PURPOSE
Increasingly broad patient groups are being treated with immune checkpoint inhibitors (ICIs) in clinical practice, but few studies have assessed their usage and outcomes in large, comprehensive real-world cohorts. We identified patients who received ICIs in the Veterans Affairs (VA) health care system and described patient characteristics and survival outcomes across multiple indications.
METHODS
We conducted a retrospective analysis using electronic health record data from VA facilities nationwide. Overall survival (OS) from time of ICI initiation for key indications was estimated by Kaplan-Meier. We also stratified OS by frailty status, as defined by a surrogate index developed in VA data. For select indications, we further compared outcomes to historic and concurrent control patients treated with standard-of-care regimens at the VA.
RESULTS
We identified 11,888 patients who were treated with ICIs and determined the cancer type and indication for which they were treated. The cohort is enriched for patient groups that are under-represented in pivotal clinical trials (PCTs), including older, non-White, and/or higher disease burdened patients. Generally, OS observed in the VA cohort is lower than that reported in PCTs. After stratifying VA patients by frailty status, OS among nonfrail patients is more similar to OS reported in PCTs for some indications. Compared with internal VA control cohorts, patients treated with ICIs generally exhibited longer OS for all indications considered.
CONCLUSION
This study describes ICI outcomes across multiple tumor types in a real-world population at the VA. For most indications, real-world survival outcomes are observed to be lower than those reported in PCTs, but patients receiving ICIs still achieve longer survival relative to patients receiving standard of care.
INTRODUCTION
The introduction of immune checkpoint inhibitors (ICIs) is one of the most important developments in cancer therapy in the past decade. Mobilizing the immune system to reverse active immune-suppressive mechanisms by targeting CTLA4, PD-1, or PDL-1 has produced remarkable antitumor effects. This has changed the landscape of cancer therapy and drug development through the approval of seven different agents in a wide range of indications, based on improved overall survival (OS), progression-free survival, or objective response rates.1-5 More than 3,000 clinical trials of ICIs alone or ICIs combined with other modalities, including radiation, chemotherapy, targeted, or other immunotherapy agents, have been completed or are ongoing, exploring essentially all types and settings of cancer.6
CONTEXT
Key Objective
To describe outcomes of patients treated with immune checkpoint inhibitors (ICIs) across multiple indications in a large real-world population at the Veterans Affairs (VA) health care system.
Knowledge Generated
The cohort of patients treated with ICIs at the VA is enriched for groups who are under-represented in pivotal clinical trials (PCTs), including older, non-White, and higher disease burdened patients. Overall survival (OS) is generally lower than that reported in PCTs, but OS among non-frail patients is more similar to that reported in PCTs. Also, VA patients receiving ICIs generally achieve significantly longer survival compared to VA patients receiving prior standard of care.
Relevance
To our knowledge, this is the most comprehensive real-world cohort of patients treated with ICIs that has been reported on. This population of patients is diverse, including older patients and patients with comorbidities who would likely be excluded from many clinical trials.
Rapid uptake into clinical use has followed, resulting in expansion to patients who do not meet the inclusion/exclusion criteria of the pivotal clinical trials (PCTs) that led to regulatory approval. Patients receiving ICIs in real-world practice may differ from those enrolled in trials in a variety of ways, including age, race, performance status, and comorbidity burden. Although such an expansion in clinical use is not uncommon for novel cancer therapies, it may be especially prominent for ICIs, given their acceptable toxicity profile relative to other therapies and their extraordinarily favorable long-term outcomes for certain patients.6,7 Empirical studies of the real-world use of ICIs have estimated that 35%-70% of patients fail to meet strict criteria of PCTs, with recent studies indicating that 55% of patients with melanoma9 and 70% of patients with non–small-cell lung cancer (NSCLC) would not satisfy eligibility.10 ICIs are also used for diverse purposes in real-world practice, including off-label usage on the basis of preliminary publications or national guidelines, such as for palliative or maintenance therapy or as an alternative for patients who would not be able to tolerate standard-of-care therapies.11-13
These discrepancies between real-world practice and clinical trials prompt a need to better understand which patients are receiving ICIs in clinical practice and the effectiveness of ICIs in such patients.14 Currently, outside of the results from PCTs, there is scarce evidence available to inform oncologists who face decisions on whether to prescribe or withhold ICIs and patients who face the financial burden of paying for ICI agents. Real-world data can help bridge this gap, notwithstanding nontrivial challenges in the analysis and interpretation of such data when treatments are nonrandomly assigned and the data are passively collected from sources not intended for research.15-17 Studies exploring the use of real-world data have found encouraging results regarding the consistency of real-world end points with one another and with clinical trial outcomes.18
A number of studies have begun to report and evaluate real-world usage and outcomes in patients treated with ICIs. Some early articles analyzed small cohorts of < 100 patients treated at specific locations and reported their outcomes.19,20 An analysis of data from community cancer care clinics considered real-world outcomes of 1,344 patients with NSCLC treated with PD-1 inhibitors found that the real-world cohort were older at treatment initiation and had shorter OS than those in clinical trials.21,22 An analysis of an even larger cohort of 5,257 patients with advanced NSCLC found there was rapid uptake of first-line ICI use and that patients with greater PDL-1 percentage staining experienced better outcomes.23 Another analysis of 1,256 older patients with NSCLC from SEER data discovered that patients receiving ICIs tended to have multiple comorbidities.24 Although these studies have provided timely data on the real-world experience, few offer a comprehensive overview of patient experience across multiple cancer types or have data on large samples with long-term follow-up. Most existing studies also have not considered assessment of outcomes relative to internal control patient groups from the same patient population receiving standard-of-care regimens without ICIs.
In this study, we describe ICI usage and outcomes in the Veterans Affairs (VA) system. The VA is one of the largest integrated health care systems in the United States, providing care to more than 9 million patients annually.25 The VA serves a population with patients who are frequently under-represented in clinical trials, including patients who are older, more frail, and more racially heterogeneous than those in typical clinical trials. In addition, the VA operates an electronic health record system (EHR) whose records are aggregated in a centralized database, enabling an up-to-date study in this real-world population. Our specific goals were as follows: (1) describe the characteristics of patients using ICIs at the VA and track the uptake of ICIs, (2) describe OS for key indications, (3) assess whether a surrogate marker of patient frailty explains differences in OS between results from the VA and PCTs, and (4) compare outcomes to those observed in historic and concurrent controls treated with previous standard-of-care regimens within the VA. In addition, this work is intended to establish a platform for additional real-world evidence studies in ICI usage and outcomes in the future.
METHODS
Patients
We conducted a retrospective study using data from the VA Corporate Data Warehouse (CDW), which centralizes EHR data for patients seen at VA facilities nationwide. Patients receiving ICIs were identified by checking whether they had any pharmacy or order records for atezolizumab, avelumab, cemiplimab, durvalumab, ipilimumab, nivolumab, or pembrolizumab up until April 2019, with follow-up extended to March 2020. We inferred the cancer type for which each patient was treated with ICIs on the basis of a combination of rules applied to data on patients’ International Classification of Diseases (ICD) diagnosis codes, treatment records, and VA Central Cancer Registry (VACCR) data where available (Data Supplement), and validated this algorithm on the basis of 1,000 randomly sampled patients (Data Supplement). Treatment indications were defined based on the inferred cancer type and prior therapy (Data Supplement). Patients who did not have any ICD diagnosis codes for cancer or for whom the cancer type remained ambiguous after applying the cancer type definitions were excluded. Patients with the date of last follow-up or date of death before initiation of the first ICI treatment were also excluded. This study was performed under a protocol approved by the VA Boston Healthcare System Research and Development Committee.
To understand and interpret outcomes in real-world patients relative to standard-of-care regimens assessed in PCTs, we defined control cohorts using pharmacy data and the VACCR to infer the diagnosis associated with standard-of-care regimens (Data Supplement). Both concurrent and historic control cohorts were defined to mitigate different sources of bias. The concurrent cohort allows for comparison of outcomes with patients receiving treatments in the same time period as patients receiving ICIs to mitigate biases from trends in outcomes over time due to changes in treatment practice. On the other hand, the historic cohort reduces biases associated with selection of ICI versus standard-of-care regimens, because ICIs were not yet available. Specifically, historic controls were defined as patients in the VACCR receiving standard-of-care therapy before the date of first use of ICI treatment of that indication at the VA, and concurrent controls were patients who received standard-of-care therapy on or after that date. On balance, the historic cohort may have less overall bias, so we only show data on the historic cohort in the main text, and data on the concurrent cohort are shown in the Data Supplement. In addition, we did not show results when the control arm had < 20 patients. In comparative analyses of patients receiving ICIs versus standard of care, we also limited the ICI cohorts to patients who had records in the VACCR and reclassified their cancer types using the same rules as for the control cohorts, to increase the comparability of the cohorts.
Study Variables and Outcome
Patients’ demographics, including age at time of treatment indication, race, sex, rurality, and region of initial treatment facility, were ascertained based on structured data from the CDW. The Charlson Comorbidity Index (CCI)26 was calculated using ICD diagnosis codes in the year before treatment initiation. To assess whether outcomes differ between patients with different degrees of vulnerability, we calculated a surrogate for patient frailty on the basis of diagnostic and procedure codes from the EHR using the VA Frailty Index (VA-FI).27 Date of death was defined based on mortality records available in the CDW, integrating data from Medicare, Social Security Administration, VA facilities, National Cemetery Administration, and death certificates.28 Time to mortality for each patient was defined to be the time from treatment initiation (ICI or standard of care) to date of mortality. Follow-up time was the time between treatment initiation and the date of the last “workload” or “fee basis” visit.
Statistical Analysis
Baseline characteristics detailing patients’ demographics and treatment characteristics were summarized by cancer type. OS was estimated separately using Kaplan-Meier curves by indication, for key indications in which results from PCTs are available from published literature. Survival rates at different time points were abstracted from Kaplan-Meier curves of publications from corresponding PCTs and displayed alongside the OS as a reference. To understand how outcomes potentially vary among patients with different degrees of frailty, we further stratified the Kaplan-Meier analyses by VA-FI status: nonfrail (VA-FI < 0.2) and frail (≥ 0.2),27 We include finer-grained VA-FI categories and CCI in the Data Supplement
In addition to describing outcomes, we also compared survival for patients receiving ICI for key indications to patients who received standard-of-care treatment of the same indication. We estimated the OS for patients treated with ICIs and the control patients by Kaplan-Meier and tested for differences between ICIs with either concurrent or historic control groups using two-sided log-rank tests. In each comparison, we limited the non-ICI arm to only those patients who had received the treatment administered in the control arm of the relevant randomized PCT, and both ICI and non-ICI arms were required to match prior treatment criteria from the specific trial, mitigating potential immortal time bias. For instance, in our treatment comparison for patients with NSCLC treated with second or subsequent line (2L+), we compared patients receiving pembrolizumab or nivolumab in the post-platinum setting to those who received docetaxel in the post-platinum setting, just as in the PCT.2
To describe trends in survival over time, we also estimated OS among historic controls stratified by year of first treatment. Univariate and multivariate Cox models were separately fit by indication to estimate hazard ratios (HRs) for ICI versus standard of care with respect to time to mortality, adjusting for age, sex, race, ethnicity, VA-FI, and body mass index at initiation in the multivariate models. An additional multivariate model with comorbidities from the R comorbidity package is also included.26 Two-sided P values based on the Wald test were calculated to test against the null hypothesis that the HR does not differ from 1.
RESULTS
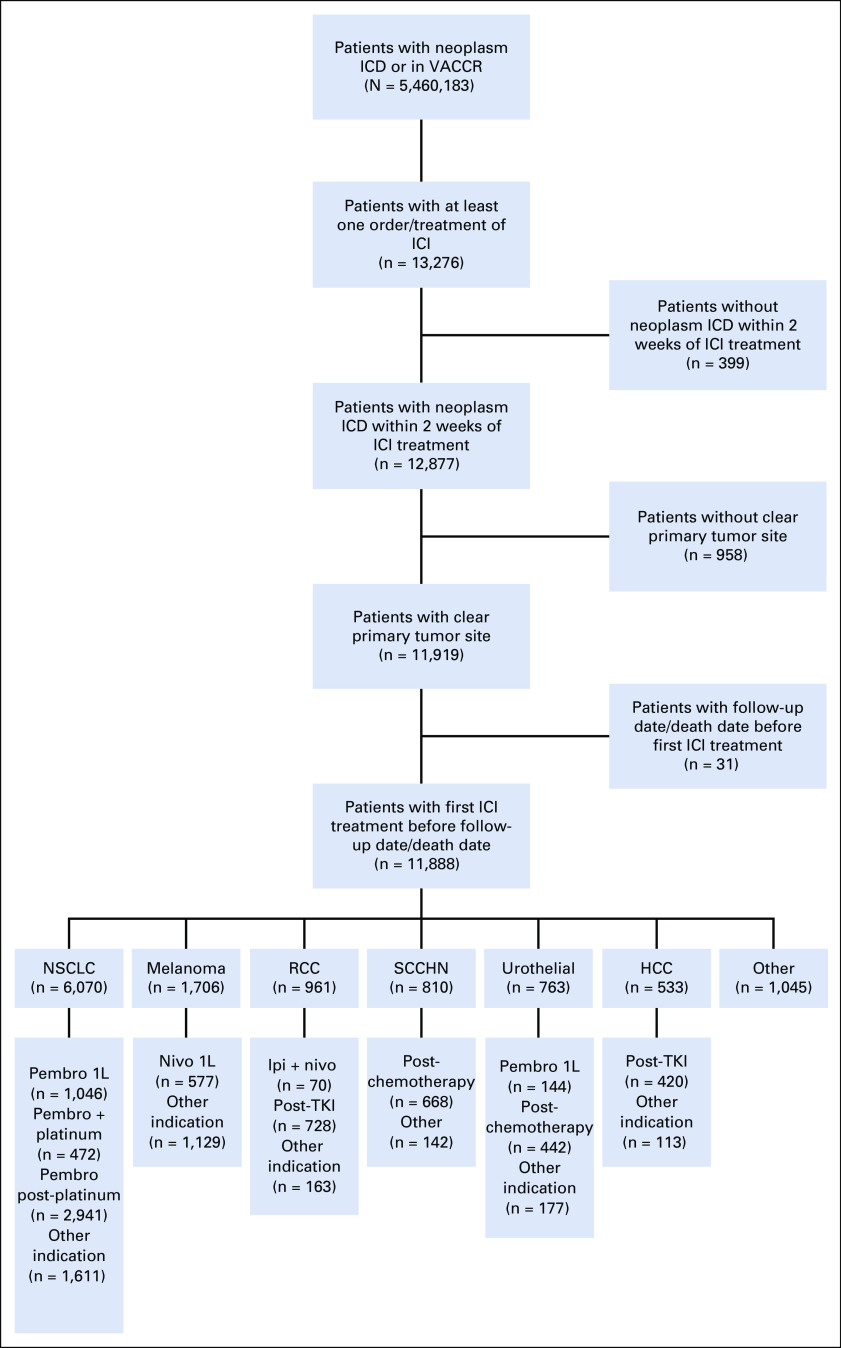
We identified 11,888 patients treated with ICIs who meet our inclusion criteria (Fig 1). The majority of patients were treated for NSCLC (6,070 patients, 51.1% of ICI cohort), followed by melanoma (1,706, 14.4%), renal cell carcinoma (RCC; 961, 8.1%), squamous cell carcinoma of the head and neck (810, 6.8%), urothelial cancer (763, 6.4%), hepatocellular carcinoma (533, 4.5%), and a number of less-common cancer types (1,045, 8.8%). In many cases, the use of ICI initiated quickly after approval and in some cases even before approval, likely through either participation of patients in clinical trials, off-label use, peer-reviewed publication, or presentation at major oncology meetings (see treatment start year in Table 1).
FIG 1.
CONSORT diagram. 1L, first line; HCC, hepatocellular carcinoma; ICD, International Classification of Diseases; ICI, immune checkpoint inhibitor; ipi, ipilimumab; nivo, nivolumab; NSCLC, non–small-cell lung cancer; pembro, pembrolizumab; RCC, renal cell carcinoma; SCCHN, squamous cell carcinoma of the head and neck; TKI, tyrosine kinase inhibitor; VACCR, Veterans Affairs Central Cancer Registry.
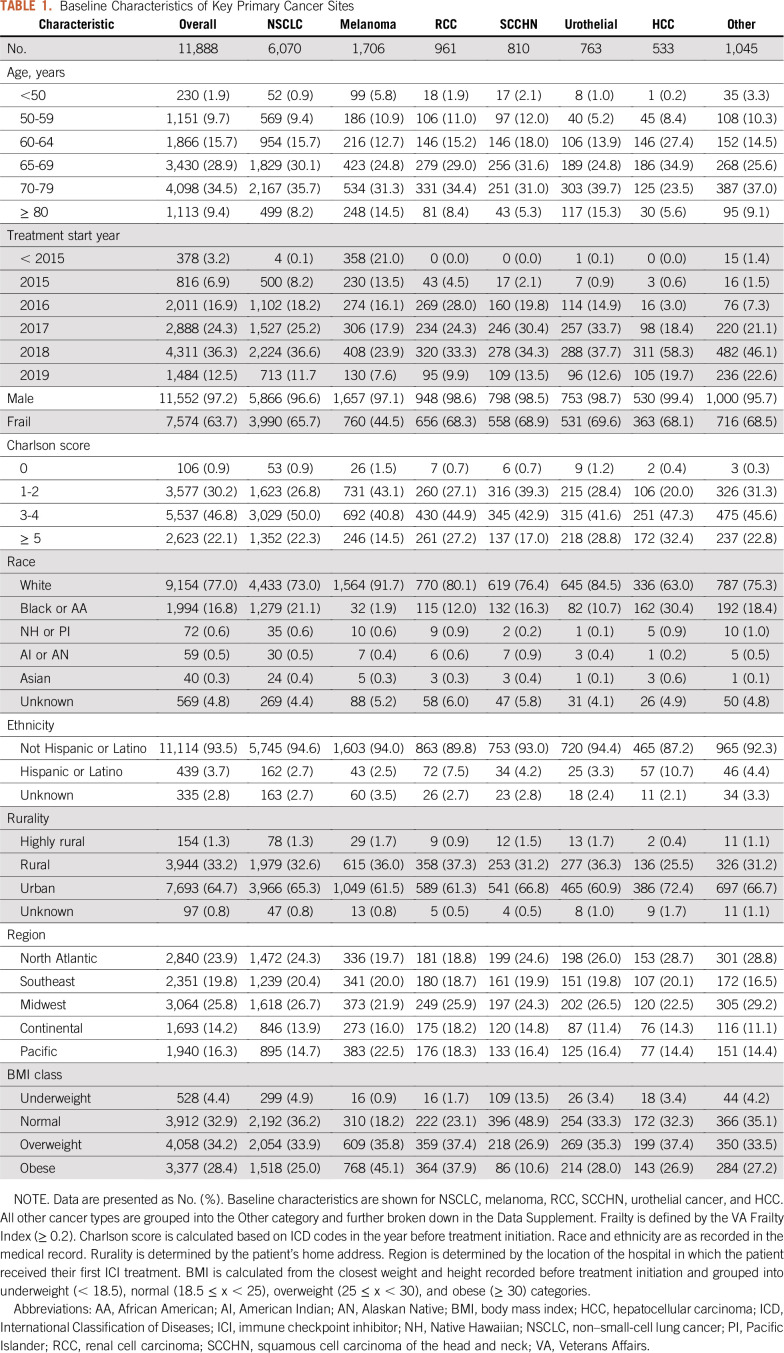
TABLE 1.
Baseline Characteristics of Key Primary Cancer Sites
Patients receiving ICIs at the VA were generally older adults (Table 1; mean age at treatment initiation, 69 years) and male (97.2%). The cohort included a substantial number of African American patients (16.8%), in addition to White patients (77.0%). Most patients initiated treatment with ICIs in 2016-2019 after the anti–PD-1 inhibitors were first approved. Patients treated with ICIs had substantial comorbidities (68.9% with CCI of ≥ 3), and a majority of patients were considered frail using the VA-FI (63.7% frail). The majority of patients were also overweight or obese (62.6%). Patients treated with ICIs were found throughout all regions of the United States, and the majority of patients resided at urban addresses (64.7%). Additional summaries of patient characteristics for patients in other cancer types are reported in the Data Supplement.
Figure 2 shows OS for nine treatment indications. Patients treated with first-line (1L) nivolumab for melanoma exhibited the longest survival, having a median survival of 25.5 months after ICI initiation. Conversely, patients treated with 2L+ ICIs for urothelial cancers had the worst outcomes, with a median survival of 6.7 months after ICI initiation. With the exception of patients treated with 1L nivolumab for melanoma and 2L+ pembrolizumab or nivolumab for NSCLC, patients receiving ICIs appear to have worse survival than patients in the corresponding PCTs, which is not unexpected, given that patients in these cohorts tend to be older and have more comorbidities.
FIG 2.
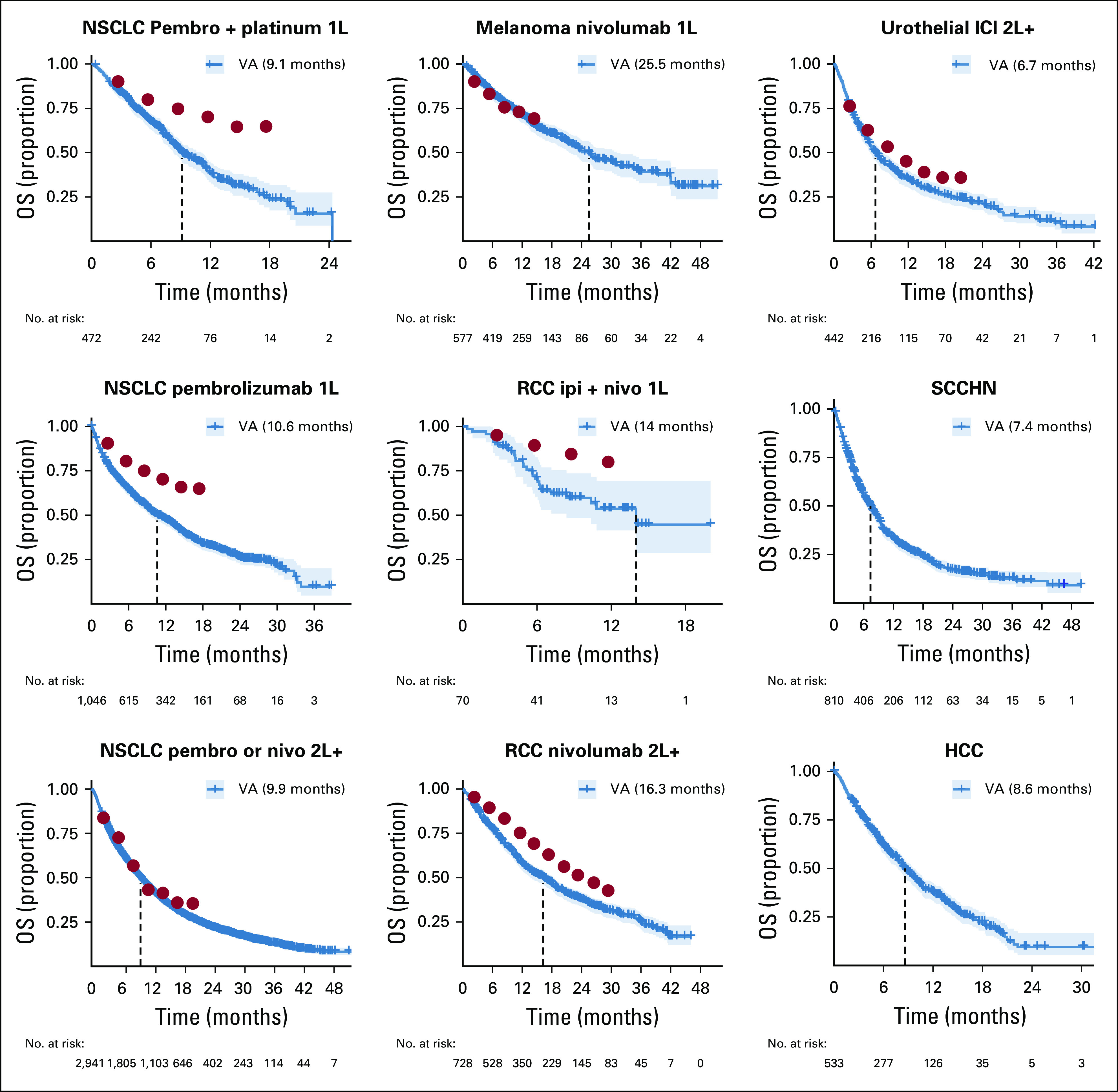
Overall survival (OS) by indication. Median survival is represented by the dashed lines and reported in the legend. Solid red circles represent estimates of survival probabilities from the corresponding pivotal clinical trials extracted using the Engauge Digitizer software. 1L, first line; 2L+, second or subsequent line; HCC, hepatocellular carcinoma; ICI, immune checkpoint inhibitor; ipi, ipilimumab; nivo, nivolumab; NSCLC, non–small-cell lung cancer; pembro, pembrolizumab; RCC, renal cell carcinoma; SCCHN, squamous cell carcinoma of the head and neck; VA, Veterans Affairs.
When OS results are stratified by frailty status as defined by the VA-FI (Fig 3), nonfrail patients tend to have higher rates of survival that are closer to those reported in PCTs than frail patients. For patients treated with 2L+ ICIs for urothelial cancer, nonfrail patients appear to have comparable survival to those in the corresponding PCT. At the same time, there was less differentiation of survival between frail and nonfrail patients treated with 1L pembrolizumab and platinum chemotherapy for NSCLC and 2L+ nivolumab for RCC. As PCTs are frequently restricted to patients with good performance status and organ function, surrogate markers such as the frailty status may help explain some of the differences with the trial results for select indications. Similar results are observed when stratifying on VA-FI with finer-grained categories (Data Supplement) and when stratifying on the Charlson score (Data Supplement). In contrast, stratifying the analyses by age did not appear to differentiate the survival as much as frailty status (Data Supplement), nor does stratification by sex, though results are difficult to interpret because of the small number of female patients (Data Supplement).
FIG 3.
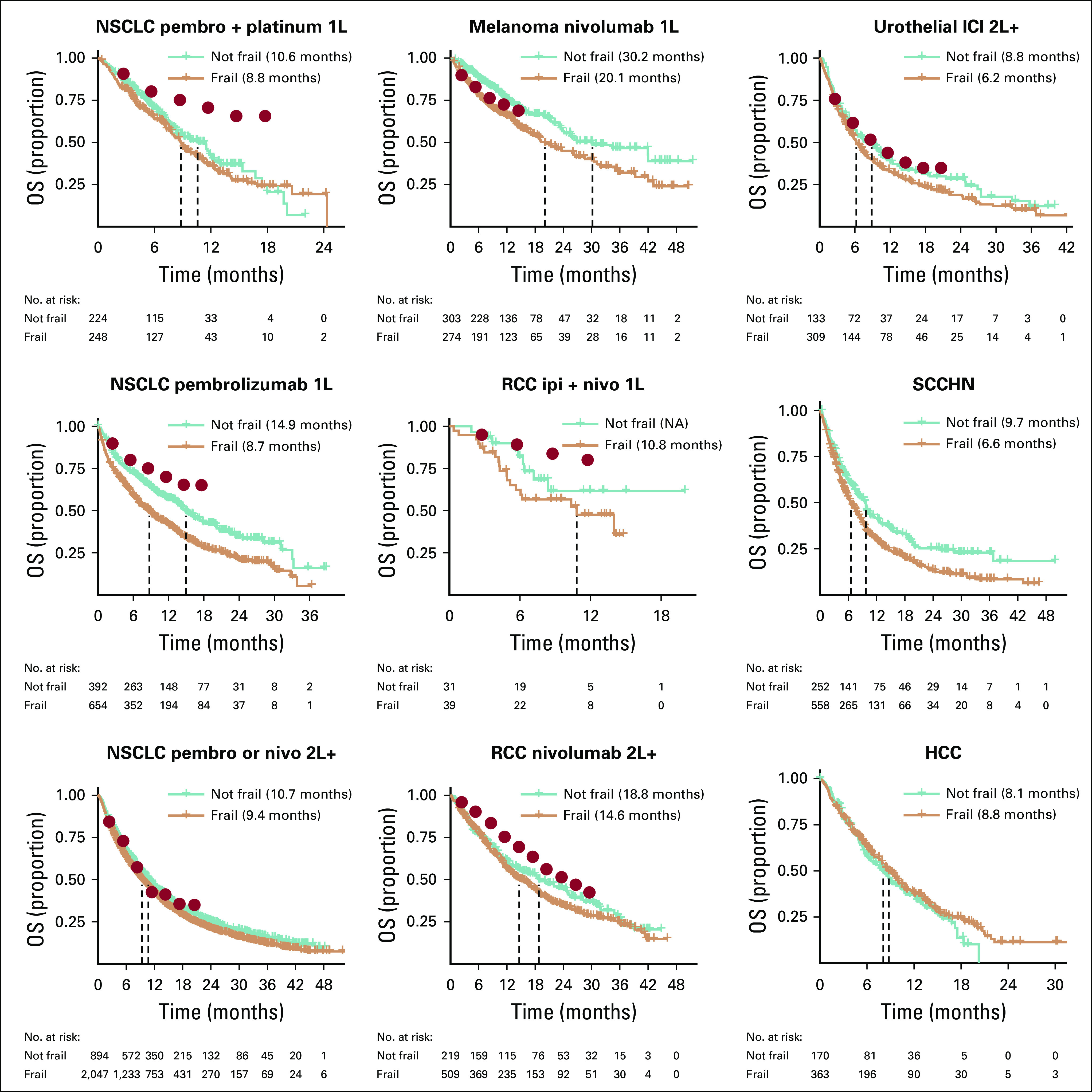
Overall survival (OS) by indication and frailty status. Median survival is represented by the dashed lines and reported in the legend. Solid red circles represent estimates of survival probabilities from the corresponding pivotal clinical trials extracted using the Engauge Digitizer software. 1L, first line; 2L+, second or subsequent line; HCC, hepatocellular carcinoma; ICI, immune checkpoint inhibitor; ipi, ipilimumab; nivo, nivolumab; NSCLC, non–small-cell lung cancer; pembro, pembrolizumab; RCC, renal cell carcinoma; SCCHN, squamous cell carcinoma of the head and neck.
For the select indications in which we defined control cohorts, we observed that patients receiving ICIs exhibited significantly longer survival than historic controls (Fig 4). Survival curves stratified by year of treatment initiation among historical controls did not show any clear temporal trends over the time period we consider in the analysis (Data Supplement). When comparing ICIs with concurrent controls, patients receiving ICIs had significantly longer survival in all indications except 1L RCC and 2L+ urothelial indications (Data Supplement). For most indications, there was early separation of survival curves for patients receiving ICIs relative to controls. One exception was pembrolizumab monotherapy, which exhibited a characteristic ICI plateau, with delayed separation of the estimated survival curve relative to chemotherapy (Fig 4).
FIG 4.
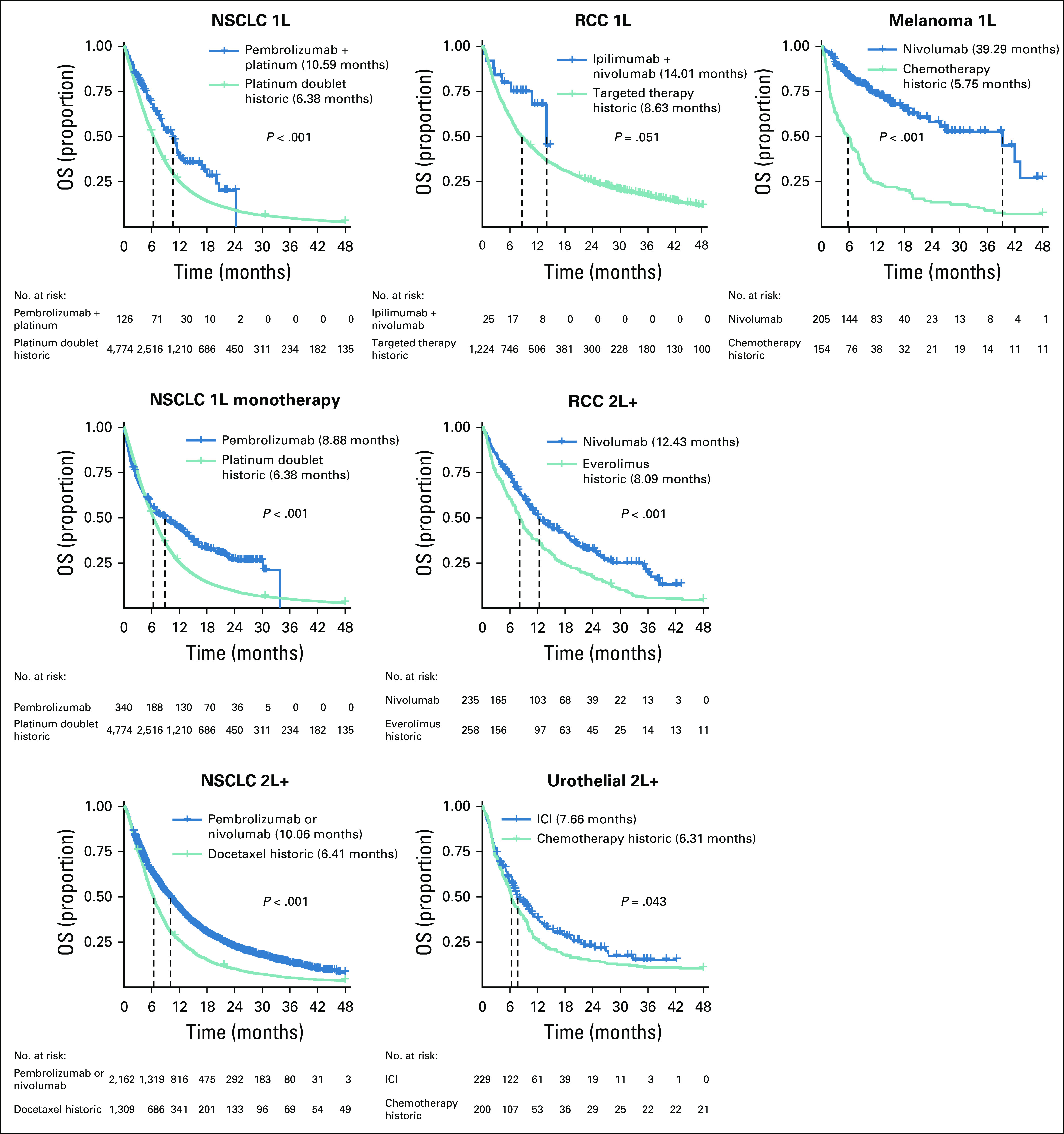
Treatment comparisons of immune checkpoint inhibitors (ICIs) versus historic controls. Median survival is represented by the dashed lines and reported in the legend. 1L, first line; 2L+, second or subsequent line; OS, overall survival; NSCLC, non–small-cell lung cancer; RCC, renal cell carcinoma.
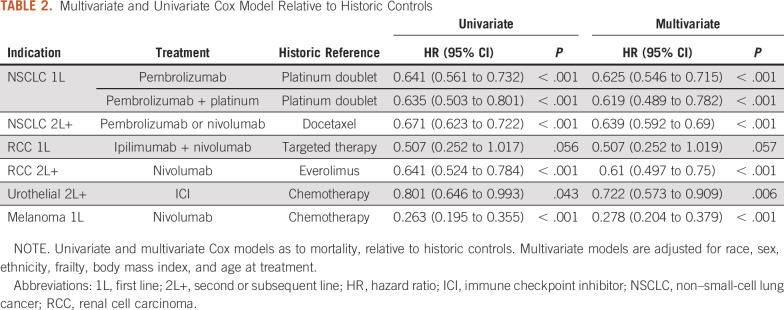
Last, we estimated HR for ICI versus previous standard-of-care treatments through Cox models. The HR estimates (Table 2 for historic controls, Data Supplement for concurrent controls) indicate that patients receiving 1L ICIs for melanoma experienced the greatest differences in survival relative to patients receiving the standard of care (adjusted HR, 0.278 v historic, with P < .001; and 0.127 v concurrent controls, with P = .006). There was also a relatively large difference in survival for patients receiving 2L+ nivolumab for RCC compared with patients receiving everolimus in the concurrent controls (adjusted HR, 0.329 v concurrent controls; P < .001). Similarly to what we observe in the Data Supplement, there was no significant difference in survival between corresponding concurrent controls compared with RCC 1L ipilimumab + nivolumab or urothelial 2L+ ICIs. For other indications, patients treated with ICIs had approximately 30%-50% reductions in the HR relative to concurrent and historic controls after adjustment. In a larger model adjusting for a number of additional comorbidities (Data Supplement), similar HR estimates are observed.
TABLE 2.
Multivariate and Univariate Cox Model Relative to Historic Controls
DISCUSSION
In this article, we described ICI use and outcomes in a large cohort of 11,888 patients treated with ICIs at the VA across multiple cancer types and treatment indications treated. To our knowledge, this is the most comprehensive real-world cohort of patients treated with ICIs that has been reported on. This population of patients is diverse, including older patients and patients with comorbidities who would likely be excluded from many ICI trials. The VA population also includes substantial numbers of African American patients and patients living in rural regions, who are frequently under-represented in clinical research. As such, these data constitute a unique resource for assessing real-world outcomes among a diverse patient population. Use of ICIs increased quickly at the VA after the approval of ICIs for most indications. This is consistent with previous analyses of ICI uptake among patients with NSCLC, which had also found that ICI use rapidly increased after approval.20,21
Our survival analysis focused on nine key indications with long-term follow-up and is more comprehensive than many previous studies of ICI therapies, which have been mostly limited to one or two cancer types or have shorter follow-up duration. As with other real-world studies of novel oncology therapies,22 the analysis of survival outcomes in ICI-treated patients showed that real-world outcomes tended to be worse than those observed in clinical trials.
A number of factors may contribute to shorter survival in the VA cohort, including both characteristics of the VA population as well as potential bias and confounding arising from use of EHR data. The VA cohort is predominantly male, a potential source of bias that needs to be taken into account when comparing with other studies. In our population, the number of females is small, so it is difficult to draw conclusions (Data Supplement), but we cannot rule out that survival could be longer in females, and this could partially explain reduced OS in the VA ICI-treated population compared with landmark trials. Similarly, the fact that the VA population is older and has a higher degree of comorbidity than in clinical trials likely plays a role in observed differences. Additional issues arise because of current limitations of our EHR-based dataset compared with prospectively collected data. We do not presently have data at hand to determine the cause for discontinuation of therapy, so we were unable to distinguish those who experienced progression, those who stopped because of not tolerating therapy, and those who stopped for some other reason. In addition, in some indications such as first-line pembrolizumab monotherapy for NSCLC, patients receiving ICI at the VA may include those receiving it for palliative purposes when chemotherapy is not deemed suitable, and these patients are expected to have worse outcomes. Last, for 2L+ indications, VA patients may be receiving ICIs after experiencing failure of multiple previous lines of therapy, whereas trials may be limited to patients who have had only one or two previous lines of therapy.
When stratifying the analyses by frailty, nonfrail patients had outcomes more similar to those observed in PCTs. This may be explained at least in part by the fact that nonfrail patients are likely to have better performance status and lower degree of comorbidities, similar to patients in PCTs. This suggests that a marker such as frailty can be used to explain some of the differences in outcome between the VA population and those enrolled in PCTs, as well as stratify within the VA population, but additional work is needed to assess this marker’s prognostic value and whether this finding might help better explain variation in prior non-VA studies of ICI real-world use.
The generally favorable survival outcome observed among patients receiving ICIs relative to both historic and concurrent controls suggests a treatment benefit for ICIs. These differences in outcomes persisted after adjusting for demographics in a multivariate Cox model for most indications. Neither concurrent nor historic controls of prior standard of care yield a perfectly reliable comparison. However, the fact that we did not observe any trend toward better outcomes under standard of care in the years leading to the uptake of ICI suggests that the superior outcomes with ICI genuinely reflect the advances observed in PCTs.
Our study’s comprehensive approach to ICI therapy and the incorporation of controls has allowed analysis of several of the most common treatment scenarios involving ICIs. This work also establishes a platform for additional up-to-date analysis of ICI outcomes and optimization of usage in real-world practice, which paves the way for future studies to help address unanswered questions involving ICIs, including study of less-common tumor types, real-world adverse event profiles, and identification of predictive covariates implicated in enhanced survival outcomes.
EQUAL CONTRIBUTION
D.T. and N.R.F. contributed equally.
SUPPORT
Supported by the VA Office of Research and Development, Cooperative Studies Program.
DISCLAIMER
The views expressed are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: Jennifer La, David Cheng, David Tuck, Nathanael R. Fillmore
Data analysis and interpretation: Jennifer La, David Cheng, David Tuck, Nathanael R. Fillmore
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/cci/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
David Cheng
Honoraria: Biogen
Consulting or Advisory Role: Blue Null Consulting Group
No other potential conflicts of interest were reported.
REFERENCES
- 1.Reck M Rodríguez-Abreu D Robinson AG, et al. : Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375:1823-1833, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Herbst RS Baas P Kim D-W, et al. : Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 387:1540-1550, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Bellmunt J de Wit R Vaughn DJ, et al. : Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 376:1015-1026, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motzer RJ Tannir NM McDermott DF, et al. : Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 378:1277-1290, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolchok JD Chiarion-Sileni V Gonzalez R, et al. : Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 377:1345-1356, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. National Institutes of Health: ClinicalTrials.gov. https://clinicaltrials.gov/
- 7.McDermott D Lebbé C Hodi FS, et al. : Durable benefit and the potential for long-term survival with immunotherapy in advanced melanoma. Cancer Treat Rev 40:1056-1064, 2014 [DOI] [PubMed] [Google Scholar]
- 8. Reference deleted.
- 9.Donia M Kimper-Karl ML Høyer KL, et al. : The majority of patients with metastatic melanoma are not represented in pivotal phase III immunotherapy trials. Eur J Cancer 74:89-95, 2017 [DOI] [PubMed] [Google Scholar]
- 10.Yoo SH Keam B Kim M, et al. : Generalization and representativeness of phase III immune checkpoint blockade trials in non-small cell lung cancer. Thorac Cancer 9:736-744, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis MP, Panikkar R: Checkpoint inhibitors, palliative care, or hospice. Curr Oncol Rep 20:2, 2018 [DOI] [PubMed] [Google Scholar]
- 12.Hansen ED Wang X Case AA, et al. : Immune checkpoint inhibitor toxicity review for the palliative care clinician. J Pain Symptom Manage 56:460-472, 2018 [DOI] [PubMed] [Google Scholar]
- 13.Parikh RB Feld EK Galsky MD, et al. : First-line immune checkpoint inhibitor use in cisplatin-eligible patients with advanced urothelial carcinoma: A secular trend analysis. Future Oncol 16:4341-4345, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhai Y Ye X Hu F, et al. : Endocrine toxicity of immune checkpoint inhibitors: A real-world study leveraging US Food and Drug Administration adverse events reporting system. J Immunother Cancer 7:286, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corrigan-Curay J, Sacks L, Woodcock J: Real-world evidence and real-world data for evaluating drug safety and effectiveness. JAMA 320:867-868, 2018 [DOI] [PubMed] [Google Scholar]
- 16.Miksad RA, Abernethy AP: Harnessing the power of real-world evidence (RWE): A checklist to ensure regulatory-grade data quality. Clin Pharmacol Ther 103:202-205, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dickerman BA García-Albéniz X Logan RW, et al. : Avoidable flaws in observational analyses: An application to statins and cancer. Nat Med 25:1601-1606, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. doi: 10.1200/CCI.18.00155. Stewart M, Norden AD, Dreyer N, et al: An exploratory analysis of real-world end points for assessing outcomes among immunotherapy-treated patients with advanced non-small-cell lung cancer. JCO Clin Cancer Inform 10.1200/CCI.18.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brustugun OT, Sprauten M, Helland Å: Real-world data on nivolumab treatment of non-small cell lung cancer. Acta Oncol 56:438-440, 2017 [DOI] [PubMed] [Google Scholar]
- 20.Wen X Ding Y Li J, et al. : The experience of immune checkpoint inhibitors in Chinese patients with metastatic melanoma: A retrospective case series. Cancer Immunol Immunother 66:1153-1162, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khozin S Abernethy AP Nussbaum NC, et al. : Characteristics of real-world metastatic non-small cell lung cancer patients treated with nivolumab and pembrolizumab during the year following approval. Oncologist 23:328-336, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khozin S Carson KR Zhi J, et al. : Real-world outcomes of patients with metastatic non-small cell lung cancer treated with programmed cell death protein 1 inhibitors in the year following US regulatory approval. Oncologist 24:648-656, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khozin S Miksad RA Adami J, et al. : Real-world progression, treatment, and survival outcomes during rapid adoption of immunotherapy for advanced non-small cell lung cancer. Cancer 125:4019-4032, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Youn B Trikalinos NA Mor V, et al. : Real-world use and survival outcomes of immune checkpoint inhibitors in older adults with non-small cell lung cancer. Cancer 126:978-985, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. US Department of Veterans Affairs: About VHA: Veterans Health Administration. https://www.va.gov/health/aboutVHA.asp.
- 26.Gasparini A: comorbidity: An R package for computing comorbidity scores. J Open Source Softw 3:648, 2018 [Google Scholar]
- 27.Orkaby AR Nussbaum L Ho Y-L, et al. : The burden of frailty among US Veterans and its association with mortality, 2002-2012. J Gerontol A Biol Sci Med Sci 74:1257-1264, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. US Department of Veterans Affairs: Ascertaining Veterans’ vital status: Data sources for mortality ascertainment and cause of death. https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/video_archive.cfm?SessionID=1242.
- 29. Reference deleted.