Abstract
Understanding the fundamental reproductive biology of a species is the first step toward identifying parameters that are critical for reproduction and for the development of assisted reproductive techniques. Ejaculates were collected from aquarium (n = 24) and in situ (n = 34) sand tiger sharks Carcharias taurus. Volume, pH, osmolarity, sperm concentration, motility, status, morphology, and plasma membrane integrity were assessed for each ejaculate. Semen with the highest proportion of motile sperm was collected between April and June for both in situ and aquarium sand tiger sharks indicating a seasonal reproductive cycle. Overall, 17 of 30 semen samples collected from aquarium sharks from April through June contained motile sperm compared to 29 of 29 of in situ sharks, demonstrating semen quality differences between aquarium and in situ sharks. Sperm motility, status, morphology, and plasma membrane integrity were significantly higher (P < 0.05) for in situ compared to aquarium sand tiger sharks. Testosterone was measured by an enzyme immunoassay validated for the species. Testosterone concentration was seasonal for both aquarium and in situ sharks with highest concentrations measured in spring and lowest in summer. In situ sharks had higher (P < 0.05) testosterone concentration in spring than aquarium sharks. This study demonstrated annual reproduction with spring seasonality for male sand tiger sharks through marked seasonal differences in testosterone and semen production. Lower testosterone and poorer semen quality was observed in aquarium sharks likely contributing to the species’ limited reproductive success in aquariums.
Keywords: assisted reproduction, conservation, fish reproduction, seasonal reproduction, semen, sperm, testosterone
During mating season, in situ sand tiger sharks Carcharias taurus have higher plasma testosterone and better semen quality than aquarium housed sand tiger sharks impairing reproductive success of aquarium populations.
Introduction
Aquariums educate and inspire their guests through the diversity of their collections, which ubiquitously include elasmobranch fishes (sharks and rays). Among the most popular sharks exhibited in aquaria are sand tigers, Carcharias taurus, owing to their large size, conspicuous dentition, docile nature, and long lifetime under managed care [1]. With nearly a quarter of elasmobranch species, including the sand tiger shark, classified as threatened with an elevated risk of extinction; research, conservation, and education are needed for responsible management of shark populations [2–5]. Removing barriers to reproduction and maintaining self-sustaining populations under managed care is one conservation measure aquariums are striving to achieve through their support of in situ and aquarium shark research [6, 7].
Sand tiger females require 8–10 years to reach maturity [1] and have a biennial or triennial reproductive cycle [8–13]. Gestation is 9–12 months and the maximum fecundity is two young, one per uterus, due to adelphophagy [11, 14–16]. Males mature after 6–8 years [1] and have an annual reproductive cycle [10]. The female’s lengthy reproductive cycle, low fecundity, and long time to reach maturity (8–10 years) results in a species that can be easily over-exploited in a short amount of time [17], imperiling the species.
Despite a long history of husbandry for sand tiger sharks dating back to the 1930s, reproduction in aquaria has been largely unsuccessful for reasons that remain poorly understood [18–21]. Further, transport of adult sharks between aquariums is logistically challenging, requiring specialized equipment and life support systems as well as highly trained and skilled husbandry professionals. Artificial insemination and assisted reproductive technologies may be one solution to help aquariums achieve self-sustaining, genetically diverse collections of elasmobranchs and preclude the need to relocate large aquatic animals [22, 23]. Before protocols for assisted reproduction through artificial insemination can be developed, safe and reliable semen collection techniques and the reproductive biology for each species in consideration need to be understood. The sand tiger shark is a highly migratory species, with onset of breeding season associated with changes in temperature and day length [14, 24–27]. These same environmental cues may be important for the reproductive seasonality of aquarium sand tiger sharks [20, 28].
Nonlethal techniques including ultrasonography, blood hormone measurements, and semen collection as well as available expertise in animal handling are now replacing lethal sampling methods to characterize reproductive status [29–31]. The objectives of this study were to: (1) develop a safe and reliable method for semen collection and assessment from sand tiger sharks; (2) investigate reproductive periodicity and seasonality of male sand tiger sharks, and (3) compare semen parameters and testosterone concentration between in situ and aquarium sand tiger sharks.
Materials and methods
Animals and housing
Mature sand tiger sharks (n = 24) housed at AZA institutions: Georgia Aquarium affiliate Marineland Dolphin Adventure, Ripley’s Aquariums, New York Aquarium, Shark Reef at Mandalay Bay, and Aquarium of the Pacific were examined one to four times annually over 3 years and six additional sharks were sampled once. Aquarium environmental conditions are shown in Table 1. In situ sharks (n = 34) were caught using longlining methods with 25–100 (16/0) circle hooks with flattened barbs baited with frozen-thawed Atlantic mackerel (Scomber scombrus). Lines were set throughout the day and soaked for 30 min–2 h before retrieval. Sharks were examined once between April and August over 2 years. Animal procedures were approved by the South-East Zoo Alliance for Reproduction & Conservation (SEZARC) Institutional Animal Care and Use Committee (IACUC) and carried out in accordance with AZA-accredited institutional guidelines.
Table 1.
Environmental characteristics for aquarium sand tiger sharks Carcharias taurus.
| Institution | Sharks (n) | Seawater | Light/photoperiod | Temperature (°C) | Temperature change (°C) | Enclosure |
|---|---|---|---|---|---|---|
| Georgia Aquarium affiliate Marineland Dolphin Adventure |
5 | Natural | Natural/natural | 14.1–29.2 | 12–15 | 248 550 l 2.1 m deep 12.2 m round |
| Ripley’s Aquariums | 2 | Artificial | Artificial/13L:11D | 17–25 | 2–8 | 208 198 l 1.7 m deep 12.2 m round |
| Wildlife Conservation Society’s New York Aquarium | 6 | Natural | Artificial with natural influence | 23 ± 1 | <2 | 357 340 l 1.3 m deep 12.2 m round |
| Aquarium of the Pacific | 1 | Natural | Natural/natural | 24.4 ± 0.6 | <2 | 16 052 l 1.8 m deep oval |
| Shark Reef Aquarium | 4 | Artificial | Artificial/15L:9D | 22.8–24.4 | <2 | 4900 kl 6.7 m deep |
Shark examinations and blood collection
Sharks with fully calcified claspers that rotate at the base and splay open at the head (rhipidion) were considered mature and sampled for semen [32]. Blood (15 ml) was collected from the ventral tail vein and immediately transferred to lithium heparin coated blood collection tubes without separator gel (BD Vacutainer, Franklin Lakes, NJ). Blood samples collected from in situ sharks were held in a cooler chilled with ice packs until processing at the end of the day. Plasma was banked in an ultralow freezer (−80 °C) and a vial stored frozen (−20 °C) for steroid hormone analysis. Ultrasonography using an Edge II with a rC60xi 5–2 MHz transducer (Fujifilm SonoSite, Inc., Bothell, WA) or Ibex Pro with a Cli3.8 2.5–5 MHz curvilinear transducer (E.I. Medical Imaging, Loveland, CO) was used to examine ampullae of males.
Semen collection
Aquarium sand tiger sharks were manually restrained and a stream of oxygenated water created from bubbling oxygen gas was directed at the shark’s mouth using a submerged pump, and respiration rate was monitored throughout restraint [33]. In situ sand tiger sharks were sampled from South Carolina (April) and Delaware Bay (June–August) and restrained boat-side or transferred to a livewell aboard the vessel for examination. Restraint included dorsoventral rotation to induce tonic immobility (TI) [34, 35] for no longer than 30 min. Semen was collected using a sterile 16-inch 18Fr polyvinyl chloride catheter with a rounded, closed tip (Kendall/Covidien, Mansfield, MA) inserted through the urogenital papilla and into an ampulla (15–20 cm). Semen was extracted using gentle suction from a catheter tip syringe (Monoject, Kendall/Covidien, Medtronic, Minneapolis, MN) while slowly withdrawing the catheter through the length of the ampulla. Semen was transferred to sterile conical tubes (Thermo Fisher Scientific, Waltham, MA) after collection.
Care was taken to preserve catheter sterility before entry into the urogenital papilla, and once introduced into an ampulla, the catheter was not removed until semen collection was complete. Semen samples were maintained in the dark at 4 °C until analysis and assessed at ambient temperature (22–25 °C) within 24 h. To investigate the prevalence of potential pathogenic organisms that might be deleterious in an artificial insemination program, semen samples (n = 7 in situ, n = 4 aquarium) from sand tiger sharks were collected onto a sterile culturette (BBL CultureSwab Plus, Becton, Dickenson and Company, Sparks, MD) immediately after collection and shipped to the University of Georgia Veterinary Diagnostic Laboratories, Athens, GA, California Microbiological Reference Laboratory, Tustin CA, or IDEXX Laboratories, Inc, ME for aerobic culture.
Semen assessment
Semen was heterogeneous with semisolid rods, translucent, and sometimes opaque, distributed within minimal and viscous seminal fluid. Semen volume was measured to the nearest ml using 15 or 50 ml conical tubes. Semen osmolarity and pH were measured using a freezing point depression method (Fiske Model 210 Micro-Osmometer, Advanced Instruments Inc., MA) and pH strips (#109584; MilliporeSigma, Billerica, MA), respectively. Sperm concentration was determined using a hemocytometer with oligospermic ejaculates defined as containing <1 × 104 sperm/ml. An aliquot of semen was preserved 1:10 in 0.1 M Sorenson’s phosphate buffer (#11610-10; Electron Microscopy Sciences, Hatfield, PA) supplemented with 0.02% CaCl2, 0.35 M sucrose, 3.2% paraformaldehyde, and 2.5% glutaraldehyde. Sperm morphology was assessed using phase contrast microscopy by examining 100 sperm per ejaculate at ×100 oil immersion and reported as a percentage.
Motility and status on a 0–5 scale with 0 for no movement and 5 for very rapid linear progression [36] were assessed in 3 μl of raw sample and after 1:10 dilution with artificial seawater (ASW; 1050 mOsM, #S9883; Sigma, St. Louis, MO) at room temperature (22 °C) using ×20 phase contrast microscopy. Progressive motility was expressed as the percentage of sperm exhibiting forward movement and total motility was expressed as a percentage that included nonprogressive and progressive motility.
Sperm plasma membrane integrity was assessed using 200 nM SYBR-14 and 24 μM propidium iodide ASW (Live/Dead stain, #L-7011; Molecular Probes, Inc., Eugene, Oregon) and counting 100 cells using an Olympus B-Max 60 epifluorescent microscope with filter cube U-M51005 for dual wavelength excitation. Spermatozoa fluorescing green over the head region were assessed as plasma membrane intact, and sperm fluorescing partially red or red over the head region was assessed as plasma membrane damaged [37]. To validate the use of the plasma membrane integrity stain for shark sperm, an aliquot of sperm suspension was frozen to −80 °C in the absence of cryoprotectant to disrupt the plasma membrane. The frozen-thawed, nonmotile sperm were added to aliquots of motile sperm in ratios of 100:0, 80:20, 60:40, 50:50, 40:60, 80:20, and 0:100. The sperm suspensions were stained and assessed for percent sperm motility and percent sperm plasma membrane integrity. To check plasma membrane integrity and motility for sperm within opaque semisolid rods, after staining, individual rods were removed to a microscope slide and a smash preparation was made by flattening beneath a coverslip.
Confocal microscopy
Whole and cross-sections of opaque semisolid rods observed in raw sand tiger ejaculate were cut by hand using single edge razor blades and immersed in 250 μl of ASW containing 200 nM SYBR and 24 μM PI for 15 min. Sections were examined in a Lab-Tek II 8 Chamber Slide (Nunc, Naperville, IL) using a laser-scanning, confocal microscope (Zeiss 710, Thornwood, NY) coupled with a Zeiss Axiophot inverted microscope in stack scanning mode, collecting 31 μm optical sections with a Zeiss Fluar 5X/0.25 numerical aperture objective, and ZEN software (Zeiss, Jena, Germany). Imaging was performed using laser excitation at 488 and 561 nm, and fluorescence was collected between 500–550 and 575–610 nm. To display 3D information in 2D images, Z-stacks were merged using maximum intensity projection in ZEN 2011.
Testosterone enzyme-linked immunoassay
Plasma samples from 24 adult aquarium sharks (n = 87 samples) collected year-round and 35 adult (n = 35 samples) and 22 juvenile (n = 22 samples) in situ sharks collected between the months of April and August were assayed for testosterone using a competitive double antibody enzyme-linked immunoassay (EIA) with a polyclonal testosterone antisera raised in New Zealand white rabbits (R156/7) [38] and corresponding horseradish peroxidase (HRP) label (Coralie Munro, University of California, Davis, CA). Antisera cross-reactivities are previously reported [39]. Precoated, preblocked, 10 μg/ml goat anti-rabbit IgG (#A009-25MG; Arbor Assays, Ann Arbor, MI) plates were maintained until use at 4 °C in zip lock bags with desiccant. All reagents and plates were brought to room temperature prior to use, and 50 μl standard or sample was added to precoated wells, followed immediately by 50 μl of HRP conjugated standard, followed by 50 μl of antibody, excluding two blank wells for a nonspecific binding control. Samples were run in duplicate at a dilution of 1:10 in a modified PBS (0.04 M NaH2PO4, 0.06 M Na2HPO4, 0.15 M NaCl, 0.1% BSA, pH 7.0). Plates were incubated (2 h) at ambient temperature (~24 °C) before washing four times with 0.008% Tween 20 (# P1379-100 ml; Sigma, St Louis, MO) in distilled water. After washing, 100 μl High Kinetic Tetramethylbenzidine (#TMBHK-1000; Moss, INC., Pasedena, MD) colorimetric substrate was added to each well and the plates were incubated at room temperature until an optical density of 1.0 at 450 nm was reached for the zero hormone standards, whereon 1 M HCl (#320331; Sigma, St Louis, MO) was added. Plates were read with a Biotek ELX808 (Biotek Instruments, INC, Winooski, VT).
Serial dilutions of pooled plasma generated dose–response curves parallel to serially diluted testosterone standard. Recovery of known amounts of testosterone added to pools of plasma was 97.6 ± 6.8% (0.6903× + 0.0065, R2 = 0.9992). As an additional validation, male sand tiger shark samples (n = 24) previously analyzed using radio-immunoassay [10] were assayed using EIA and were correlated (R2 = 0.863). Interassay coefficients of variation (CVs) were 11 and 15% for high and low biological controls and intraassay CVs were 2.46 ± 0.09%. Assay sensitivity (the value obtained at 90–95% binding) was 0.04 ng/ml for testosterone. Hormone values were reported as ng/ml.
Statistics
Descriptive statistics were used for semen and sperm parameters. Pearson’s correlation coefficient was calculated to assess the relationship between plasma membrane integrity and total motility for semen after dilution in ASW to validate the Live/Dead stain for use with elasmobranch sperm. Semen parameters and testosterone concentration were compared by using the single best sample collected for each aquarium-housed male based on sperm motility (when data for more than 1 month was available) and in situ sand tiger sharks for April to June using a two-sample Wilcoxon rank-sum (Mann–Whitney) test. In situ semen parameters were compared between months using a Kruskal–Wallis test and Dunn post-hoc pairwise comparisons with Benjamini–Hochberg adjusted P-values.
Testosterone concentration between months for in situ males was compared using one-way analysis of variance (ANOVA) with Tukey post-hoc comparisons. For the summer season, testosterone concentration for mature versus immature in situ sharks was compared using an independent sample t-test. Linear mixed modeling (LMM) was used to assess the significance of month on testosterone concentration for aquarium sharks. A single plasma sample was collected from an aquarium shark during the months of July and December; therefore, these months were removed from analysis. Mixed models included individual as a random factor to account for unbalanced repeated measures for aquarium sharks with month as the factor or explanatory variable. Testosterone concentrations were compared between shared seasons (spring, March to May and summer, June to August) for aquarium and in situ sharks using LMM with shark as a random factor to account for unbalanced repeated measures for aquarium sharks and season and population (in situ or aquarium) and their interaction modeled sequentially. Model fit was compared using likelihood ratio tests (LRT). For models with a significant LRT, explanatory factors were retained if the model Bayesian Information Criteria (BIC) was reduced [40]. Final models were fit by restricted maximum likelihood (REML) with error degrees of freedom calculated using the Satterthwaite method. Differences for significant factors were explored using post-hoc pairwise comparisons with Tukey adjusted P-values and Kenward–Roger adjusted degrees of freedom. Statistical analyses were conducted using R Version 3.3.1 [41] with packages lme4 Version 1.1–14 [42], lmerTest Version 2.0–33 [43], emmeans Vesion 1.3.1 [44], and ggplot2 Version 2.2.1.0 [45]. An alpha value of P < 0.05 was considered significant.
Results
Animal handling
In this study, 24 aquarium sharks were examined collectively 94 times without incident. Handling for 30 min or less did not result in observable negative effects, and aquarium males swam normally upon release and resumed eating within 24 h. In situ sharks were handled similarly and swam normally upon release.
Semen and spermatozoa characteristics
Ejaculates were collected from 58 sharks. Ultrasonography informed if semen was available for collection in the bilateral ampullae (Figure 1). With the transducer in transverse orientation cranial to the pelvic girdle, ampullae cross-sections were oval in shape with heterogeneous hypoechoic centers when engorged with semen and were collapsed or flat when empty. Correlation between the ultrasonographic appearance and ability to collect an ejaculate was confirmed by three unsuccessful attempts when ampullae appeared flat or collapsed versus 100% success when ampullae appeared engorged or full. Collection was not attempted further for sharks when ultrasound indicated collapsed or flat ampullae. Semen cultures were positive for Pseudomonas sp. and Staphlyococcus sp. in two of seven in situ sharks and one of four aquarium sharks.
Figure 1.
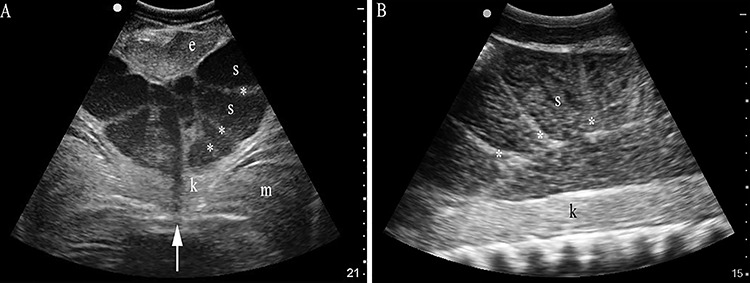
Semen localization in ampulla epididymis by ultrasonography in spring mating season. With the transducer in transverse orientation (A) and positioned on midline (arrow) or sagittal and positioned laterally (B) immediately cranial to the pelvic girdle, semen (s) in the ampulla lumen is hypoechoic compared to kidney (k), dorsal musculature (m), and the ventral and caudal epigonal (e). Semen is separated by septae (asterisk) forming partial chambers in the terminal portion of the ampulla. Imaging depth (cm) is given on the right of each sonograph.
Sand tiger shark seminal fluid contained semisolid translucent rods 5–10 mm in length and 2–3 mm in diameter. Semen contained free sperm in the seminal fluid and within opaque rods or spermatophores (Figure 2A). Spermatophores were smaller than translucent rods and contained small groups of aligned sperm, randomly dispersed throughout the center of the opaque rod [46–48] (Figure 2B). Spermic ejaculates contained either free sperm or a combination of spermatophores and free sperm; spermatophores were never observed without free sperm. Spermatophores were most common in aquarium shark ejaculates collected during May and in situ sharks during April (Table 2).
Figure 2.
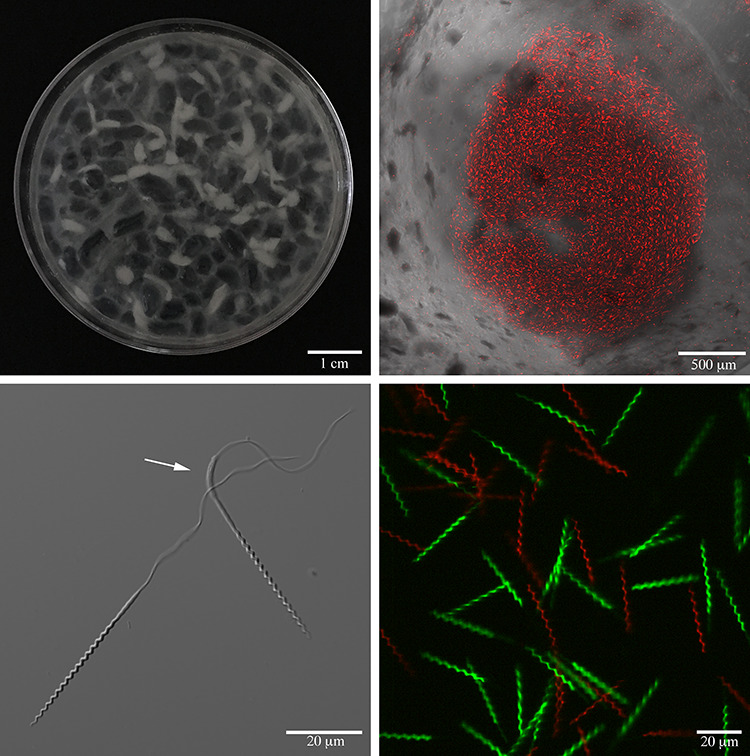
Sand tiger shark semen (A) is composed of semisolid translucent rods, opaque spermatophores, and free sperm in the minimal and viscous seminal fluid. A spermatophore cross section (B) reveals small groups of aligned sperm, randomly dispersed throughout the center of the opaque rod. Sand tiger shark spermatozoa (C) consisted of a helical head with an acrosome, a midpiece, and a flagellum with a transient cytoplasmic sleeve (arrow) located at the junction of the midpiece and tail that was shed from most sperm soon after acquiring motility. Nuclei (D) of plasma membrane intact (green) and damaged (red) spermatozoa after staining with sperm Live/Dead stain.
Table 2.
Semen collection by month for aquarium and in situ sand tiger sharks Carcharias taurus.
| Jan | Feb | March | April | May | June | July | Aug | Sept | Oct | Nov | Dec | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aquarium (n = 24) | ||||||||||||
| Sharks | 3 | 12 | 6 | 6 | 15 | 6 | 10 | 6 | 6 | 3 | 1 | |
| Collection attempts | 3 | 13 | 8 | 8 | 17 | 11 | 15 | 6 | 7 | 5 | 1 | |
| Semen obtained | 0% | 31% | 25% | 38% | 88% | 36% | 27% | 17% | 14% | 0% | 0% | |
| Motility observed | 0% | 23% | 25% | 25% | 65% | 36% | 0% | 0% | 0% | 0% | 0% | |
| Spermatophores present | 0% | 8% | 13% | 13% | 71% | 36% | 7% | 0% | 0% | 0% | 0% | |
| In situ (n = 34) | ||||||||||||
| Sharks | 17 | 7 | 7 | 3 | ||||||||
| Collection attempts | 17 | 7 | 7 | 0 | ||||||||
| Semen obtained | 100% | 100% | 86% | 0% | ||||||||
| Motility observed | 100% | 100% | 86% | 0% | ||||||||
| Spermatophores present | 100% | 86% | 57% | 0% | ||||||||
C. taurus spermatozoa (Figure 2C) consisted of a helical head (36.1 ± 0.24 μm) with 16 gyres, a midpiece with 4 gyres (8.9 ± 0.06 μm), and a flagellum (64.6 ± 0.22 μm). A cytoplasmic sleeve, located at the junction of the midpiece and tail, was present on freshly collected sperm. Upon activation of sperm motility by dilution in ASW, the cytoplasmic sleeve moved toward the distal end of the tail and for most sperm was shed from the sperm within minutes.
Sperm were nonmotile or had minimal motility in raw ejaculates and acquired motility only after dilution in ASW, demonstrating a helical forward movement akin to a drilling motion. In smash preps of spermatophores, sperm were nonmotile with the rare exception of sperm near the periphery of the preparation from select in situ samples. Spermatozoa displayed backwards motility on occasion. In each case of backward motility, the sperm’s forward progression was halted by matrix or other cellular debris within the sample and resulted in the sperm reversing its rotation axis and “unscrewing” itself from the obstacle. After a short distance of reverse motility, the sperm would cease all motility briefly before resuming progressive forward motility.
Semen parameters and seasonality
In situ sharks were examined in April, June, July, and August, and semen was present in ampullae April to July, but not August (Table 2). All ejaculates from in situ sharks were spermic, and sperm concentration was significantly higher for samples collected in July (n = 6) compared to June (n = 7) and April (n = 17), but semen volume was significantly lower in July and semen was not available for collection in August marking the end of the reproductive season (Table 3). Morphology, PMI, and motility were all significantly lower in July compared to April and June (Table 3.) In comparison, only 38% of collection attempts for aquarium males were successful in April, increasing to 88% in May, and decreasing to 36% in June. Outside of those months (January to March and August to December), the percentage of successful collections was lower confirming a similar seasonal window for sperm production for aquarium males.
Table 3.
Monthly semen characteristics for in situ sand tiger sharks Carcharias taurus.
| Month | n | Mean ± SE | Median | Range | Group* |
|---|---|---|---|---|---|
| Sperm concentration (106/ml) | |||||
| April | 17 | 13.15 ± 3.06 | 10.15 | 0.08–39.5 | a |
| June | 7 | 196.29 ± 65.45 | 119 | 28–466 | ab |
| July | 6 | 490.5 ± 214.19 | 350.5 | 117–1540 | b |
| Volume (ml) | |||||
| April | 17 | 218.8 ± 41.6 | 120 | 50–580 | a |
| June | 7 | 103.2 ± 30.8 | 130 | 7.5–200 | ab |
| July | 6 | 12.6 ± 3.0 | 12 | 3.5–25 | b |
| Morphology (%) | |||||
| April | 15 | 96 ± 1 | 98 | 87–100 | a |
| June | 7 | 90 ± 6 | 96 | 58–100 | a |
| July | 6 | 64 ± 10 | 72.5 | 32–95 | b |
| Plasma membrane integrity (%) | |||||
| April | 12 | 67.8 ± 8.0 | 77.5 | 10–95 | a |
| June | 7 | 53.7 ± 10.4 | 45 | 22.8–85 | ab |
| July | 6 | 22.7 ± 5.4 | 21.7 | 5–45 | b |
| Total motility (%) | |||||
| April | 16 | 61.9 ± 7.7 | 70.25 | 10–95 | a |
| June | 7 | 45.2 ± 10.3 | 33.1 | 15–80 | ab |
| July | 6 | 14.6 ± 2.6 | 16.25 | 5–20 | b |
| Progressive motility (%) | |||||
| April | 16 | 19.8 ± 3.9 | 20 | 0–50 | a |
| June | 7 | 14.4 ± 4.8 | 9.3 | 5–35 | a |
| July | 6 | 0.8 ± 0.4 | 0.5 | 0–2.5 | b |
*Letters indicate group membership from Kruskal–Wallis test and Dunn post-hoc pairwise comparisons with Benjamini–Hochberg adjusted P-values. Groups that share a letter are not different using a significance threshold of P < 0.05.
For semen collected from April to June, pH, osmolarity, semen volume, and sperm concentration were similar between in situ and aquarium sharks, but sperm morphology, PMI, motility, and status were significantly higher for in situ semen (Table 4). Normal sperm morphology percentages were generally high throughout sampling months (58–100% in situ, 42–96% aquarium sharks) with the predominant sperm abnormalities observed as partial or complete unfurling of the head gyres, swelling or blebbing of the head and midpiece, bent midpiece, and coiled or bent tails. Assessment of PMI using the SYBR-PI combination stain revealed that percentages of green and red stained sperm were correlated with ratios of frozen-plasma membrane damaged and nondamaged sperm (r = 0.95; P < 0.001), validating the stain as an indicator of shark sperm plasma membrane integrity (Figure 2D). In smash preps of spermatophores stained with SYBR-PI, sperms were plasma membrane damaged, with the rare exception of a few in situ samples.
Table 4.
Semen characteristics and plasma testosterone for aquarium and in situ sand tiger sharks Carcharias taurus during the mating season, April to June.
| Aquarium | In situ | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| P * | n | Mean ± SE | Median | Range | n | Mean ± SE | Median | Range | |
| Sperm concentration (106/ml) | 0.087 | 14 | 12.05 ± 3.71 | 7.5 | 0.62–48.8 | 24 | 66.57 ± 25.14 | 17.35 | 0.08–466 |
| Volume (ml) | 0.370 | 14 | 209.8 ± 75.2 | 74.5 | 20–1000 | 24 | 185.1 ± 32.4 | 125 | 7.5–580 |
| Morphology (%) | 0.000 | 12 | 79 ± 4 | 81 | 42–96 | 22 | 93.95 ± 1.99 | 97.5 | 58–100 |
| Plasma membrane integrity (%) | 0.010 | 13 | 32.7 ± 9.3 | 20 | 0–87.9 | 19 | 62.6 ± 6.3 | 75 | 10–95 |
| Total motility (%) | 0.004 | 14 | 27 ± 8 | 11 | 0–80 | 23 | 57 ± 6 | 70 | 10–95 |
| Progressive motility (%) | 0.002 | 14 | 5 ± 2 | 0 | 0–26 | 23 | 18 ± 3 | 20 | 0–50 |
| Status | 0.008 | 14 | 2.3 ± 0.4 | 2 | 0–4 | 23 | 3.6 ± 0.2 | 4 | 2–4 |
| pH | 0.051 | 14 | 7.1 ± 0.1 | 7.12 | 6.25–7.7 | 24 | 6.8 ± 0.1 | 6.75 | 6.5–7.75 |
| Osmolarity (mOsm) | 0.322 | 13 | 1088 ± 25 | 1060 | 989–1328 | 22 | 1104 ± 16 | 1108 | 935–1254 |
| Plasma testosterone (ng/ml) | 0.000 | 13 | 9.35 ± 6.34 | 1.2 | 0.2–84.6 | 21 | 28.57 ± 5.83 | 20.8 | 1.4–103.4 |
*Wilcoxon rank-sum (Mann–Whitney) test P-value.
Testosterone
Mature in situ male sharks (n = 19) had higher testosterone concentrations than juvenile sharks (n = 22) for samples collected during summer (June to August), the only season when samples from both age classes were collected (P = 0.045; Figure 3A). Mature in situ shark testosterone concentrations were significantly higher (P < 0.05) in April than June, July, or August (Figure 3B). Mature in situ sharks had higher testosterone than aquarium sharks (P < 0.05) during spring, but no difference was observed during the summer season (Figure 4). Testosterone concentrations in aquarium sharks were highest in February and lowest in summer and fall (Figure 5). Elevated testosterone in spring coincided with the breeding season when ejaculates of the best quality were collected, whereas summer testosterone concentrations were significantly lower (P < 0.05) than spring and coincided with the end of breeding season and cessation of semen production.
Figure 3.
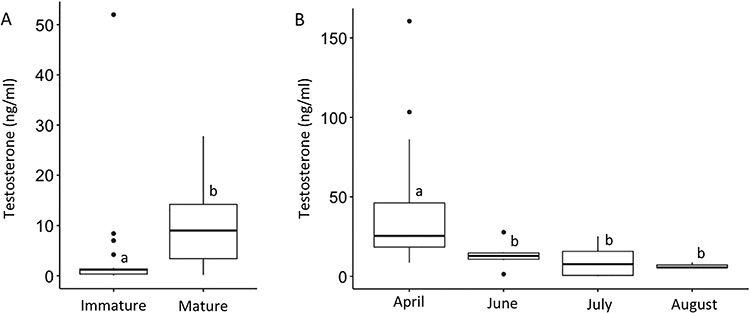
Testosterone concentration for in situ sand tiger sharks. Immature sharks have lower plasma testosterone than mature sharks during summer (A). For mature sharks, testosterone is highest in April, the breeding season, and decreases to a nadir by late summer when they are no longer breeding (B). Different letters indicate significant (P < 0.05) differences among the groups.
Figure 4.
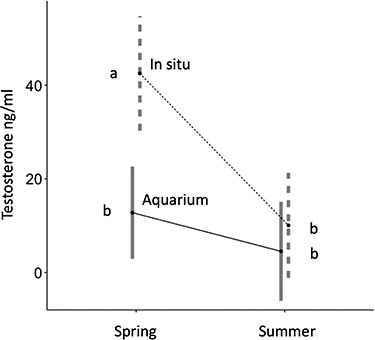
Testosterone concentration (estimated marginal mean and 95% confidence interval) for in situ and aquarium sharks during spring (breeding season) and summer (nonbreeding season). In situ shark testosterone concentration was highest during spring and decreased to baseline levels in summer. Aquarium shark testosterone concentration was not different between seasons. In situ sharks had higher testosterone concentration than aquarium sharks during the breeding season. Different letters indicate significant (P < 0.05) differences among the groups.
Figure 5.
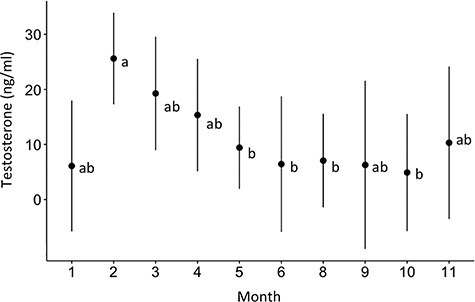
Annual testosterone concentration (estimated marginal mean and 95% confidence interval) for aquarium sharks. Testosterone concentration was highest in February to initiate spermatogenesis in preparation for the reproductive season. Testosterone remained elevated throughout spring, the breeding season, and decreased to baseline in late summer. Different letters indicate significant (P < 0.05) differences between months.
Discussion
Seasonal patterns of testosterone and sperm production confirm that the reproductive cycle of male sand tiger sharks is annual with a breeding season from April to June. However, in situ samples were not available for March; therefore, it is possible that the breeding season commences before April. All biological samples were collected from live animals demonstrating that nonlethal techniques are effective to characterize reproduction, an important consideration for research with species that are protected or endangered. Careful monitoring of shark health during examinations and limiting animal handling to 30 min were effective in minimizing risk of adverse effects after examinations. These methods have since been safely employed to collect blood and semen samples in multiple elasmobranch species (data not shown), including zebra sharks (Stegostoma tigirinum), leopard sharks (Triakis semifasciata), sharpnose sharks (Rhizoprionodon terraenovae), blacknose sharks (Carcharias acronotus), smooth dogfish (Mustelus canis), southern stingrays (Hypanus americanus), shovelnose guitarfish (Rhinobatos productus), and blacktip reef sharks (Carcharhinus melanopterus), demonstrating the practicality of this sampling approach.
Reproduction of aquarium sand tiger sharks resulting in live young has been realized by only four aquaria worldwide [21]. Comparatively, poor semen quality and lower blood plasma testosterone for aquarium sharks compared to in situ sharks during the breeding season may contribute to the lack of reproductive success. Similar observations of impaired reproduction due to reduced quantity and quality of semen have been described also among teleost fishes in aquaculture where exogenous hormones and photothermal regimes are used to stimulate gametogenesis [49–53]. Reproductive dysfunctions observed for aquaculture fish include a lower gonadosomatic index (GSI) and truncated or temporally shifted spawning seasons [54–56] compared to their in situ counterparts. In situ greater amberjack Seriola dumerili (Risso, 1810) had higher concentrations of androgens during spermatogenesis and progestins during spermiation compared to aquaculture fish [56]. Also common with sand tiger sharks, lower sperm motility has been observed for aquaculture fish compared to in situ fish [50, 55, 57]. Collectively, the male reproductive system is affected by managed care for many fishes.
Sand tiger shark spermatozoa were consistent in morphology to other elasmobranch species and composed of a helical shaped head, midpiece with cytoplasmic sleeve, flagellum, and acrosome [47]. The cytoplasmic sleeve of sand tiger spermatozoa slid off the midpiece and along the entire length of the flagellum until it was shed completely, an event that typically took only minutes after spermatozoa acquired motility. In this case, the cytoplasmic sleeve is analogous to the mammalian Hermes body or cytoplasmic droplet and a measure of maturation [58]. This indicates that sand tiger sperm undergo a post-testicular maturation process analogous to capacitation. Similar to other elasmobranchs studied, sand tiger shark spermatozoa were either nonmotile or minimally motile until diluted in ASW or other iso-osmotic electrolyte solutions (data not shown). This is consistent with observations in the banded houndshark (Triakis scyllium) where it was suggested that dilution of the ejaculate in electrolyte solutions similar to seawater or uterine fluid would mimic conditions during breeding and initiate forward progression [59]. Studies in teleosts show that changes in the concentration of specific ions rather than total osmolarity are responsible for the initiation of motility [60].
The observation of backward sperm motility in the sand tiger shark was unexpected, though backwards sperm motility in elasmobranchs was described already for T. scyllium [59]. Reverse motility is not a common spermatozoa characteristic, but spermatozoa from gastropods and fruit flies also have reverse motility [61–63]. Both fruit flies and gastropods demonstrate postcopulatory sexual selection [64, 65], and the sand tiger shark has been shown to demonstrate behavioral polyandry [66]. Polyandry is associated with postcopulatory sperm competition in animals with internal fertilization and generally favors large ejaculates. Because each sand tiger shark ampulla contains approximately a liter of semen and females mate with multiple males, it is reasonable to suppose that sperm competition occurs in this species and that backwards motility may allow further postcopulatory sperm selection.
Sand tiger shark semen contained free and individual sperm as well as spermatophores. Spermatophores are broadly defined as fully encapsulated sperm packages and are observed commonly among arthropods and molluscs [67]. Spermatophores occasionally are described in vertebrates including select amphibians, teleosts, and chondrichthyans [67]. Among chondrichthyans, spermatophores have been described for sharks [46–48, 68, 69] and chimeras [70, 71] as ovoid to spherical aggregates of laterally aligned bundles of sperm embedded in a matrix forming a cellular medulla with an acellular cortex. When spermatophores were observed in sand tiger shark semen, they were dispersed within an ejaculate that included acellular hyaline rods and free sperm. Similar translucent acellular gelatinous rods have been described in ejaculates from blue Prionace glauca [72] and basking Cetorhinus maximus [68] sharks. Spermatophores are indicative of testicular spermatogenesis and accessory sex gland secretory activity, but seminal fluid and acellular hyaline rods reflect only accessory gland secretory activity. Shark spermatophores are posited to be a mechanism for sperm storage and slow release after insemination, but the current study found that for sand tiger spermatophores, only rarely were plasma membrane intact sperm capable of motility observed. In contrast to blue shark spermatophores [72], sand tiger spermatophores do not breakdown in the cold-stored raw ejaculate or after dilution in ASW for months (data not shown), suggesting that enzymatic action is required for spermatozoa liberation. Experiments establishing the fertilization capability of spermatozoa released from spermatophores are needed to understand their role in sand tiger reproduction. Polyandrous sand tiger sharks may utilize both postcopulatory sperm competition and postfertilization competition via adelphophagy, affording females an additional selection mechanism to ensure the single young per uterus is offspring from the male with the fittest genes [66].
Elasmobranch reproduction is regulated by the hypothalamic–pituitary–gonadal (HPG) axis through hormone production. Endocrine profiles have been measured and related to testis size, sexual conflicts, and reproductive season for several shark [73–78] and ray [79–81] species. The endocrinology of male sand tigers has received little attention with no studies measuring hormones for in situ sharks prior to this study and only two studies for aquarium sharks [10, 28]. This study found higher testosterone during the breeding season among in situ compared to aquarium sharks. Previous studies found that aquarium sharks that engaged in seasonal precopulatory conflicts had elevated progesterone (P4), T and dihydrotestosterone (DHT) compared to aquarium sharks that were not observed to engage in sexual conflicts, and T and DHT varied individually based on a dominance hierarchy for males housed together [10], supporting a link between testosterone and reproductive activity and performance/quality in this species.
Aquarium and in situ male testosterone profiles and semen seasonality support an annual reproductive cycle with spermatogenesis initiated by increasing testosterone concentration in late winter that peaks before and remains elevated during mating season in spring. Testosterone concentration was higher for in situ males in April to June compared to aquarium males, but for aquarium sharks, the highest concentration of T was measured during February and no in situ samples from February were available for comparison. If in situ and aquarium males follow a similar seasonality, then the concentration of T for in situ males in winter is expected to be higher than observed for aquarium sharks in winter. Testosterone has been associated with gonadal recrudescence, spermiation, and precopulatory behavior [73, 79–83]. Therefore, persistently low testosterone could result in aspermic or oligospermic ejaculates as well as lack of precopulatory and copulatory behaviors. The androgens DHT and 11-keto-testosterone (11-KT), estrogen, and progesterone also are produced by elasmobranchs [10, 28, 77, 78, 82] and may influence spermatogenesis and spermiation in sand tiger sharks but were not measured in this study.
For seasonally reproducing species, a combination of environmental and social cues initiates the reproductive cycle and maintains its periodicity [82, 84–86]. In situ sand tiger sharks undertake large seasonal migrations related to their reproductive habits, [12, 13, 24, 25, 87, 88] and as a result, experience pronounced changes in social structure, nutrition, photoperiod, and temperature. Of these major environmental factors, seasonal temperature change is shared among aquaria with successful sand tiger shark reproduction [20, 21]. The highest testosterone concentration and sperm motility among aquarium sharks was measured in samples from males exposed to seasonal photoperiod and temperature cycles. The specific role of environmental and social factors in the reproductive cycle of aquarium and in situ sand tiger sharks requires more research.
There are challenges in manipulating environmental cues such as temperature for species maintained in aquariums in large mixed exhibits, as a positive change for one species may have a negative impact on other species. The rate and magnitude of temperature change required for such cues to be effective is dependent on the life history of each species. For many sharks, in situ research is needed to inform and optimize aquarium husbandry. Finally, when environmental cues of the appropriate magnitude are provided, it is also unknown how quickly the sharks can be expected to respond. Evidence for temperature-associated shifts in elasmobranch reproduction has been observed for in situ [82, 89, 90] and aquarium [28, 73] elasmobranchs. Among sand tigers, precopulatory behavior and T and DHT hormone peaks shifted to 4 months earlier than previous years for three aquarium males after a prolonged period of elevated temperatures during the prior summer [28]. Shifting of reproductive season for one or both sexes can result in asynchronous cycling and prevent successful insemination.
Cultures from sand tiger semen support a low risk for introduction of infectious agents through transferring semen between animals, which is an important consideration when moving gametes. Artificial insemination with semen from in situ sharks would increase genetic diversity of the aquarium shark population and promote sustainability of managed populations through introduction of new genetics with little or no expected impact on in situ populations. Artificial insemination using in situ semen may be necessary if the poor semen quality observed among aquarium males is inadequate for successful reproduction. Studies are underway to identify underlying causes for poor semen quality in aquarium sand tigers. This study describes the first steps toward developing techniques for assisted reproduction in sand tiger sharks, including proving a technique for semen collection and assessment and describing the periodicity and seasonality for semen production through examination of in situ and aquarium sharks.
Acknowledgments
A project of this undertaking requires the collaboration, cooperation, and expertise of many people and in 2015 North Carolina Aquariums initiated collaboration between aquariums that has grown to be the Sand Tiger Shark Consortium. We thank husbandry and veterinary professionals at Georgia Aquarium and their affiliate Marineland Dolphin Adventure, Ripley’s Aquariums, North Carolina Aquariums, Aquarium of the Pacific, New York Aquarium, and Shark Reef Aquarium at Mandalay Bay. We thank Adventure Aquarium, Jenkinson’s Aquarium, and Audubon Aquarium of the Americas for providing opportunistic samples. Alan Henningsen from the National Aquarium provided sand tiger shark plasma samples for hormone assay validation. This project benefited from support from the AZA Sand Tiger Species Survival Plan (SSP). The authors are extremely grateful for in situ samples and boat time provided by Ripley’s Aquariums, North Carolina Aquariums, the National Aquarium, and through the Cooperative Atlantic States Pupping and Nursery Survey (COASTSPAN) and Cooperative Shark Tagging Program (CSTP) administered by the Apex Predators Program (APP).
Conference Presentation: Presented in part at the Regional Aquatics Workshop, 14–18 May, 2018, Tampa, FL and [29].
Footnotes
† Grant support: This work was supported by a grant from the SeaWorld & Busch Gardens Conservation Fund to JTW and LMP. Confocal microscope access was supported by grants from the NIH-NIGMS (P20 GM103446), the NSF (IIA-1301765), and the State of Delaware and equipment was acquired with the Delaware INBRE Grant P20 GM103446.
References
- [1]. Goldman KJ, Branstetter S, Musick JA. A re-examination of the age and growth of sand tiger sharks, Carcharias taurus, in the western North Atlantic: The importance of ageing protocols and use of multiple back-calculation techniques. Environ Biol Fishes 2006; 77:241–252. [Google Scholar]
- [2]. Carlson J, McCandless C, Cortés E, Grubbs R, Andrews K, MacNeil M, Musick J. Update on the Status of the Sand Tiger Shark, Carcharias taurus in the Northwest Atlantic Ocean . Tech Memo NMFS-SEFSC-585 Panama City, FL: NOAA, National Marine Fisheries Service; 2009. [Google Scholar]
- [3]. Dulvy NK, Fowler SL, Musick JA, Cavanagh RD, Kyne PM, Harrison LR, Carlson JK, Davidson LN, Fordham SV, Francis MP, Pollock CM, Simpfendorfer CA et al. Extinction risk and conservation of the world’s sharks and rays. Elife 2014; 3:e00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Dulvy NK, Simpfendorfer CA, Davidson LN, Fordham SV, Bräutigam A, Sant G, Welch DJ. Challenges and priorities in shark and ray conservation. Curr Biol 2017; 27:R565–R572. [DOI] [PubMed] [Google Scholar]
- [5]. Pollard D, Smith A. Carcharias taurus The IUCN Red List of Threatened Species; 2009: e.T3854A10132481 10.2305/IUCN.UK.2009-2.RLTS.T3854A10132481.en. [DOI] [Google Scholar]
- [6]. Haulsee DE, Fox DA, Breece MW, Clauss TM, Oliver MJ. Implantation and recovery of long-term archival transceivers in a migratory shark with high site fidelity. PLoS One 2016; 11:e0148617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Sheldon JD, Allender MC, George RH, Bulman F, Abney K. Reproductive hormone patterns in male and female cownose rays (Rhinoptera bonasus) in an aquarium setting and correlation to ultrasonographic staging. J Zoo Wildl Med 2018; 49:638–647. [DOI] [PubMed] [Google Scholar]
- [8]. Branstetter S, Musick JA. Age and growth estimates for the sand tiger in the northwestern Atlantic Ocean. Trans Am Fish Soc 1994; 123:242–254. [Google Scholar]
- [9]. Bansemer C, Bennett M. Reproductive periodicity, localised movements and behavioural segregation of pregnant Carcharias taurus at Wolf Rock, Southeast Queensland, Australia. Mar Ecol Prog Ser 2009; 374:215–227. [Google Scholar]
- [10]. Henningsen AD, Murru FL, Rasmussen LEL, Whitaker BR, Violetta GC. Serum levels of reproductive steroid hormones in captive sand tiger sharks, Carcharias taurus (Rafinesque), and comments on their relation to sexual conflicts. Fish Physiol Biochem 2008; 34:437–446. [DOI] [PubMed] [Google Scholar]
- [11]. Castro JI. Observations on the reproductive cycles of some vivparous North American sharks. Aqua Int J Ichthyol 2009; 15:205–222. [Google Scholar]
- [12]. Dicken M, Smale M, Booth A. Spatial and seasonal distribution patterns of the ragged-tooth shark Carcharias taurus along the coast of South Africa. African J Mar Sci 2006; 28:603–616. [Google Scholar]
- [13]. Lucifora L, Menni RC, Escalante AH. Reproductive ecology and abundance of the sand tiger shark, Carcharias taurus, from the southwestern Atlantic. ICES J Mar Sci 2002; 59:553–561. [Google Scholar]
- [14]. Gilmore G, Dodrill JW, Linley PA. Reproduction and embryonic development of the sand tiger shark, Odontaspis taurus (Rafinesque). Fish Bull 1983; 81:201–224. [Google Scholar]
- [15]. Springer S. Oviphagous embryos of the sand shark, Carcharias taurus. Copeia 1948; 1948:153. [Google Scholar]
- [16]. Gilmore RG, Putz O, Dodrill J. Oophagy, intrauterine cannibalism and reproductive strategy in lamnoid sharks In: Hamlett WC. (ed.), Reproductive Biology and Phylogeny of Chondrichthyes: Sharks, Batoids and Chimaeras. Enfield, NJ: Science Publishers; 2005: 435–462. [Google Scholar]
- [17]. Otway NM, Bradshaw CJ, Harcourt RG. Estimating the rate of quasi-extinction of the Australian grey nurse shark (Carcharias taurus) population using deterministic age- and stage-classified models. Biol Conserv 2004; 119:341–350. [Google Scholar]
- [18]. Sin Y. Age, Growth, and Reproductive Biology of Whitespotted Bamboo Shark (Chiloscyllium plagiosum) from Hong Kong and Adjacent Waters. Hong Kong: University of Hong Kong; 2009. [Google Scholar]
- [19]. Koob TJ. Elasmobranchs in the public aquarium: 1860 to 1930 In: The Elasmobranch Husbandry Manual: Captive Care of Sharks, Rays and Their Relatives. Columbus, Ohio: Ohio Biological Survey; 2004: 1–14. [Google Scholar]
- [20]. Willson K, Smith M. Reproduction of the sand tiger shark, Carcharias taurus (Rafinesque, 1810), at UnderWater World SEA LIFE Mooloolaba from 1992–2012 In: Smith M, Warmolts D, Thoney D, Hueter R, M M, Ezcurra J (eds.), The Elasmobranch Husbandry Manual II: Recent Advances in the Care of Sharks, Rays and their Relatives. Columbus, Ohio: Special Publication of the Ohio Biological Survey; 2017: 391–401. [Google Scholar]
- [21]. Henningsen A, Street EP, Claus E, Street EP, Littlehale D, Choromanski J, Parkway K, Gordon I, Willson K. Reproduction of the sand tiger sharks, Carcharias taurus, in aquaria: a framework for a managed breeding program In: Smith M, Warmolts D, Thoney D, Hueter R, M M, Ezcurra J (eds.), The Elasmobranch Husbandry Manual II: Recent Advances in the Care of Sharks, Rays and their Relatives. Columbus, Ohio: Special Publication of the Ohio Biological Survey; 2017: 375–390. [Google Scholar]
- [22]. Buckley KA, Crook DA, Pillans RD, Smith L, Kyne PM. Sustainability of threatened species displayed in public aquaria, with a case study of Australian sharks and rays. Rev Fish Biol Fish 2018; 28:137–151. [Google Scholar]
- [23]. Daly J, Jones R. The use of reproductive technologies in breeding programs for elasmobranchs in aquaria In: Smith M, Warmolts D, Thoney D, Hueter R, Murray M, Ezcurra J (eds.), The Elasmobranch Husbandry Manual II: Recent Advances in the Care of Sharks, Rays and their Relatives. Columbus, Ohio: Special Publication of the Ohio Biological Survey; 2017: 363–374. [Google Scholar]
- [24]. Haulsee DE, Fox DA, Breece MW, Brown LM, Kneebone J, Skomal GB, Oliver MJ. Social network analysis reveals potential fission-fusion behavior in a shark. Sci Rep 2016; 6:34087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Teter SM, Wetherbee BM, Fox DA, Lam CH, Kiefer DA, Shivji M. Migratory patterns and habitat use of the sand tiger shark (Carcharias taurus) in the western North Atlantic. Mar Freshw Res 2015; 66:158. [Google Scholar]
- [26]. Kneebone J, Chisholm J, Skomal G. Movement patterns of juvenile sand tigers (Carcharias taurus) along the east coast of the USA. Mar Biol 2014; 161:1149–1163. [Google Scholar]
- [27]. Haulsee DE, Breece MW, Brown LM, Wetherbee BM, Fox DA, Oliver MJ. Spatial ecology of Carcharias taurus in the northwestern mid-Atlantic coastal ocean. Mar Ecol Prog Ser 2018; 597:191–206. [Google Scholar]
- [28]. Henningsen AD, Whitaker BR, Kight K, Hess DL, Hadfield C, Zohar Y. The use of a gonadotropin releasing hormone antagonist in captive sand tiger sharks, Carcharias taurus, and the serum levels of the antagonist and reproductive steroid hormones. J Zoo Aquarium Res 2015; 3:107–115. [Google Scholar]
- [29]. Penfold LM, Wyffels JT. Reproductive science in sharks and rays In: Comizzoli P, Brown J, Holt W (eds.), Advances in Experimental Medicine and Biology, vol. 1200 Cham: Springer; 2019: 465–488. [DOI] [PubMed] [Google Scholar]
- [30]. Sulikowski JA, Driggers WB, Ingram GW, Kneebone J, Ferguson DE, Tsang PCW. Profiling plasma steroid hormones: a non-lethal approach for the study of skate reproductive biology and its potential use in conservation management. Environ Biol Fishes 2007; 80:285–292. [Google Scholar]
- [31]. Awruch CA, Frusher SD, Pankhurst NW, Stevens JD. Non-lethal assessment of reproductive characteristics for management and conservation of sharks. Mar Ecol Prog Ser 2008; 355:277–285. [Google Scholar]
- [32]. Clarke E, von Schmidt K. Sharks of the central gulf coast of Florida. Bull Mar Sci 1965; 15:15–83. [Google Scholar]
- [33]. Stamper MA. Immobilization of Elasmobranchs In: Smith M, Warmolts D, Thoney D, Hueter R (eds.), The Elasmobranch Husbandry Manual: Captive Care of Sharks, Rays and their Relatives. Columbus, Ohio: Ohio Biological Survey, Inc.; 2004: 281–296. [Google Scholar]
- [34]. Kessel ST, Hussey NE. Tonic immobility as an anaesthetic for elasmobranchs during surgical implantation procedures. Can J Fish Aquat Sci 2015; 72:1287–1291. [Google Scholar]
- [35]. Henningsen AD. Tonic immobility in twelve elasmobranchs: use as an aid in captive husbandry. Zoo Biol 1994; 13:325–332. [Google Scholar]
- [36]. Howard J, Bush M, Wildt DE. Semen collection, analysis and cryopreservation in nondomestic mammals In: Morrow DA. (ed.), Current Therapy in Theriogenology. Philadelphia, PA;WB Saunders; 1986: 1047–1053. [Google Scholar]
- [37]. Garner DL, Johnson LA, Yue ST, Roth BL, Haugland RP. Dual DNA staining assessment of bovine sperm viability using SYBR-14 and propidium iodide. J Androl 1994; 15:620–629. [PubMed] [Google Scholar]
- [38]. Munro CJ, Lasley BL. Non-radiometric methods for immunoassay of steroid hormones. Prog Clin Biol Res 1988; 285:289–329. [PubMed] [Google Scholar]
- [39]. Munro C, Stabenfeldt G. Development of a microtitre plate enzyme immunoassay for the determination of progesterone. J Endocrinol 1984; 101:41–49. [DOI] [PubMed] [Google Scholar]
- [40]. Schwarz G. Estimating the dimension of a model. Ann Stat 1978; 6:461–464. [Google Scholar]
- [41]. R Core Team. R A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. [Google Scholar]
- [42]. Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw 2015; 67:1–48. [Google Scholar]
- [43]. Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest package: tests in linear mixed effects models. J Stat Softw 2017; 82:1–26. [Google Scholar]
- [44]. Lenth R. Emmeans: Estimated marginal means, aka least-squares means. 2018; R package version 1.3.1. https://CRAN.R-project.org/package=emmean.
- [45]. Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag; 2009. [Google Scholar]
- [46]. Gilmore RG. Reproductive biology of lamnoid sharks. Environ Biol Fishes 1993; 38:95–114. [Google Scholar]
- [47]. Tanaka S, Kurokawa H, Masako H. Comparative morphology of the sperm in chondrichthyan fishes. Mémoires Du Muséum Natl d’Histoire Nat 1995; 166:321–332. [Google Scholar]
- [48]. Pratt HL, Tanaka S. Sperm storage in male elasmobranchs: a description and survey. J Morphol 1994; 219:297–308. [DOI] [PubMed] [Google Scholar]
- [49]. Mylonas CC, Duncan NJ, Asturiano JF. Hormonal manipulations for the enhancement of sperm production in cultured fish and evaluation of sperm quality. Aquaculture 2017; 472:21–44. [Google Scholar]
- [50]. Gilroy CE, Litvak MK. Swimming kinematics and temperature effects on spermatozoa from wild and captive shortnose sturgeon (Acipenser brevirostrum). Anim Reprod Sci 2019; 204:171–182. [DOI] [PubMed] [Google Scholar]
- [51]. Cabrita E, Soares F, Dinis MT. Characterization of Senegalese sole, Solea senegalensis, male broodstock in terms of sperm production and quality. Aquaculture 2006; 261:967–975. [Google Scholar]
- [52]. Valcarce DG, Robles V. Effect of captivity and cryopreservation on ROS production in Solea senegalensis spermatozoa. Reproduction 2016; 152:439–446. [DOI] [PubMed] [Google Scholar]
- [53]. Zohar Y, Mylonas CC. Endocrine manipulation of spawning induction in cultured fish from hormone to gene. Aquac Int 2001; 197:99–139. [Google Scholar]
- [54]. Zupa R, Fauvel C, Mylonas CC, Pousis C, Santamaria N, Papadaki FI, Cicirelli V, Mangano S, Passantino L, Lacalandra GM, Corriero A. Rearing in captivity affects spermatogenesis and sperm quality in greater amberjack, Seriola dumerili (Risso, 1810). J Anim Sci 2017; 95:4085–4100. [DOI] [PubMed] [Google Scholar]
- [55]. Zupa R, Fauvel C, Mylonas CC, Santamaria N, Valentini L, Pousis C, Papadaki M, Suquet M, De la Gándara F, Bello G, De Metrio G, Corriero A. Comparative analysis of male germ cell proliferation and apoptosis in wild and captive Atlantic bluefin tuna (Thunnus thynnus L.). J Appl Ichthyol 2013; 29:71–81. [Google Scholar]
- [56]. Zupa R, Rodrõâguez C, Mylonas CC, Rosenfeld H, Fakriadis I, Papadaki M, Peârez JA, Pousis C, Basilone G, Corriero A. Comparative study of reproductive development in wild and captive-reared greater amberjack Seriola dumerili (Risso, 1810). PLoS One 2017; 12:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57]. Locatello L, Bertotto D, Cerri R, Parmeggiani A, Govoni N, Trocino A, Xiccato G, Mordenti O. Sperm quality in wild-caught and farmed males of the European eel (Anguilla anguilla). Anim Reprod Sci 2018; 198:167–176. [DOI] [PubMed] [Google Scholar]
- [58]. Jones RC, Jones N, Djakiew D. Luminal composition and maturation of spermatozoa in the male genital ducts of the Port Jackson shark, Heterodontus portusjacksoni. J Exp Zool 1984; 230:417–426. [Google Scholar]
- [59]. Minamikawa S, Morisawa M. Acquisition, initiation and maintenance of sperm motility in the shark, Triakis scyllia. Comp Biochem Physiol Part A Physiol 1996; 113:387–392. [Google Scholar]
- [60]. Morisawa M, Suzuki K. Osmolality and potassium ion: their roles in initiation of sperm motility in teleosts. Science 1980; 210:1145–1147. [DOI] [PubMed] [Google Scholar]
- [61]. Buckland-Nicks JA, Chia FS. Locomotion of the filiform sperm of littorina (Gastropoda, Prosobranchia). Cell Tissue Res 1981; 219:27–39. [DOI] [PubMed] [Google Scholar]
- [62]. Shiba K, Shibata D, Inaba K. Autonomous changes in the swimming direction of sperm in the gastropod Strombus luhuanus. J Exp Biol 2014; 217:986–996. [DOI] [PubMed] [Google Scholar]
- [63]. Köttgen M, Hofherr A, Li W, Chu K, Cook S, Montell C, Watnick T. Drosophila sperm swim backwards in the female reproductive tract and are activated via TRPP2 ion channels. PLoS One 2011; 6:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64]. Taylor PW, Yuval B. Postcopulatory sexual selection in Mediterranean fruit flies: advantages for large and protein-fed males. 1999; 58:247–254. [DOI] [PubMed] [Google Scholar]
- [65]. Paterson IG, Partridge V, Buckland-Nicks J. Multiple paternity in Littorina obtusata (Gastropoda, Littorinidae) revealed by microsatellite analyses. Biol Bull 2001; 200:261–267. [DOI] [PubMed] [Google Scholar]
- [66]. Chapman DD, Wintner SP, Abercrombie DL, Ashe J, Bernard AM, Shivji MS, Feldheim K a. The behavioural and genetic mating system of the sand tiger shark, Carcharias taurus, an intrauterine cannibal. Biol Lett 2013; 9:20130003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67]. Mann T. Spermatophores: Development, Structure, Biochemical Attributes, and Role in the Transfer of Spermatozoa. New York: Springer-Verlag; 1984. [Google Scholar]
- [68]. Matthews LH. Reproduction in the basking shark, Cetorhinus maximus (Gunner). Philos Trans R Soc B Biol Sci 1950; 234:247–316. [DOI] [PubMed] [Google Scholar]
- [69]. Pratt H. Reproduction in the male white shark In: K AP, Ainley D (eds.), Great White Sharks: The Biology of Carcharodon carcharias. Waltham: Academic Press; 1996: 131–138. [Google Scholar]
- [70]. Hamlett WC, Reardon M, Clark J, Walker TI. Ultrastructure of sperm storage and male genital ducts in a male holocephalan, the elephant fish, Callorhynchus milii. J Exp Zool 2002; 292:111–128. [DOI] [PubMed] [Google Scholar]
- [71]. Reardon MB, Walker TI, Hamlett WC. Microanatomy of spermatophore formation and male genital ducts in the holocephalan, Callorhynchus milii. Mar Freshw Res 2002; 53:591–600. [Google Scholar]
- [72]. Pratt HL. Reproduction in the blue shark Prionace glauca. Fish Bull 1979; 77:445–470. [Google Scholar]
- [73]. Heupel MR, Whittier JM, Bennett MB. Plasma steroid hormone profiles and reproductive biology of the epaulette shark, Hemiscyllium ocellatum. J Exp Zool 1999; 284:586–594. [PubMed] [Google Scholar]
- [74]. Hoffmayer ER, Sulikowski JA, Hendon JM, Parsons GR. Plasma steroid concentrations of adult male Atlantic sharpnose sharks, Rhizoprionodon terraenovae, in the northern Gulf of Mexico, with notes on potential long term shifts in reproductive timing. Environ Biol Fishes 2010; 88:1–7. [Google Scholar]
- [75]. Awruch CA, Pankhurst NEDW, Frusher SD. Endocrine and morphological correlates of reproduction in the draughtboard shark Cephaloscyllium laticeps (Elasmobranchii: Scyliorhinidae). J Exp Zool 2008; 309A:184–197. [DOI] [PubMed] [Google Scholar]
- [76]. Parsons GR, Grier HJ. Seasonal changes in shark testicular structure and spermatogenesis. J Exp Zool 1992; 261:173–184. [Google Scholar]
- [77]. Garnier HG, Sourdaine P, Jegou B. Seasonal variations in sex steroids and male sexual characteristics in Scyliorhinus canicula. Gen Comp Endocrinol 1999; 116:281–290. [DOI] [PubMed] [Google Scholar]
- [78]. Manire CA, Rasmussen LE. Serum concentrations of steroid hormones in the mature male bonnethead shark, Sphyrna tiburo. Gen Comp Endocrinol 1997; 107:414–420. [DOI] [PubMed] [Google Scholar]
- [79]. Tricas TC, Maruska KP, Rasmussen LE. Annual cycles of steroid hormone production, gonad development, and reproductive behavior in the Atlantic stingray. Gen Comp Endocrinol 2000; 118:209–225. [DOI] [PubMed] [Google Scholar]
- [80]. Snelson FF, Rasmussen LE, Johnson MR, Hess DL. Serum concentrations of steroid hormones during reproduction in the Atlantic stingray, Dasyatis sabina. Gen Comp Endocrinol 1997; 108:67–79. [DOI] [PubMed] [Google Scholar]
- [81]. Sulikowski JA, Tsang PCW, Huntting Howell W. An annual cycle of steroid hormone concentrations and gonad development in the winter skate, Leucoraja ocellata, from the western Gulf of Maine. Mar Biol 2004; 144:845–853. [Google Scholar]
- [82]. Mull CG, Lowe CG, Young KA. Photoperiod and water temperature regulation of seasonal reproduction in male round stingrays (Urobatis halleri). Comp Biochem Physiol Part A Mol Integr Physiol 2008; 151:717–725. [DOI] [PubMed] [Google Scholar]
- [83]. Rasmussen LE, Hess DL, Luer CA. Alterations in serum steroid concentrations in the clearnose skate, Raja eglanteria: correlations with season and reproductive status. J Exp Zool 1999; 284:575–585. [DOI] [PubMed] [Google Scholar]
- [84]. Elisio M, Colonello JH, Cortés F, Jaureguizar AJ, Somoza GM, Macchi GJ. Aggregations and reproductive events of the narrownose smooth-hound shark (Mustelus schmitti) in relation to temperature and depth in coastal waters of the south-western Atlantic Ocean (38–42°S). Mar Freshw Res 2017; 68:732–742. [Google Scholar]
- [85]. Mull CG, Lowe CG, Young KA. Seasonal reproduction of female round stingrays (Urobatis halleri): Steroid hormone profiles and assessing reproductive state. Gen Comp Endocrinol 2010; 166:379–387. [DOI] [PubMed] [Google Scholar]
- [86]. Dharmadi F, White WT. Species composition and aspects of the biology of Orectolobiformes from Indonesian waters. J Fish Biol 2015; 86:484–492. [DOI] [PubMed] [Google Scholar]
- [87]. Bansemer CS, Bennett MB. Sex- and maturity-based differences in movement and migration patterns of grey nurse shark, Carcharias taurus, along the eastern coast of Australia. Mar Freshw Res 2011; 62:596–606. [Google Scholar]
- [88]. Smale MJ, Cliff G, Dicken ML, Booth AJ. Spatial and seasonal distribution patterns of juvenile and adult raggedtooth sharks (Carcharias taurus) tagged off the east coast of South Africa. Mar Freshw Res 2007; 58:127–134. [Google Scholar]
- [89]. Gervais CR, Nay TJ, Renshaw G, Johansen JL, Steffensen JF, Rummer JL. Too hot to handle? Using movement to alleviate effects of elevated temperatures in a benthic elasmobranch, Hemiscyllium ocellatum. Mar Biol 2018; 165:162. [Google Scholar]
- [90]. Bangley CW, Paramore L, Shiffman DS, Rulifson RA. Increased abundance and nursery habitat use of the bull shark (Carcharhinus leucas) in response to a changing environment in a warm-temperate estuary. Sci Rep 2018; 8:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]


