Abstract
Purpose
Telomere biology, especially tissue-specific ultra-short telomeres, might provide a strong contribution to our current knowledge in COPD development as well as a predictive marker for prognosis. To test this hypothesis, we investigated telomere lengths in lung tissue and leukocytes in patients diagnosed with COPD.
Patients and Methods
Thirty-two patients were included in the current study. All patients showed a post-bronchodilator ratio of less than 70% post-bronchodilator predicted value of forced expiratory volume in second (FEV1%), mean 56%; range [19% to 86%]. To be able to investigate ultra-short telomeres, universal single telomere length analysis (U-STELA) was used.
Results
Our results showed a higher level of the ultra-short telomere presence in bronchoalveolar lavage (BAL) cells when compared to leukocytes with statistical significance t(62)=5.771, p<0.00001. The FEV1% was lower in subjects with ultra-short telomeres in BAL (50.6% vs 81.6%: p<0.001) and in ultra-short telomeres in blood leukocytes (37.3% vs 58.5%: p=0.051) when compared to subjects without ultra-short telomeres in leukocytes. Furthermore, the patients who had ultra-short telomeres in BAL samples were significantly older (p=0.014) than patients who did not have ultra-short telomeres. Ultra-short telomeres in BAL (p=0.05) but not in leukocytes (p=0.33) were associated with FEV1% in a regressions model adjusting for age (p<0.0001), ever smoking (p<0.0001) and sex (p=0.71). The patients with ultra-short telomeres were graded higher in the Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification (p=0.006).
Conclusion
This study emphasizes the need to investigate the correct tissue to get a representative evaluation of the stage or advancedness of COPD. To our knowledge, this is the first study to show that there is a correlation between the presence of ultra-short telomeres in lung tissue and COPD severity. Our results suggest that ultra-short telomeres are involved in the molecular pathogenesis of COPD and might be used as a tissue-specific predictive biomarker.
Keywords: COPD, ultra-short telomeres, BAL, tissue-specific
Introduction
Chronic obstructive pulmonary disease (COPD) is a disease with progressive chronic airflow limitation. COPD generally starts to get symptomatic after the age of 60 years although some more susceptible subjects such as people with an alpha-1 antitrypsin deficiency may develop symptoms at an earlier age.1 Phenotypic characteristics of patients diagnosed with COPD are small airway obstruction2 followed by emphysema.3 These phenotypes are often coupled with frequent exacerbations which are increased as the severity of COPD increases.4 The molecular mechanisms of these phenotypes are similar despite different pathologies and the most predominant mechanisms underlying these phenotypes are inflammation,5 oxidative stress,6 senescence7 and apoptosis.8
In 2018 a systematic review revealed that COPD prevalence is 9.3% in males and 6.2% in females.9 Smoking is the greatest risk factor for developing COPD although exposure to tobacco smoke indirectly, air pollution, and dietary choices are also known risk factors for COPD.10 Oxidative stress is induced by cigarette smoke in the pulmonary epithelium, which leads to inflammation.11 In COPD cases, the exacerbations due to oxidative stress provide the necessary signals for the recruitment of the macrophages.12
In patients diagnosed with COPD, apoptosis seems to be accelerated and remains persistent even after the patients had stopped smoking.13 Increased apoptosis results in emphysema which is a major factor in COPD development. Thus, apoptosis can be both a result and a reason for COPD manifestations.14 Taken together, oxidative stress and inflammation accelerate aging in the lungs simply by increasing cell turnover together with apoptosis. Aging of the lung tissue plays a significant role, although the contribution of aging to the molecular mechanisms for developing COPD is still not fully understood.15
One possible mechanism that can lead to apoptosis and senescence in the lung is replicative “exhaustion”, caused by the long-term renewal of lung tissue as observed in patients with emphysema. This kind of senescence is tightly linked to telomere shortening. Previous studies showed that telomere loss in white blood cells (WBC) is accelerated by oxidative stress and inflammation in vitro. It was postulated that the accelerated WBC telomere shortening occurs as a result of long-term exposure to chronic inflammation.16
Telomeres are complex DNA-protein structures located at the outmost end of eukaryotic chromosomes. Telomere length shortens with age in all replicating somatic cells. The chromosomes’ ends are of a special structure composed of 5–15 kb of repeated DNA, containing tandem repeats of the sequence TTAGGG, and surrounded by sheltering proteins.17 In humans, most somatic cells gradually lose their telomeres due to the fact replication machinery cannot replicate the utmost ends of chromosomes, and possibly also due to unrepaired telomeric DNA damage. It is believed that when telomeres reach a sufficiently short length the telomeric loop is opened, exposing the chromosome end, which is detected as a double-stranded break, sending the cell into senescence.18 This extensive shortening results in ultra-short telomeres which are telomeres shorter than 1500 kb.
It has been previously suggested that damage and inflammation have a high correlation with short telomeres, but the precise mechanism remains unclear.19 Despite the fact that telomere length and its association with COPD risk is still contradictory, constitutive short telomeres and deficient telomere sheltering might be the underlying molecular mechanisms behind the COPD.
Telomere biology, especially tissue-specific ultra-short telomeres, might provide a strong contribution to our current knowledge in COPD development as well as a predictive marker for prognosis. To test this hypothesis, we investigated telomere lengths in lung tissue and leukocytes in patients diagnosed with COPD. To be able to investigate ultra-short telomeres, Universal Single Telomere Length Analysis (U-STELA) was used,20–22 which was previously developed by our group.
Patients and Methods
Selection of Subjects
Patients were recruited from the lung department at Erciyes University Hospital Bronchoscopy Unit with a median age of 63 years; range [31 to 73 years]. Thirty-two subjects were included. Seventeen patients (53%) were diagnosed with lung cancer, 1 patient had lung metastasis (3%) and 14 patients (44%) had no pathology by the bronchoscopy. Among all 32 patients, all had persistent airway obstruction, defined as a post-bronchodilator ratio of less than 70%. Lung function was expressed as post-bronchodilator predicted value of forced expiratory volume in second (FEV1%) mean 56%; range [19% to 86%]. All subjects, therefore, had from mild to severe COPD.23
Tissue Selection and DNA Isolation
The determination of ultra-short telomeres was done by using leukocytes and cells that were collected with bronchoalveolar lavage BAL (lung tissue cells, i.e. alveolar cells, neutrophils, macrophages) from each patient that are eligible for a bronchoscopy for this study. A previously established and telomeric length well-characterized mesenchymal stem cell line was used as an experimental internal control for U-STELA. These cells were cultured from previously immortalized stock24 and telomere length and existence of ultra-short telomeres were shown previously with our group studies. Peripheral blood (leukocytes) and BAL samples cells were collected from all 32 patients. DNAs were isolated using DNA isolation kits from RTA technologies for both blood and tissue samples. Near East University granted Ethical approval to carry out the study (Ethical Application Ref: YDU-2015/30-202) with informed consent from participants. All procedures were carried out according to the Declaration of Helsinki.
Universal STELA (U-STELA)
U-STELA was carried out with isolated DNA as described previously21 with minor alterations. In short, 1 µg of isolated DNA was digested with 1 µL of MseI and 0.5 µL of NdeI in 50 µL of the volume containing 5 µL cutsmart buffer. It was then incubated at 37°C for 1 hour followed by 20 minutes of inactivation at 65°C. 0.05 µg of digested DNA is mixed with 3 µL of 12 mer and 42 mer panhandles in 7 µL of volume. The temperature was decreased from 65°C to 16°C in 49 minutes. Twenty units of T4 DNA ligase and 1.5 µL of T4 DNA ligase buffer were quickly added to the mixture and volume was increased to 15 µL with 6 µL dH2O at 16°C and samples were incubated overnight. Subsequently, 20 units of T4 DNA ligase and 2.5 µL telorette working solution was added with 1 µL of T4 DNA ligase buffer and volume was increased to 25 µL with 6 µL dH2O. Samples were incubated at 35°C overnight and the reaction was inactivated by 20 minutes incubation at 65°C. This was then followed by a PCR reaction containing, 40 pg ligated DNA, 0.1 µM adapter and teltail primers, 6 µL of failsafe master mix and 0.5 µL of the failsafe enzyme with a total volume of 12 µL. PCR conditions were exact to the previously described.21 U-STELA products were separated by gel electrophoresis on a 0.8% gel at 70 V for 3 hours. The separated DNA was transferred to a positively charged nylon membrane (Amersham). DNA fragments in the blot were hybridized to the DIG-labeled telomeric probe overnight at room temperature and incubated with a DIG-specific antibody with AP fragments. Chemiluminescence was detected with CDP-Star (Roche). All experiments were triplicated. The threshold for the ultra-short telomeres was defined as 1.5 kb as established previously.
Statistical Analysis
The descriptive statistics for quantitative variables were represented as arithmetic mean ± standard deviation. The categorical data were represented with frequency and percentages. The relationship between the presence of ultra-short telomeres with age, sex, smoking status, and COPD grades was obtained by either the Fisher Exact Chi-Square test for categorical variables or Mann–Whitney U-test for continuous variables. p-values ≤0.05 were regarded as statistically significant. To test the hypothesis that ultra-short telomeres (either in cells that collected with BAL or leukocytes) lead to decline in lung function, multiple linear regression (entry method) was used to assess the associations FEV1% adjusted for age, sex and smoking status (ever smoker/never smoked). SPSS (IBM SPSS version 23.0) statistical program was used for all statistical analyses.
Results
Presence of Ultra-Short Telomeres
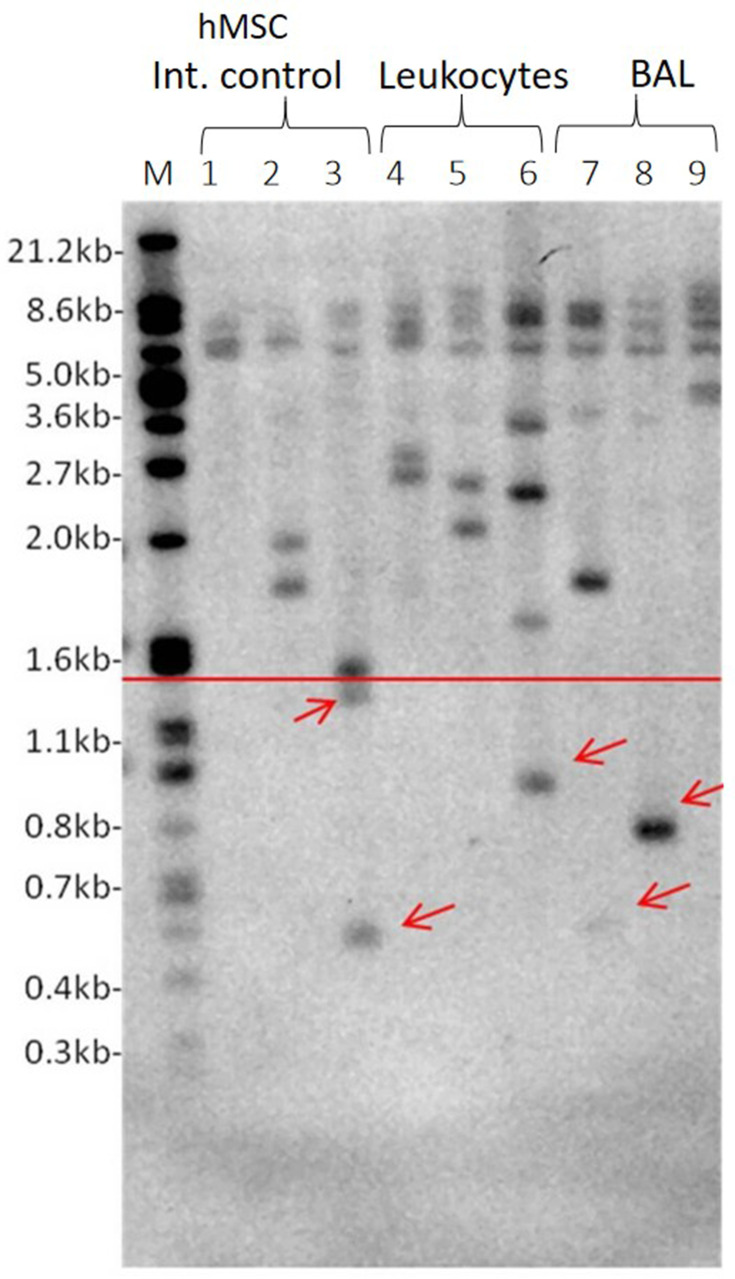
In total, 26 patients’ BAL samples cells (81.1%) had ultra-short telomeres while only three patients had ultra-short telomeres in leukocytes (9.3%). Twenty-two subjects were either current or former smokers (69%) and of those 18 subjects (81.8%) had ultra-short telomeres. The ultra-short telomere determination is shown in Figure 1. There were no differences between gender with regards to the presence of ultra-short telomeres in the tissues that have been tested (lung tissue cells in BAL versus Leukocytes). Thirteen patients of the 17 patients with lung cancer, had ultra-short telomeres (76.5%). On the other hand, 15 patients who did not had lung cancer, 13 of whom had ultra-short telomeres and 2 did not (86.7%). The presence of ultra-short telomeres and incidence of lung cancer is not associated (p=0.659).
Figure 1.
Representative USTELA. 1: Marker, 2–4: hMSC internal control at different passages (p X, X, X), 5–7: Leukocyte samples, 8–10: BAL samples. Red arrows indicate the presence of ultra-short telomeres that are below the threshold of 1.5kb shown as red horizontal line.
Presence of Ultra-Short Telomeres and Lung Function
FEV1% was lower in ever smokers (current or former) with ultra-short telomeres than in never smokers with ultra-short telomeres (44.6% versus 64.4%; p=0.014). The FEV1% was lower subjects with ultra-short telomeres in BAL (50.6% vs 81.6%: p<0.001) compared to subjects without ultra-short telomeres. The FEV1% was lower in subjects with ultra-short telomeres in blood leukocytes (37.3% vs 58.5%: p=0.051) compared to subjects without ultra-short telomeres in leukocytes. Furthermore, the patients who had ultra-short telomeres in BAL samples were significantly older (p=0.014) than patients who did not have ultra-short telomeres. Ultra-short telomeres in BAL (p=0.05) but not in leukocytes (p=0.33) was associated FEV1% in a regressions model adjusting for age (p<0.0001), ever smoking (p<0.0001) and sex (p=0.71).
Tissue-Specific Ultra-Short Telomeres According to COPD Groups
The categorical analysis of COPD patients is shown in Table 1. The presence of ultra-short telomeres in BAL cells compared to individuals’ leukocytes were statistically significant t(62)=5.771, p<0.00001. These results indicate the importance of a correct tissue when telomere length is to be evaluated. To evaluate whether lung tissue cells that were in BAL samples and leukocyte telomere shortening is associated with the progression of COPD, we compared patients with and without ultra-short telomeres. The main outcome was that the patients with ultra-short telomeres were graded higher in terms of GOLD classification (p=0.006) (Table 2). In contrast, the presence of ultra-short telomeres in leukocytes does not represent an association with COPD grade (p>0.05) as well as age (71.7), gender, and smoking status.
Table 1.
Categorical Analysis of COPD Patients
| COPD Patients (n=32) Mean ± SD |
|
|---|---|
| Age (years) | 60.0 ± 10.5 |
| n (%) | |
| Smoking History Smoker Former Smoker Non Smoker |
13 (40.1) 9 (28.1) 10(31.3) |
| Gender Male Female |
26 (81.3) 6 (18.7) |
| Presence of ultra-short telomeres in leukocytes | 3 (9.3) |
| Presence of ultra-short telomeres in BAL | 26 (81.3) |
| COPD Grade Grade 1–2 Grade 3–4 |
15 (46.9) 17 (53,1) |
Note: Ultra-short telomeres were present in 26 BAL samples and in contrast to 3 leukocyte samples.
Table 2.
The Relationship Between the Presence of Ultra-Short Telomeres and Age, Smoking History, and COPD Grading
| BAL + (n=26) | BAL – (n=6) | p | Leu + (n=3) | Leu – (n=29) | p | |
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||
| Age (years) | 62.6 7.8 | 48.8 14.2 | 0.014* | 71.7 1.2 | 58.8 10.3 | NaN |
| n (%) | n (%) | n (%) | n (%) | |||
| Smoking History Ever Smoker Never Smoker |
18 (81.2) 8 (80) |
4(11.8) 2 (20) |
1.00** |
3 (13.6) 0 (0.0) |
19 (86.4) 10 (100.0) |
0.534** |
| COPD Grade Grade 1–2 Grade 3–4 |
9 (60.0) 17 (100.0) |
6 (40.0) 0 (0.0) |
0.006 ** |
1 (6.7) 2 (11.8) |
14 (93.3) 15 (88.2) |
1.00** |
Notes: *Results were obtained with the Mann–Whitney U-test; **Results were obtained with Fisher Exact test; NaN, No statistics were calculated due to inadequate sample size in one Leu (+) group (n=3); Bold indicates statistically significant values.
Abbreviations: BAL+, presence of ultra-short telomeres in BAL samples; BAL-, absence of ultra-short telomeres in Bal samples; Leu+, presence of ultra-short telomeres in leukocyte samples; Leu-, absence of ultra-short telomeres in leukocyte samples.
Discussion
Our results demonstrated that evaluating tissue-specific sampling lung tissue cells (BAL) instead of WBC seems to be a better choice to detect the possible association between inflammatory disorders like COPD and ultra-short telomeres. To the best of our knowledge, this is the first study to show that there is a correlation between the presence of ultra-short telomeres in lung tissue and COPD which could explain the importance of the selection of representative tissue instead of leukocytes to provide a more appropriate insight towards the molecular basis of given disease such as COPD. This study shows the potential use of ultra-short telomeres as a biomarker in a simple and cost-effective way.
Telomere length measurements were often done only in leukocytes.23–25 This approach is generally acceptable and sufficient to provide base aging-related telomere attrition but as evidenced here, in the case of diseases with high cell turnover such as COPD, tissue and cell-specific methods and evaluation of ultra-short telomeres are more effective in the characterization of the molecular mechanisms underlying chronic diseases.
As COPD is a chronic and progressive disease, older patients diagnosed with COPD are more likely to be at an advanced stage. Thus, our results are in line with the previous evidence that reflects the fact that abrupt telomere shortening increases with aging. The investigation of ultra-short telomeres in senescence-associated diseases will help for a better understanding of the disease mechanism since a single ultra-short telomere may induce senescence rather than overall short telomeres.20
In addition to these, we demonstrated that telomere dynamics might play a role in disease progression as well as development. The cellular aging due to advanced age might also contribute to accelerated telomere shortening,26 thus explain the presence of ultra-short telomeres in BAL samples. The renewal rate of leukocytes is higher than the renewal rate of lung tissue due to respective stem cell activities and homing.27 In light of our result, we hypothesize that ultra-short telomeres can be considered as a potential prognostic marker in COPD patients who have undergone bronchoscopy at regular intervals.
The limitations of the study are: 80% of the sample had ultra-short telomeres in BAL cells and even though our sample covered a comprehensive range of post-bronchodilator obstruction (19–86%) a control group of non-smoking non-obstructive subjects would have been ideal but at the time for sampling, no subjects fulfilled these criteria for a bronchoscopy. Therefore, all the patients included in this study were COPD patients. Previous studies indicate the macrophage counts increasing in BAL samples from COPD patients when compared with healthy controls. Additionally, macrophage number correlates with the severity of COPD.12 Furthermore, the sorting of the cells in BAL may have the strength of the association as well as quantifying the ultra-short telomeres. Yet in our study significant association has been observed. Smoking is attributed to be the main risk factor for COPD, in the current study, no significant direct tissue relationship was found to the COPD groups but a significant association with lung function was found. This difference could be explained by the small sample size, lack of exacerbation registration. Ultra-short telomeres serving as a potential biomarker remained to be studied in larger cohorts. Factors such as air pollution, lifestyle, and occupation might have influenced our results. Since we did not acquire data on the influence of these variables, these factors cannot be ruled out.
Conclusion
Our results suggest that ultra-short telomeres are involved in the molecular pathogenesis of COPD. Ultra-short telomeres might be used as a tissue-specific predictive biomarker for COPD. Furthermore, this study emphasizes the need to investigate the correct specific tissue to get a representative evaluation of the stage or advancedness of a given disease, such as COPD.
Acknowledgments
We would like to thank for the support provided by the Centre of Excellence at Near East University.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author, NS, upon reasonable request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest with regard to this manuscript neither commercially nor financially.
References
- 1.Strange C. Airway disease in alpha-1 antitrypsin deficiency. COPD. 2013;1:68–73. doi: 10.3109/15412555.2013.764404 [DOI] [PubMed] [Google Scholar]
- 2.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158 [DOI] [PubMed] [Google Scholar]
- 3.McDonough JE, Yuan R, Suzuki M, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365:1567–1575. doi: 10.1056/NEJMoa1106955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–1138. doi: 10.1056/NEJMoa0909883 [DOI] [PubMed] [Google Scholar]
- 5.Hassett DJ, Borchers MT, Panos RJ. Chronic obstructive pulmonary disease (COPD): evaluation from clinical, immunological and bacterial pathogenesis perspectives. J Microbiol. 2014;52:211–226. [DOI] [PubMed] [Google Scholar]
- 6.Domej W, Oettl K. Oxidative stress and free radicals in COPD – implications and relevance for treatment. Int J Chron Obstruct Pulmon Dis. 2014;9:1207–1224. doi: 10.2147/COPD.S51226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aoshiba K, Nagai A. Senescence hypothesis for the pathogenetic mechanism of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009;6:596–601. doi: 10.1513/pats.200904-017RM [DOI] [PubMed] [Google Scholar]
- 8.Aoshiba K, Zhou F, Tsuji T, et al. DNA damage as a molecular link in the pathogenesis of COPD in smokers. Eur Respir J. 2012;39:1368–1376. doi: 10.1183/09031936.00050211 [DOI] [PubMed] [Google Scholar]
- 9.Ntritsos G, Franek J, Belbasis L, et al. Gender-specific estimates of COPD prevalence: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2018;13:1507–1514. doi: 10.2147/COPD.S146390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Postma DS, Bush A, Van den Berge M. Risk factors and early origins of chronic obstructive pulmonary disease. Lancet. 2015;385:899–909. doi: 10.1016/S0140-6736(14)60446-3 [DOI] [PubMed] [Google Scholar]
- 11.Puig-Vilanova E, Rodriguez DA, Lloreta J, et al. Oxidative stress, redox signaling pathways, and autophagy in cachectic muscles of male patients with advanced COPD and lung cancer. Free Radic Biol Med. 2015;79:91–108. doi: 10.1016/j.freeradbiomed.2014.11.006 [DOI] [PubMed] [Google Scholar]
- 12.Vlahos R, Bozinovski S. Role of alveolar macrophages in chronic obstructive pulmonary disease. Front Immunol. 2014;5:435. doi: 10.3389/fimmu.2014.00435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodge S, Hodge G, Holmes M, et al. Increased airway epithelial and T-cell apoptosis in COPD remains despite smoking cessation. Eur Respir J. 2005;25:447–454. doi: 10.1183/09031936.05.00077604 [DOI] [PubMed] [Google Scholar]
- 14.Podowski M, Calvi CL, Cheadle C, et al. Complex integration of matrix, oxidative stress, and apoptosis in genetic emphysema. Am J Pathol. 2009;175:84–96. doi: 10.2353/ajpath.2009.080870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mercado N, Ito K, Barnes PJ. Accelerated ageing of the lung in COPD: new concepts. Thorax. 2015;70:482–489. doi: 10.1136/thoraxjnl-2014-206084 [DOI] [PubMed] [Google Scholar]
- 16.Steer SE, Williams FM, Kato B, et al. Reduced telomere length in rheumatoid arthritis is independent of disease activity and duration. Ann Rheum Dis. 2007;66:476–480. doi: 10.1136/ard.2006.059188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350 [DOI] [PubMed] [Google Scholar]
- 18.Shay JW, Wright WE. Senescence and immortalization: role of telomeres and telomerase. Carcinogenesis. 2005;26:867–874. doi: 10.1093/carcin/bgh296 [DOI] [PubMed] [Google Scholar]
- 19.Amsellem V, Gary-Bobo G, Marcos E, et al. Telomere dysfunction causes sustained inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;184:1358–1366. doi: 10.1164/rccm.201105-0802OC [DOI] [PubMed] [Google Scholar]
- 20.Harbo M, Delaisse JM, Kjaersgaard-Andersen P, et al. The relationship between ultra-short telomeres, aging of articular cartilage and the development of human hip osteoarthritis. Mech Ageing Dev. 2013;134:367–372. doi: 10.1016/j.mad.2013.07.002 [DOI] [PubMed] [Google Scholar]
- 21.Bendix L, Horn PB, Jensen UB, et al. The load of short telomeres, estimated by a new method, universal STELA, correlates with number of senescent cells. Aging Cell. 2010;9:383–397. doi: 10.1111/j.1474-9726.2010.00568.x [DOI] [PubMed] [Google Scholar]
- 22.Serakinci N, Cagsin H, Mavis M. Use of U-STELA for accurate measurement of extremely short telomeres In: Turksen K, editor. Stem Cells and Aging, Methods in Molecular Biology. New York: Humana; 2019:217–224. [DOI] [PubMed] [Google Scholar]
- 23.Karloh M, Mayer AF, Maurici R, et al. The COPD assessment test: what do we know so far? A systematic review and meta-analysis about clinical outcomes prediction and classification of patients into GOLD stages. Chest. 2016;149:413–425. doi: 10.1378/chest.15-1752 [DOI] [PubMed] [Google Scholar]
- 24.Mui TSY, Man JM, McElhaney JE, et al. Telomere length and chronic obstructive pulmonary disease: evidence of accelerated aging. J Am Geriatr Soc. 2009;57:2372–2374. doi: 10.1111/j.1532-5415.2009.02589.x [DOI] [PubMed] [Google Scholar]
- 25.Lee J, Sandford AJ, Connett JE, et al. The relationship between telomere length and mortality in chronic obstructive pulmonary Disease (COPD). PLoS One. 2012;7:e35567. doi: 10.1371/journal.pone.0035567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vidacek NS, Cukusic A, Ivankovic M, et al. Abrupt telomere shortening in normal human fibroblasts. Exp Gerontol. 2010;45:235–242. doi: 10.1016/j.exger.2010.01.009 [DOI] [PubMed] [Google Scholar]
- 27.Yamada M, Fujino N, Ichinose M. Inflammatory responses in the initiation of lung repair and regeneration: their role in stimulating lung resident stem cells. Inflamm Regen. 2016;36:15. doi: 10.1186/s41232-016-0020-7 [DOI] [PMC free article] [PubMed] [Google Scholar]