of best overall prostate-specific antigen (PSA) decline from pretaxane baseline during therapy with docetaxel or cabazitaxel according to (A) Epic Sciences and (B) Johns Hopkins androgen receptor splice variant 7 (AR-V7) status. (*) Indicates that PSA decline was confirmed with second subsequent value per Prostate Cancer Working Group 2/3 guidelines.
TABLE A1.
Baseline Pretaxane Characteristics of Patients Enrolled According to Johns Hopkins AR-V7 Status
TABLE A2.
Summary of Clinical Outcomes of Patients Treated With Abiraterone or Enzalutamide According to Pretreatment AR-V7 Status
TABLE A3.
Summary of Clinical Outcomes of Patients Treated With Taxane Chemotherapy According to CTC AR-V7 Status After Abiraterone or Enzalutamide and Before Taxane
TABLE A4.
Percentage of Agreement in CTC AR-V7 Detection Between Epic Sciences and Johns Hopkins Testing
PRIOR PRESENTATION
Presented in part at the ASCO/Society of Urologic Oncology/American Society for Radiation Oncology Genitourinary Cancers Symposium, San Francisco, CA, February 13-15, 2020.
SUPPORT
Supported by a grant from the Prostate Cancer Foundation and Movember; by the Department of Defense Prostate Cancer Clinical Trials Consortium, which provided infrastructural support; by the National Institutes of Health and Grant 1R01CA233585-01, Duke Cancer Institute (DCI) Grant No. P30 CA014236, and DCI shared resources for biostatistics, flow cytometry, and sequencing and genomic technologies (A.J.A.); in part by Department of Defense Grants No. W81XWH-13-PCRP-CCA, W81XWH-17-2-0021, and W81XWH-14-2-0179 (D.J.G., A.J.A.; Duke University), W81XWH-14-2-0159 (D.M.N.; Weill Cornell), W81XWH-15-2-0018 (R.Z.S.; University of Chicago), W81XWH-15-2-0018 (H.I.S.; Memorial Sloan Kettering Cancer Center [MSKCC]), and W81XWH-16-PCRP-CCRSA (E.S.A., Johns Hopkins); in part by National Cancer Institute (NCI) Grant No. P30CA008748 and the MSKCC Sidney Kimmel Center for Prostate and Urologic Cancers (D.C.D., H.I.S.); NCI Grant No. P30CA006973 (E.S.A.); in part by NCI Grant No. T32CA062948; and in part by Clinical and Translational Science Center Grants No. UL1 TR002384-01 and P30 CA014236.
AUTHOR CONTRIBUTIONS
Conception and design: Andrew J. Armstrong, Jun Luo, David M. Nanus, Paraskevi Giannakakou, Daniel C. Danila, Michael R. Harrison, Howard I. Scher, Richard Wenstrup, Emmanuel S. Antonarakis, Daniel J. George, Susan Halabi
Financial support: Andrew J. Armstrong, Jun Luo, Emmanuel S. Antonarakis
Administrative support: Andrew J. Armstrong, Monika Anand, Scott T. Tagawa
Provision of study material or patients: Andrew J. Armstrong, Jun Luo, David M. Nanus, Russell Z. Szmulewitz, William R. Berry, Tian Zhang, Michael R. Harrison, Scott T. Tagawa, Emmanuel S. Antonarakis
Collection and assembly of data: Andrew J. Armstrong, Jun Luo, David M. Nanus, Russell Z. Szmulewitz, Daniel C. Danila, Monika Anand, William R. Berry, Tian Zhang, Michael R. Harrison, Changxue Lu, Yan Chen, Giuseppe Galletti, Joseph D. Schonhoft, Howard I. Scher, Scott T. Tagawa, Emmanuel S. Antonarakis, Susan Halabi
Data analysis and interpretation: Andrew J. Armstrong, Jun Luo, Paraskevi Giannakakou, Russell Z. Szmulewitz, Patrick Healy, Monika Anand, William R. Berry, Michael R. Harrison, Changxue Lu, Giuseppe Galletti, Joseph D. Schonhoft, Howard I. Scher, Richard Wenstrup, Scott T. Tagawa, Daniel J. George, Susan Halabi
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Andrew J. Armstrong
Honoraria: Dendreon, Janssen Oncology
Consulting or Advisory Role: Bayer, Sanofi, Dendreon, Medivation, Janssen Biotech, Pfizer, Astellas Scientific and Medical Affairs, Clovis Oncology, AstraZeneca
Speakers’ Bureau: Dendreon, Bayer
Research Funding: Dendreon (Inst), Sanofi (Inst), Bayer (Inst), Pfizer (Inst), Novartis (Inst), Janssen Oncology (Inst), Medivation (Inst), Astellas Pharma (Inst), Gilead Sciences (Inst), Roche/Genentech (Inst), Active Biotech (Inst), Bristol Myers Squibb (Inst), Constellation Pharmaceuticals (Inst), Merck (Inst)
Patents, Royalties, Other Intellectual Property: Circulating tumor cell novel capture technology (Inst)
Travel, Accommodations, Expenses: Dendreon, Janssen Biotech, Bayer, Astellas Scientific and Medical Affairs
Jun Luo
Consulting or Advisory Role: Sun Pharma, Janssen Oncology, Tolero Pharmaceuticals
Research Funding: Sanofi (Inst), Orion Pharma (Inst), Mirati Therapeutics (Inst), Gilead Sciences (Inst), Astellas Pharma (Inst), Constellation Pharmaceuticals (Inst), Calibr (Inst), Cardiff Oncology (Inst)
Patents, Royalties, Other Intellectual Property: Coinventor of a technology assigned to Johns Hopkins University, which licensed to Tokai Pharmaceuticals (Inst); coinventor of a technology licensed to Qiagen (Inst); coinventor of a technology licensed to A&G Pharmaceuticals (Inst)
David M. Nanus
Consulting or Advisory Role: Roche/Genentech
Research Funding: Novartis (Inst), Boehringer Ingelheim (Inst), Zenith Epigenetics (Inst), AstraZeneca (Inst), Immumedics (Inst), Janssen (Inst), Clovis Oncology (Inst), Pfizer (Inst)
Paraskevi Giannakakou
Employment: Novartis (I)
Stock and Other Ownership Interests: Novartis (I)
Patents, Royalties, Other Intellectual Property: Coinventor on international patent application docket No. 1676.083WO1“Identifying taxane sensitivity in prostate cancer patients”
Russell Z. Szmulewitz
Honoraria: Astellas Pharma
Consulting or Advisory Role: AstraZeneca, AbbVie, Exelixis, Merck, Amgen, Janssen Oncology, Sanofi, Astellas Pharma, Pfizer
Research Funding: AbbVie, Astellas Pharma, Incyte, Macrogenics, Janssen Oncology
Patents, Royalties, Other Intellectual Property: Coinventor on patent licensed by University of Chicago to Corcept Therapeutics for combination AR/GR inhibition in prostate cancer
Travel, Accommodations, Expenses: Corcept Therapeutics
Daniel C. Danila
Honoraria: Angle, Bayer, ScreenCell, Janssen Oncology, Pfizer, AxImmune, Pfizer, Clovis Oncology, Astellas Pharma
Consulting or Advisory Role: Angle, Bayer, Sanador, AxImmune, Pfizer, Clovis Oncology, Astellas Pharma, Janssen Scientific Affairs
Research Funding: Prostate Cancer Foundation, Genentech, Janssen Research & Development (Inst)
Patents, Royalties, Other Intellectual Property: Gene expression profile associated with prostate cancer
Travel, Accommodations, Expenses: Cambridge Healthtech Institute, Prostate Cancer Foundation, ScreenCell, StopCancer, American Austrian Open Medical Institute, Janssen Biotech, Genzyme, Pfizer, Janssen Scientific Affairs, Astellas Pharma
Patrick Healy
Consulting or Advisory Role: Parexel
William R. Berry
Honoraria: Pfizer, Merck, Genomic Health, Janssen Oncology
Consulting or Advisory Role: Merck, Pfizer, Genomic Health, Janssen Oncology
Research Funding: Merck (Inst)
Travel, Accommodations, Expenses: Merck, Pfizer, Genomic Health, Janssen Oncology
Tian Zhang
Leadership: Capio BioSciences (I), Archimmune Therapeutics (I)
Stock and Other Ownership Interests: Capio Biosciences (I), Archimmune Therapeutics (I), Nanorobotics (I)
Honoraria: Exelixis, Genentech/Roche, MJH Life Sciences, Pacific Genuity
Consulting or Advisory Role: Janssen, Genentech/Roche, Sanofi, Exelixis, AstraZeneca, Pfizer, Bristol Myers Squibb, Foundation Medicine, Pharmacyclics, Amgen, Merck, Seattle Genetics
Speakers’ Bureau: Exelixis, Genentech/Roche, Genomic Health, Sanofi
Research Funding: Janssen (Inst), Acerta Pharma (Inst), Pfizer (Inst), Merrimack (Inst), Stem CentRx (Inst), Novartis (Inst), OmniSeq (Inst), Personal Genome Diagnostics (Inst), Regeneron (Inst), Merck (Inst), Mirati Therapeutics (Inst), Astellas Pharma
Patents, Royalties, Other Intellectual Property: Circulating tumor cell novel capture by c-MET technology (Inst); prochelators as targeted prodrugs for prostate cancer (Inst)
Travel, Accommodations, Expenses: Acerta Pharma, Genomic Health, AstraZeneca
Michael R. Harrison
Consulting or Advisory Role: Bayer, Sanofi, Exelixis, Genentech, Argos Therapeutics, Fujifilm, Janssen Oncology, AstraZeneca, Pfizer, Bristol Myers Squibb
Speakers’ Bureau: Genentech, Exelixis
Research Funding: Argos Therapeutics (Inst), Bristol Myers Squibb (Inst), Genentech (Inst), Pfizer (Inst), Medivation/Astellas Pharma (Inst), Merck (Inst), Clovis Oncology (Inst), Acerta Pharma (Inst), AstraZeneca (Inst)
Changxue Lu
Patents, Royalties, Other Intellectual Property: Inventor of the patent that was licensed to Qiagen and received royalty
Joseph D. Schonhoft
Employment: Epic Sciences
Stock and Other Ownership Interests: Epic Sciences
Howard I. Scher
Leadership: Asterias Biotherapeutics
Stock and Other Ownership Interests: Asterias Biotherapeutics
Honoraria: Research to Practice
Consulting or Advisory Role: Janssen Biotech, Amgen, Janssen Research & Development, Menarini Silicon Biosystems, WIRB-Copernicus Group, ESSA, Sanofi, Ambry Genetics, Konica Minolta, Pfizer, Bayer
Research Funding: Janssen (Inst), Illumina (Inst), Epic Sciences (Inst), Menarini Silicon Biosystems (Inst), Thermofisher Scientific Biomarkers (Inst)
Travel, Accommodations, Expenses: Asterias Biotherapeutics, Menarini Silicon Biosystems, Amgen, WIRB-Copernicus Group, Konica Minolta, ESSA, Prostate Cancer Foundation, Sanofi, Bayer, Phosplatin Therapeutics
Richard Wenstrup
Employment: Epic Sciences
Leadership: Epic Sciences
Stock and Other Ownership Interests: Epic Sciences
Consulting or Advisory Role: Blueprint Genetics, Resolys
Patents, Royalties, Other Intellectual Property: Patent royalties from AssurexHealth
Travel, Accommodations, Expenses: Epic Systems
Scott T. Tagawa
Consulting or Advisory Role: Medivation, Astellas Pharma, Dendreon, Janssen, Bayer, Genentech, Endocyte, Immunomedics, Karyopharm Therapeutics, AbbVie, Tolmar, QED, Amgen, Sanofi, Pfizer, Clovis Oncology, Novartis, Genomic Health, POINT Biopharma
Research Funding: Lilly (Inst), Sanofi (Inst), Janssen (Inst), Astellas Pharma (Inst), Progenics (Inst), Millennium Pharmaceuticals (Inst), Amgen (Inst), Bristol Myers Squibb (Inst), Dendreon (Inst), Rexahn Pharmaceuticals (Inst), Bayer (Inst), Genentech (Inst), Newlink Genetics (Inst), Inovio Pharmaceuticals (Inst), AstraZeneca (Inst), Immunomedics (Inst), Novartis (Inst), AVEO (Inst), Boehringer Ingelheim (Inst), Merck (Inst), Stem CentRx (Inst), Karyopharm Therapeutics (Inst), AbbVie (Inst), Medivation (Inst), Endocyte (Inst), Exelixis (Inst), Clovis Oncology (Inst)
Travel, Accommodations, Expenses: Sanofi, Immunomedics, Amgen
Uncompensated Relationships: Telix Pharmaceuticals, ATLAB Pharma, Phosplatin Therapeutics
Emmanuel S. Antonarakis
Honoraria: Sanofi, Dendreon, Medivation, Janssen Biotech, ESSA, Astellas Pharma, Merck, AstraZeneca, Clovis Oncology
Consulting or Advisory Role: Sanofi, Dendreon, Janssen Biotech, ESSA, Merck, AstraZeneca, Clovis Oncology, Lilly, Bayer
Research Funding: Janssen Biotech (Inst), Johnson & Johnson (Inst), Sanofi (Inst), Dendreon (Inst), Aragon Pharmaceuticals (Inst), Exelixis (Inst), Millennium Pharmaceuticals (Inst), Genentech (Inst), Novartis (Inst), Astellas Pharma (Inst), Tokai Pharmaceuticals (Inst), Merck (Inst), AstraZeneca (Inst), Clovis Oncology (Inst), Constellation Pharmaceuticals (Inst)
Patents, Royalties, Other Intellectual Property: Coinventor of a biomarker technology that has been licensed to Qiagen
Travel, Accommodations, Expenses: Sanofi, Dendreon, Medivation
Daniel J. George
Leadership: Capio BioSciences
Honoraria: Sanofi, Bayer, Exelixis, EMD Serono, OncLive, Pfizer, UroToday, Acceleron Pharma, American Association for Cancer Research, Axess Oncology, Janssen Oncology, Millennium Medical Publishing
Consulting or Advisory Role: Bayer, Exelixis, Pfizer, Sanofi, Astellas Pharma, Innocrin Pharma, Bristol Myers Squibb, Genentech, Janssen, Merck Sharp & Dohme, Myovant Sciences, AstraZeneca, Michael J. Hennessy Associates, Vizuri Health Sciences
Speakers’ Bureau: Sanofi, Bayer, Exelixis
Research Funding: Exelixis (Inst), Janssen Oncology (Inst), Novartis (Inst), Pfizer (Inst), Astellas Pharma (Inst), Bristol Myers Squibb (Inst), Acerta Pharma (Inst), Bayer (Inst), Dendreon (Inst), Innocrin Pharma (Inst), Calithera Biosciences (Inst), Sanofi (Inst)
Travel, Accommodations, Expenses: Bayer, Exelixis, Merck, Pfizer, Sanofi, Janssen Oncology, UroToday
Susan Halabi
Employment: ASCO Targeted Agent and Profiling Utilization Registry
Consulting or Advisory Role: Eisai, Ferring Pharmaceuticals, Bayer
No other potential conflicts of interest were reported.
REFERENCES
- 1.Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–433. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 3. doi: 10.1056/NEJMoa1209096. Ryan CJ, Smith MR, de Bono JS, et al: Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 368:138-148, 2013 [Erratum: N Engl J Med 368:584, 2013] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 5.de Wit R, de Bono J, Sternberg CN, et al. Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N Engl J Med. 2019;381:2506–2518. doi: 10.1056/NEJMoa1911206. [DOI] [PubMed] [Google Scholar]
- 6.Khalaf DJ, Annala M, Taavitsainen S, et al. Optimal sequencing of enzalutamide and abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer: A multicentre, randomised, open-label, phase 2, crossover trial. Lancet Oncol. 2019;20:1730–1739. doi: 10.1016/S1470-2045(19)30688-6. [DOI] [PubMed] [Google Scholar]
- 7.Noonan KL, North S, Bitting RL, et al. Clinical activity of abiraterone acetate in patients with metastatic castration-resistant prostate cancer progressing after enzalutamide. Ann Oncol. 2013;24:1802–1807. doi: 10.1093/annonc/mdt138. [DOI] [PubMed] [Google Scholar]
- 8. doi: 10.1016/j.eururo.2013.06.042. Schrader AJ, Boegemann M, Ohlmann CH, et al: Enzalutamide in castration-resistant prostate cancer patients progressing after docetaxel and abiraterone. Eur Urol 65:30-36, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong AJ, Halabi S, Luo J, et al. Prospective multicenter validation of androgen receptor splice variant 7 and hormone therapy resistance in high-risk castration-resistant prostate cancer: The PROPHECY study. J Clin Oncol. 2019;37:1120–1129. doi: 10.1200/JCO.18.01731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–1038. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scher HI, Graf RP, Schreiber NA, et al. Assessment of the validity of nuclear-localized androgen receptor splice variant 7 in circulating tumor cells as a predictive biomarker for castration-resistant prostate cancer. JAMA Oncol. 2018;4:1179–1186. doi: 10.1001/jamaoncol.2018.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. doi: 10.1001/jamaoncol.2016.1828. Scher HI, Lu D, Schreiber NA, et al: Association of AR-V7 on circulating tumor cells as a treatment-specific biomarker with outcomes and survival in castration-resistant prostate cancer. JAMA Oncol 2:1441-1449, 2016 [Erratum: JAMA Oncol 2:1511, 2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antonarakis ES, Lu C, Luber B, et al. Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration-resistant prostate cancer. JAMA Oncol. 2015;1:582–591. doi: 10.1001/jamaoncol.2015.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halabi S, Lin CY, Kelly WK, et al. Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2014;32:671–677. doi: 10.1200/JCO.2013.52.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halabi S, Small EJ, Kantoff PW, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003;21:1232–1237. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]
- 16.Morris MJ, Basch EM, Wilding G, et al. Department of Defense prostate cancer clinical trials consortium: A new instrument for prostate cancer clinical research. Clin Genitourin Cancer. 2009;7:51–57. doi: 10.3816/CGC.2009.n.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. doi: 10.1158/1078-0432.CCR-08-0872. de Bono JS, Scher HI, Montgomery RB, et al: Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 14:6302-6309, 2008 [Erratum: Clin Cancer Res 15:1506, 2009] [DOI] [PubMed] [Google Scholar]
- 18.Scher HI, Heller G, Molina A, et al. Circulating tumor cell biomarker panel as an individual-level surrogate for survival in metastatic castration-resistant prostate cancer. J Clin Oncol. 2015;33:1348–1355. doi: 10.1200/JCO.2014.55.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antonarakis ES, Lu C, Luber B, et al. Clinical significance of androgen receptor splice variant-7 mRNA detection in circulating tumor cells of men with metastatic castration-resistant prostate cancer treated with first- and second-line abiraterone and enzalutamide. J Clin Oncol. 2017;35:2149–2156. doi: 10.1200/JCO.2016.70.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lokhandwala PM, Riel SL, Haley L, et al. Analytical validation of androgen receptor splice variant 7 detection in a Clinical Laboratory Improvement Amendments (CLIA) laboratory setting. J Mol Diagn. 2017;19:115–125. doi: 10.1016/j.jmoldx.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 21. Markowski MC, Silberstein JL, Eshleman JR, et al: Clinical utility of CLIA-grade AR-V7 testing in patients with metastatic castration-resistant prostate cancer. JCO Precis Oncol doi:10.1200/PO.17.00127. [DOI] [PMC free article] [PubMed]
- 22. doi: 10.1200/JCO.2015.64.2702. Scher HI, Morris MJ, Stadler WM, et al: Trial design and objectives for castration-resistant prostate cancer: Updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol 34:1402-1418, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisenhauer EA, Therasse P, Bogaerts J, et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 24.Halabi S, Lin CY, Small EJ, et al. Prognostic model predicting metastatic castration-resistant prostate cancer survival in men treated with second-line chemotherapy. J Natl Cancer Inst. 2013;105:1729–1737. doi: 10.1093/jnci/djt280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scher HI, Graf RP, Schreiber NA, et al. Phenotypic heterogeneity of circulating tumor cells informs clinical decisions between AR signaling inhibitors and taxanes in metastatic prostate cancer. Cancer Res. 2017;77:5687–5698. doi: 10.1158/0008-5472.CAN-17-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eisenberger M, Hardy-Bessard AC, Kim CS, et al. Phase III study comparing a reduced dose of cabazitaxel (20 mg/m2) and the currently approved dose (25 mg/m2) in postdocetaxel patients with metastatic castration-resistant prostate cancer: PROSELICA. J Clin Oncol. 2017;35:3198–3206. doi: 10.1200/JCO.2016.72.1076. [DOI] [PubMed] [Google Scholar]
- 27.Oudard S, Fizazi K, Sengeløv L, et al. Cabazitaxel versus docetaxel as first-line therapy for patients with metastatic castration-resistant prostate cancer: A randomized phase III trial—FIRSTANA. J Clin Oncol. 2017;35:3189–3197. doi: 10.1200/JCO.2016.72.1068. [DOI] [PubMed] [Google Scholar]
- 28.Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: A randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;37:2974–2986. doi: 10.1200/JCO.19.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fizazi K, Chi KN. Abiraterone in metastatic prostate cancer. N Engl J Med. 2017;377:1697–1698. doi: 10.1056/NEJMc1711029. [DOI] [PubMed] [Google Scholar]
- 30.Fizazi K, Shore N, Tammela TL, et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2019;380:1235–1246. doi: 10.1056/NEJMoa1815671. [DOI] [PubMed] [Google Scholar]
- 31.Smith MR, Yu MK, Small EJ. Apalutamide and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378:2542. doi: 10.1056/NEJMc1806189. [DOI] [PubMed] [Google Scholar]
- 32.Sternberg CN, Fizazi K, Saad F, et al. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2020;382:2197–2206. doi: 10.1056/NEJMoa2003892. [DOI] [PubMed] [Google Scholar]
- 33.Grünwald V, Hidalgo M. Development of the epidermal growth factor receptor inhibitor OSI-774. Semin Oncol. 2003;30(suppl 6):23–31. doi: 10.1016/s0093-7754(03)70022-0. [DOI] [PubMed] [Google Scholar]
- 34.Beltran H, Prandi D, Mosquera JM, et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat Med. 2016;22:298–305. doi: 10.1038/nm.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mu P, Zhang Z, Benelli M, et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science. 2017;355:84–88. doi: 10.1126/science.aah4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aggarwal R, Huang J, Alumkal JJ, et al. Clinical and genomic characterization of treatment-emergent small-cell neuroendocrine prostate cancer: A multi-institutional prospective study. J Clin Oncol. 2018;36:2492–2503. doi: 10.1200/JCO.2017.77.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arora VK, Schenkein E, Murali R, et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell. 2013;155:1309–1322. doi: 10.1016/j.cell.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Annala M, Vandekerkhove G, Khalaf D, et al. Circulating tumor DNA genomics correlate with resistance to abiraterone and enzalutamide in prostate cancer. Cancer Discov. 2018;8:444–457. doi: 10.1158/2159-8290.CD-17-0937. [DOI] [PubMed] [Google Scholar]
- 39.Romanel A, Gasi Tandefelt D, Conteduca V, et al. Plasma AR and abiraterone-resistant prostate cancer. Sci Transl Med. 2015;7:312re10. doi: 10.1126/scitranslmed.aac9511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wyatt AW, Azad AA, Volik SV, et al. Genomic alterations in cell-free DNA and enzalutamide resistance in castration-resistant prostate cancer. JAMA Oncol. 2016;2:1598–1606. doi: 10.1001/jamaoncol.2016.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Laere B, van Dam PJ, Whitington T, et al. Comprehensive profiling of the androgen receptor in liquid biopsies from castration-resistant prostate cancer reveals novel intra-AR structural variation and splice variant expression patterns. Eur Urol. 2017;72:192–200. doi: 10.1016/j.eururo.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 42.Henzler C, Li Y, Yang R, et al. Truncation and constitutive activation of the androgen receptor by diverse genomic rearrangements in prostate cancer. Nat Commun. 2016;7:13668. doi: 10.1038/ncomms13668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kohli M, Ho Y, Hillman DW, et al. Androgen receptor variant AR-V9 is coexpressed with AR-V7 in prostate cancer metastases and predicts abiraterone resistance. Clin Cancer Res. 2017;23:4704–4715. doi: 10.1158/1078-0432.CCR-17-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abida W, Cyrta J, Heller G, et al. Genomic correlates of clinical outcome in advanced prostate cancer. Proc Natl Acad Sci USA. 2019;116:11428–11436. doi: 10.1073/pnas.1902651116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. doi: 10.1038/s41588-018-0078-z. Armenia J, Wankowicz SAM, Liu D, et al: The long tail of oncogenic drivers in prostate cancer. Nat Genet 50:645-651, 2018 [Erratum: Nat Genet 51:1194, 2019] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chakraborty G, Armenia J, Mazzu YZ, et al. Significance of BRCA2 and RB1 co-loss in aggressive prostate cancer progression. Clin Cancer Res. 2020;26:2047–2064. doi: 10.1158/1078-0432.CCR-19-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Laere B, Oeyen S, Mayrhofer M, et al. TP53 outperforms other androgen receptor biomarkers to predict abiraterone or enzalutamide outcome in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2019;25:1766–1773. doi: 10.1158/1078-0432.CCR-18-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]