Abstract
Cognitive impairment is viewed as a core symptom of schizophrenia (SCZ), but its pathophysiological mechanism remains unclear. White matter (WM) disruption is considered to be a central abnormality that may contribute to cognitive impairment in SCZ patients. However, few studies have addressed the association between cognition and WM integrity in never-treated first-episode (NTFE) patients with SCZ. In this study, we used the MATRICS Consensus Cognitive Battery (MCCB) to evaluate cognitive function in NTFE patients (n = 39) and healthy controls (n = 30), and associated it with whole-brain fractional anisotropy (FA) values obtained via voxel-based diffusion tensor imaging. We found that FA was lower in five brain areas of SCZ patients, including the cingulate gyrus, internal capsule, corpus callosum, cerebellum, and brainstem. Compared with the healthy control group, the MCCB’s total score and 8 out of 10 subscores were significantly lower in NTFE patients (all p < 0.001). Moreover, in patients but not healthy controls, the performance in the Trail Making Test was negatively correlated with the FA value in the left cingulate. Our findings provide evidence that WM disconnection is involved in some cognitive impairment in the early course of SCZ.
Subject terms: Neuroscience, Schizophrenia
Introduction
Impaired cognitive function is viewed as a fundamental feature of schizophrenia (SCZ)1–3. Cognitive declines exist in the prodromal stage of psychosis and persist throughout the course of illness regardless of how symptoms change4–6. Patients show different cognitive spectrum deficits including global cognitive functioning, executive functioning, processing speed, working memory, verbal fluency, attention, and social cognition7–9. These impairments influence various dimensions of functional outcome including independent living ability, social functioning, and occupational functioning10, and have more negative impacts on brain function than the clinical symptoms of SCZ11,12. However, as the pathophysiological mechanisms of cognitive impairment in SCZ patients are not clear, adjunctive pharmacological treatments only produce very limited efficacy13,14.
Diffusion tensor imaging (DTI) is a promising approach for measuring microstructural changes with neuropathology, which can quantify the fiber orientation and the white matter (WM) integrity pathways within neural networks15–17. Fractional anisotropy (FA), a common DTI measure of directional dependence of water diffusion, reflects fiber structural integrity, fiber tract coherence, degree of myelination, and packing density in WM18,19. Widespread FA reduction has been observed in several brain regions and fiber tracts in SCZ patients relative to healthy controls20–22. A meta-analysis23 has shown WM tract abnormalities in chronic SCZ, with lower FA in the medial frontal/anterior cingulate cortex, anterior corpus callosum, left temporal WM, and right anterior limb of the internal capsule, indicating disconnections within affected gray matter regions of SCZ patients.
Previous studies about WM FA mainly used patients with chronic SCZ and there were relatively few studies using first-episode SCZ patients24. Yao et al.25. identified consistent WM FA reduction in first-episode patients in the left deep temporal lobe, including the inferior fronto-occipital fasciculus and inferior longitudinal fasciculus. Based on these neuroimaging results, the disconnection hypothesis of SCZ has been put forward, proposing that SCZ arises from functional deterioration of the brain caused by lack of connections between different regions26–28. However, the findings have been inconsistent. Although most previous studies indicated lower FA in first-episode patients29–33, other research failed to replicate the finding34–36 or even found elevated FA37.
WM fiber tracts are the neural architectures supporting high-speed interactions between different regions of the brain, and thus clinical and cognitive symptoms related to SCZ could be attributed to the alterations of WM pathways38. Previous studies have revealed the association between WM connections and cognitive performance in SCZ patients39–43. For example, executive and motor functioning deficits are linked to reduced WM integrity in the temporal and frontal connectivity in first-episode SCZ patients32. Worse visual and verbal learning abilities, as well as processing speed, associate with lower FA values in the left inferior longitudinal fasciculus and left inferior fronto-occipital fasciculus in SCZ38. These findings suggest that disconnectivity of WM tracts may contribute to cognitive deficits in SCZ and provide support for the disconnection hypothesis of SCZ. However, there have also been divergent findings, possibly due to the wide heterogeneity in the study populations and in the imaging methodologies used. In particular, previous DTI studies have mainly evaluated SCZ patients with anti-psychiatric treatment, which is an important confounder and may impact WM microstructure features and brain anatomy44,45.
To our best knowledge, no study has investigated the relationship between voxel-based DTI measures and cognitive performance in never-treated first-episode (NTFE) patients with SCZ. Evaluating NTFE patients has particularly advantages for understanding the neurobiology mechanism of SCZ, which can minimize the confounding effects of drug treatment, disease duration, and comorbidities caused by illness chronicity46. Therefore, our study involved NTFE patients in measuring FA and cognitive function to examine the relationship between WM disruption and cognitive impairment in SCZ.
Methods
Subjects
A total of 39 Chinese Han NTFE in-patients with SCZ were recruited from Beijing Hui-Long-Guan hospital, a largest public psychiatric hospital in Beijing with almost 1400 beds. Patients were assessed at the time of admission and during 3~6 months of follow-up to establish a DSM-IV diagnosis of SCZ. Inclusion criteria for SCZ group were as follows: (1) in an acute episode period and met the diagnostic criteria of SCZ, which was conducted by two independent and experienced psychiatrists using Structured Clinical Interview for DSM-IV; (2) the symptom duration shorter than 60 months; (3) no history of taking either antipsychotic or non-antipsychotic medications; and (4) age between 18 and 40 years old. In addition, in this study, the definition for first episode was first onset of psychotic symptoms. The mean age in patients group was 28.9 ± 10.2 years and the mean illness duration was 26.6 ± 19.3 months (Table 1).
Table 1.
Demographics and cognition of first-episode patient and healthy control subjects.
| Schizophrenia (n = 39) | Control (n = 30) | F/X2 | p | |
|---|---|---|---|---|
| Age | 28.9 ± 10.2 | 27.5 ± 7.9 | 0.39 | 0.54 |
| Gender (male/female) | 16/23 | 13/17 | 0.04 | 0.85 |
| Education | 12.4 ± 3.1 | 12.3 ± 4.0 | 0.03 | 0.87 |
| MCCB | ||||
| MSCEIT | 50.6 ± 10.4 | 52.4 ± 7.7 | 1.02 | 0.32 |
| Category fluency | 52.1 ± 10.8 | 58.0 ± 15.5 | 3.28 | 0.08 |
| Symbol coding | 41.2 ± 9.7 | 65.2 ± 9.3 | 96.2 | <0.001 |
| Trail Making A | 46.5 ± 8.2 | 58.8 ± 8.4 | 32.1 | <0.001 |
| CPT-IP | 40.9 ± 11.1 | 58.7 ± 8.0 | 58.0 | <0.001 |
| Spatial span total | 41.3 ± 15.3 | 62.3 ± 12.4 | 38.8 | <0.001 |
| Digital sequence test | 45.3 ± 11.9 | 61.9 ± 9.2 | 40.3 | <0.001 |
| HVLT-R total | 50.6 ± 10.2 | 63.7 ± 8.9 | 34.9 | <0.001 |
| BVMT-R total | 47.9 ± 9.8 | 60.8 ± 8.9 | 27.6 | <0.001 |
| Mazes (NAB) total | 43.7 ± 10.0 | 64.5 ± 10.0 | 70.4 | <0.001 |
| MCCB total | 44.5 ± 10.6 | 67.4 ± 11.8 | 64.9 | <0.001 |
BVMT-R Brief Visuospatial Memory Test-Revised, CPI-IP Continuous Performance Test-Identical Pairs, HVLT-R Hopkins Verbal Learning Test-Revised, MCCB Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery (MCCB), MSCEIT Mayer–Salovey–Caruso Emotional Intelligence Test, NAB Neuropsychological Assessment Battery.
Thirty healthy controls were recruited by advertising in the local community. The age, gender, and education in control group were matched with the above SCZ group (Table 1). None of subjects in control group had an individual or family history of mental disorders.
We collected all subjects’ medical history and physical examination and laboratory test results through clinical records. Exclusion criteria for all subjects were as follows: (1) with major medical illness; (2) with a history of drug or alcohol abuse/dependence; and (3) without a written informed consent. In addition, all SCZ and control subjects were right-handed.
All subjects gave written informed consent before entering this study. The research proposal was approved by the Institutional Review Committee of Beijing Hui-Long-Guan Hospital.
Cognitive tests
Cognitive functioning was measured by the standardized neurocognitive battery called the “Measurement and Treatment Research to Improve Cognition in Schizophrenia” (MATRICS) or the “MATRICS Consensus Cognitive Battery” (MCCB)47,48. The MCCB includes ten standardized tests measuring functions in seven cognitive domain as follows: (1) speed of processing (Trail Making Test Part A, BACS Symbol Coding Test, Category Fluency Test); (2) attention/vigilance (Continuous Performance Test-Identical Pairs); (3) working memory (Letter-Number Span, Wechsler Memory Scale Spatial Span); (4) verbal learning (Hopkins Verbal Learning Test); (5) visual learning (Brief Visuospatial Memory Test); (6) reasoning and problem solving (Neuropsychological Assessment Battery Mazes); (7) social cognition (Mayer–Salovey–Caruso Emotional Intelligence Test (MSCEIT) Managing Emotions Branch). The MCCB provides a score for each cognitive domain as well as a composite score derived from the seven domain scores. In this study, the Chinese version of the MCCB49 was employed in all subjects.
Psychopathological assessment
Two trained experienced psychiatrists evaluated psychopathological symptoms in each patient using the Positive and Negative Syndrome Scale (PANSS), with an inter-evaluator reliability being greater than 0.8 in terms of the total score.
Imaging acquisition and analysis
DTI data were acquired by a 3.0 Tesla General Electric scanner using an eight-channel phased array coil and a single-shot echo-planar imaging sequence. Scanning parameters were as follows: repetition time, 13,525 ms; echo time, 77.3 ms; slices, 50; thickness, 2.4 mm; gap, 2.4 mm; matrix size, 128 × 128; field of view, 256 × 256 mm2; b-value, 1000 s/mm2; 19 gradient directions; two averages. The b = 0 images were scanned three times. The preprocessing was performed applying FSL (FMRIB’s Software Library) freely available at (http://www.fmrib.ox.ac.uk/). First, with the FMRIB’s Diffusion Toolbox, FA images were from the DTI data constructed by fitting a tensor model to raw images. Brain extraction was subsequently conducted using the Brain Extraction Tool50. Third, with the FMRIB’s Nonlinear Registration Tool, FA data of all subjects were normalized into Montreal Neurological Institute space, employing the b-spline representation warp field51. Fourth, the normalized images were resampled into 2 × 2 × 2 mm3, producing a standard space version of each FA image. Fifth, a mean FA image (threshold of 0.2)38 was created and thinned to generate a mean FA skeleton representing the centers of all tracts common to the group. The maximum FA value obtained in a direction perpendicular to each tract was subject to each skeleton voxel. Sixth, the aligned FA map of each subject was projected onto this skeleton and the resulting data were fed into voxel-wise cross-subject statistical analyses38. Spatial smoothing was then undertaken using a 6 mm full-width half-maximum Gaussian kernel.
Statistical analysis
Univariate analyses of covariance were performed on the MCCB scores to compare the cognitive performance between SCZ and the control groups, with age, gender, and education as covariates. Group-level analyses were conducted on brain areas with significantly detectable WM abnormalities in SCZ vs. control groups. FA differences were compared between groups by utilizing a parametric two-sample t-test implemented in the Statistical Parametric Mapping 8 software (Wellcome Department of Imaging Neuroscience, London, UK), with age, gender, and education as covariates. Contrasts were carried out to detect between-group FA changes, with a significance threshold of p < 0.05 corrected using false discovery rate (FDR). Pearson’s correlation analyses were performed to examine the influence of demographic features on FA values in the entire sample or healthy controls or patients respectively. The correlations between FA and clinical variables were described in our previous study33 and therefore were not included here. Partial correlations (controlling for age, gender, and education) were calculated between FA values and MCCB scores in the two groups, respectively. Only those mean FA values obtained from the regions with observed group differences were included. Bonferroni correction was used when multiple testing was conducted. Given that 12 components of the MCCB were measured and lower FA were identified in five brain regions, a p-value of 0.00083 (0.05/60) was considered significant. Finally, a stepwise multiple regression analysis was conducted to examine the relationships between cognition indicated by MCCB total and index scores, psychotic symptoms, and FA values in the five brain regions.
Results
Cognitive performance
The total and index scores of MCCB were respectively compared between SCZ patients and controls in Table 1. Except for non-significant differences in the MSCEIT (p = 0.32) and marginally significant differences in category fluency (p = 0.08), the cognitive scores on the MCCB total and other tests were significantly lower in patients than healthy controls (all p < 0.001). These differences remained significant after controlling for age, gender, and education (all p < 0.001).
FA values
Compared with controls, patients displayed an overall lower FA values in five brain regions as follows: the left cingulate gyrus, right internal capsule, right corpus callosum, left cerebellum, and right brainstem (all p < 0.05, FDR corrected; Table 2). These values did not show any significant correlations with subjects’ age, gender, and education either in the entire sample or in healthy controls or in SCZ patients (all p > 0.05). In the patient group, no significant correlation was found between FA values and the age of onset, course of disease, age of hospitalization, and family history of psychosis (all p > 0.05).
Table 2.
Fractional anisotropy (FA) in the brain regions showing differences between schizophrenia and control subjects.
| Fractional anisotropy | |||||
|---|---|---|---|---|---|
| Region | Hemisphere | Schizophrenia | Control | t | p |
| Cingulate gyrus | Left | 0.445 ± 0.028 | 0.463 ± 0.026 | 3.62 | <0.05 |
| Internal capsule | Right | 0.359 ± 0.016 | 0.375 ± 0.013 | 4.13 | <0.01 |
| Corpus callosum | Right | 0.478 ± 0.028 | 0.493 ± 0.020 | 4.14 | <0.01 |
| Cerebellum | Left | 0.279 ± 0.012 | 0.287 ± 0.017 | 3.59 | <0.05 |
| Brainstem | Right | 0.396 ± 0.012 | 0.409 ± 0.013 | 4.14 | <0.01 |
Results were thresholded at false discovery rate (FDR)-corrected p < 0.05. All p < 0.01 after adjusting for age, gender, and education.
Correlations between FA values and cognitive performance
In SCZ patients, the FA value in the left cingulate gyrus was negatively correlated with the Trail Making Test A score (r = −0.53, df = 30, p = 0.003; Fig. 1). After controlling for age, gender, education level, and duration of illness, the correlation remained significant (r = −0.44, df = 25, p = 0.023). However, it did not survive Bonferroni correction (the p-value should be < 0.00083). In the healthy control group, FA did not correlate with any index or the total score of the MCCB (all p > 0.05), probably related to a ceiling effect of MCCB scores and the small sample size.
Fig. 1. The correlation between the fractional anisotropy (FA) value in the left cingulate gyrus and performance in the Trail Making Test in never-treated first-episode patients with schizophrenia.
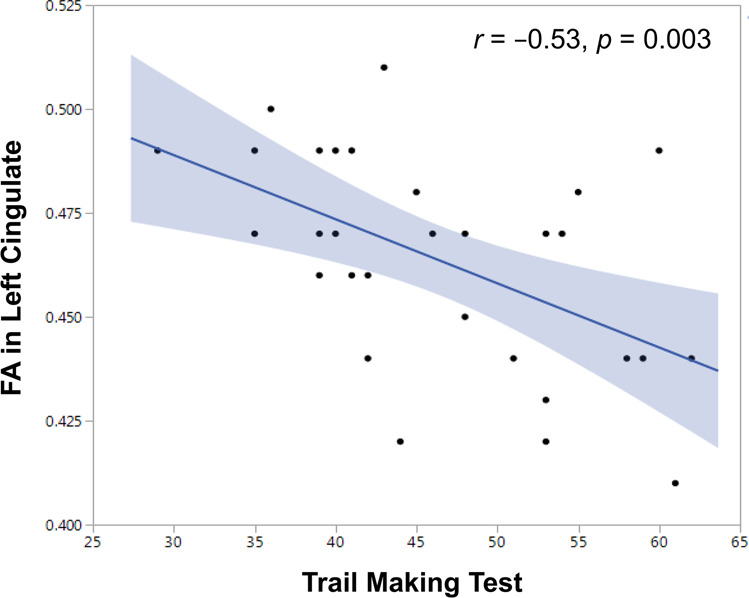
The FA in the left cingulate was negatively associated with the Trail Making Test A index (r = −0.53, df = 30, p = 0.003).
The multiple regression analysis revealed that neither any of the MCCB indices nor its total score was contributed by FA in healthy controls (all p > 0.05). In the patient group, the PANSS positive symptom (β = −0.36, t = −2.17, p = 0.04) and FA in the left cingulate gyrus (β = −0.38, t = −2.24, p = 0.034) were independent contributors to the Trail Making Test index, with adjusted R2 = 0.19. However, FA did not contribute to the other MCCB indices or the total score in this group.
Discussion
To our knowledge, this is the first study to address the association between cognitive function and WM integrity in NTFE patients with SCZ in comparison to healthy individuals. The present study had three main findings. (1) The patients showed significant disruption in WM integrity indicated by widespread reduced FA in five brain regions, involving the cingulate gyrus, internal capsule, corpus callosum, cerebellum, and brainstem, in the early stage of SCZ. (2) The patients displayed widespread neurocognitive impairment, and the total score of the MCCB and almost all of its subscales except for the MSCEIT index were lower than those in the control group. (3) FA in the left cingulate gyrus negatively correlated with the Trail Making Test index in the patient group, suggesting that WM disconnection may be one of the causes of cognitive deficits in SCZ.
Widespread FA reduction in the five brain regions in NTFE patients with SCZ is in line with most previous findings29,31,52, in particular those obtained in first-episode SCZ30,32,33,53,54, which indicates that WM abnormalities already exist at illness onset. There is accumulating evidence, including our current findings, for lower FA in multiple brain regions, suggesting that WM interruptions in SCZ are widespread rather than focal30,55. These findings also support the disconnection hypothesis of SCZ56, suggesting that the widespread disruption of WM integrity might be associated with the pathogenesis of SCZ.
Cognitive performance on the MCCB in NTFE patients with SCZ was overall worse than that in healthy controls, with significant group differences in eight of the ten scores and marginally significant group differences in category fluency. This is consistent with the majority of research on cognition in patients with chronic and first-episode SCZ7–9,57,58, including previous studies in Chinese patients59 as well as those in the United States using the MCCB60,61. Our findings suggest that SCZ patients have neuropsychological damage in the early stage of the disorder. The impairment in cognitive function seems to be an intrinsic characteristic of SCZ and occurs at illness onset.
Furthermore, we found that FA values in the left cingulate gyrus were negatively associated with the Trail Making Test index of the MCCB in NTFE patients with SCZ. This correlation remained significant after controlling for demographic and clinical variables. It is in line with previous evidence. The Trail Making Test examines processing speed and executive function62 and structural changes in the cingulate gyrus have been shown to be related to this cognitive domain63,64. A number of studies have reported the association of abnormal WM integration with neurocognitive deficits SCZ39,40,42,43,65,66. The findings involve various domains of cognitive functioning, including attention67, working memory68, processing speed69, language and visual learning ability38, executive and motor function32, and facial emotion perception70. There has also been evidence that FA measurements predict the global neurocognitive performance in SCZ patients and healthy subjects, notably for reaction time indicating a complex constellation of WM disruption responsible for altered behavioral performance in SCZ patients relative to healthy individuals55,71. Taken together, these findings, including ours in the present study support the proposal that the disruption of WM connectivity is related to neurocognitive deficits in SCZ.
However, it is noteworthy that WM disruption in SCZ may be observed in different brain areas due to the regional specificity of neurocognitive function in SCZ55. For example, lower FA in the left inferior longitudinal fasciculus and left inferior fronto-occipital fasciculus was shown to associate with the weakening of processing speed and language and visual learning38. Working memory performance was found to be correlated with FA changes in the cingulate fasciculi72 and the left superior longitudinal fasciculus41, as well as the covariance of cortical thickness between the posterior cingulate gyrus and ventral medial prefrontal cortex73. Lower FA in the cingulate gyrus was correlated with lower intelligence74. Facial emotion perception associated with FA in a variety of brain regions, including left forceps major, inferior longitudinal fasciculus, inferior fronto-occipital fasciculus, left splenium of the corpus callosum, and left longitudinal fasciculus, but no correlation was found in healthy individuals70. Interestingly, Roalf et al.71 reported that better general cognitive ability, indexed by lower across-task within-individual variability for performance speed on a computerized neurocognitive battery, was correlated with lower FA in the left cingulum bundle and left frontal-occipital fasciculus in healthy subjects, but not in SCZ patients. A recent study involving patients with SCZ, bipolar disorder, and major depressive disorder found that there was no significant association between reduced FA and cognitive performance in SCZ and bipolar disorder, while FA in the corpus callosum and right cingulum was significantly correlated with attention and cognitive composite performance in patients with major depressive disorder75. Therefore, the findings on WM-cognition association may vary greatly between studies despite the well-established hypothesis that WM tracts or certain brain structures are implicated in cognitive deficits in SCZ.
There is another point noteworthy, i.e., the negative association of the FA values in the left cingulate in SCZ patients with the performance on the Trail Making Test, which is against our prediction. Although lower FA in the cingulate is common in SCZ76, it has been shown to be associated with poorer ability on attention orientation77, with longer response time and higher response time variability in the Stroop task71. Based on the evidence, one would postulate a positive correlation between FA in the left cingulate and the Trail Making Test index. However, there is also evidence that less impaired cognition could be predicted by either higher or lower FA in certain brain areas in SCZ, with better neurocognitive performance related to more diffusion characteristics (negative correlation) and greater directionality (positive correlation) of regional WM55,71. The high heterogeneity of the association between brain connectivity and cognitive functioning in SCZ may reflect the complexity of information processing in the brain. For example, lower FA in particular areas may represent brain regions with cross-fibers78 or those of diffused regional connectivity with the surrounding cortex79, which may both be required for optimal cognitive functioning55. In our study, the microscopic neuroanatomical substrates of FA alterations in regional WM that might explain the negative correlation between FA values in the left cingulate and the Trial Making Test are unknown. Lower FA may reflect the changes of myelin structure, myelin content, or axon diameter76.
In summary, compared with health controls, NTFE patients with SCZ displayed lower FA in five brain areas, involving the cingulate gyrus, internal capsule, corpus callosum, cerebellum, and brainstem in, suggesting that the widespread disruption of WM integrity may contribute to the pathogenesis of SCZ. Also, patients showed a series of cognitive deficits at the early stage of illness. Interestingly, we found that lower FA in the left cingulate was associated with better performance in cognitive processing speed and executive functioning. However, these results need to be carefully considered for the following three reasons. (1) FA in the left cingulate was correlated with only one of the ten subscores (Trail Making Test) in the MCCB and the multiple comparisons did not hold up with Bonferroni corrections, although there were significant differences in the MCCB index and total scores between patient and controls. (2) The correlational finding is contrary to our expectation, with a negative rather than positive association between the FA values in the left cingulate and the Trail Making Test performance. We speculate that this negative association may be due to the more diffuse properties of regional WM in the left cingulate, leading to better performance in cognitive processing speed and executive functioning. (3) Although some related factors were adjusted in the main analyses, many other important factors associated with FA values, clinical symptoms and cognition were missing. These factors are especially important as a number of them are at more severe levels in patients with first-episode SCZ, such as stress, anxiety, depression, and sleep disorders, which should be remedied in future studies. (4) The voxel-based morphometry used in the data analysis in the current research has been considered as a substandard approach in DTI studies80,81. However, tract-based spatial statistics82 is considered to produce more rigorous results in our future DTI studies. (5) Future studies need to use a large sample of NTFE patients with SCZ to conduct longitudinal and prospective research on how cognition is related to brain connectivity in SCZ.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (grant numbers 61806042 and 31600880).
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shan Gao, Email: gaoshan@uestc.edu.cn.
Xiangyang Zhang, Email: zhangxy@psych.ac.cn.
References
- 1.Antonucci LA, et al. A pattern of cognitive deficits stratified for genetic and environmental risk reliably classifies patients with schizophrenia from healthy control subjects. Biol. Psychiatry. 2020;87:697–707. doi: 10.1016/j.biopsych.2019.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Kar SK, Jain M. Current understandings about cognition and the neurobiological correlates in schizophrenia. J. Neurosci. Rural Pract. 2016;7:412–418. doi: 10.4103/0976-3147.176185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang, X. Y. et al. Glucose disturbances, cognitive deficits and white matter abnormalities in first-episode drug-naive schizophrenia. Mol. Psychiatry. 10.1038/s41380-019-0478-1 (2019). [DOI] [PubMed]
- 4.Nuechterlein KH, Ventura J, Subotnik KL, Bartzokis G. The early longitudinal course of cognitive deficits in schizophrenia. J. Clin. Psychiatry. 2014;75(Suppl 2):25–29. doi: 10.4088/JCP.13065su1.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irani F, et al. Computerized neurocognitive test performance in schizophrenia: a lifespan analysis. Am. J. Geriatr. Psychiatry. 2012;20:41–52. doi: 10.1097/JGP.0b013e3182051a7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheffield JM, Karcher NR, Barch DM. Cognitive deficits in psychotic disorders: a lifespan perspective. Neuropsychol. Rev. 2018;28:509–533. doi: 10.1007/s11065-018-9388-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green MF, Horan WP, Lee J. Nonsocial and social cognition in schizophrenia: current evidence and future directions. World Psychiatry. 2019;18:146–161. doi: 10.1002/wps.20624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo JY, Ragland JD, Carter CS. Memory and cognition in schizophrenia. Mol. Psychiatry. 2018;24:633–642. doi: 10.1038/s41380-018-0231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harvey PD, Isner EC. Cognition, social cognition, and functional capacity in early-onset schizophrenia. Child Adolesc. Psychiatr. Clin. N. Am. 2020;29:171–182. doi: 10.1016/j.chc.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Lepage M, Bodnar M, Bowie CR. Neurocognition: clinical and functional outcomes in schizophrenia. Can. J. Psychiatry. 2014;59:5–12. doi: 10.1177/070674371405900103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalache SM, et al. The impact of aging, cognition, and symptoms on functional competence in individuals with schizophrenia across the lifespan. Schizophr. Bull. 2015;41:374–381. doi: 10.1093/schbul/sbu114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheffield JM, Barch DM. Cognition and resting-state functional connectivity in schizophrenia. Neurosci. Biobehav. Rev. 2016;61:108–120. doi: 10.1016/j.neubiorev.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barch DM. Pharmacological strategies for enhancing cognition in schizophrenia. Curr. Top. Behav. Neurosci. 2010;4:43–96. doi: 10.1007/7854_2010_39. [DOI] [PubMed] [Google Scholar]
- 14.Goff DC, Hill M, Barch D. The treatment of cognitive impairment in schizophrenia. Pharm. Biochem. Behav. 2011;99:245–253. doi: 10.1016/j.pbb.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epstein KA, et al. White matter abnormalities and cognitive impairment in early-onset schizophrenia-spectrum disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2014;53:362–372. e361-362. doi: 10.1016/j.jaac.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li R, et al. Disrupted structural and functional rich club organization of the brain connectome in patients with generalized tonic-clonic seizure. Hum. Brain Mapp. 2016;37:4487–4499. doi: 10.1002/hbm.23323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett IJ, Madden DJ, Vaidya CJ, Howard DV, Howard JH., Jr Age-related differences in multiple measures of white matter integrity: a diffusion tensor imaging study of healthy aging. Hum. Brain Mapp. 2010;31:378–390. doi: 10.1002/hbm.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scholz J, Klein MC, Behrens TE, Johansen-Berg H. Training induces changes in white-matter architecture. Nat. Neurosci. 2009;12:1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amodio A, et al. Avolition-apathy and white matter connectivity in schizophrenia: Reduced fractional anisotropy between amygdala and insular cortex. Clin. EEG Neurosci. 2018;49:55–65. doi: 10.1177/1550059417745934. [DOI] [PubMed] [Google Scholar]
- 21.Asmal L, et al. Insight and white matter fractional anisotropy in first-episode schizophrenia. Schizophr. Res. 2017;183:88–94. doi: 10.1016/j.schres.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Harms MP, Akhter KD, Csernansky JG, Mori S, Barch DM. Fractional anisotropy in individuals with schizophrenia and their nonpsychotic siblings. Psychiatry Res. 2015;231:87–91. doi: 10.1016/j.pscychresns.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellison-Wright I, Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr. Res. 2009;108:3–10. doi: 10.1016/j.schres.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 24.Quan M, et al. White matter tract abnormalities between rostral middle frontal gyrus, inferior frontal gyrus and striatum in first-episode schizophrenia. Schizophr. Res. 2013;145:1–10. doi: 10.1016/j.schres.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao L, et al. White matter deficits in first episode schizophrenia: an activation likelihood estimation meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;45:100–106. doi: 10.1016/j.pnpbp.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 26.Leroux E, Vandevelde A, Trehout M, Dollfus S. Abnormalities of fronto-subcortical pathways in schizophrenia and the differential impacts of antipsychotic treatment: a DTI-based tractography study. Psychiatry Res. Neuroimaging. 2018;280:22–29. doi: 10.1016/j.pscychresns.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Konrad A, Winterer G. Disturbed structural connectivity in schizophrenia primary factor in pathology or epiphenomenon? Schizophr. Bull. 2008;34:72–92. doi: 10.1093/schbul/sbm034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pettersson-Yeo W, Allen P, Benetti S, McGuire P, Mechelli A. Dysconnectivity in schizophrenia: where are we now? Neurosci. Biobehav. Rev. 2011;35:1110–1124. doi: 10.1016/j.neubiorev.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Levitt JJ, et al. Reduced structural connectivity in frontostriatal white matter tracts in the associative loop in schizophrenia. Am. J. Psychiatry. 2017;174:1102–1111. doi: 10.1176/appi.ajp.2017.16091046. [DOI] [PubMed] [Google Scholar]
- 30.Meng L, et al. Widespread white-matter microstructure integrity reduction in first-episode schizophrenia patients after acute antipsychotic treatment. Schizophr. Res. 2019;204:238–244. doi: 10.1016/j.schres.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 31.Kyriakopoulos M, Frangou S. Recent diffusion tensor imaging findings in early stages of schizophrenia. Curr. Opin. Psychiatry. 2009;22:168–176. doi: 10.1097/YCO.0b013e328325aa23. [DOI] [PubMed] [Google Scholar]
- 32.Perez-Iglesias R, et al. White matter integrity and cognitive impairment in first-episode psychosis. Am. J. Psychiatry. 2010;167:451–458. doi: 10.1176/appi.ajp.2009.09050716. [DOI] [PubMed] [Google Scholar]
- 33.Zhang XY, et al. Extensive white matter abnormalities and clinical symptoms in drug-naive patients with first-episode schizophrenia: a voxel-based diffusion tensor imaging study. J. Clin. Psychiatry. 2016;77:205–211. doi: 10.4088/JCP.14m09374. [DOI] [PubMed] [Google Scholar]
- 34.Lu LH, Zhou XJ, Keedy SK, Reilly JL, Sweeney JA. White matter microstructure in untreated first episode bipolar disorder with psychosis: comparison with schizophrenia. Bipolar Disord. 2011;13:604–613. doi: 10.1111/j.1399-5618.2011.00958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moriya J, et al. Gray and white matter volumetric and diffusion tensor imaging (DTI) analyses in the early stage of first-episode schizophrenia. Schizophr. Res. 2010;116:196–203. doi: 10.1016/j.schres.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 36.White T, et al. Global white matter abnormalities in schizophrenia: a multisite diffusion tensor imaging study. Schizophr. Bull. 2011;37:222–232. doi: 10.1093/schbul/sbp088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Federspiel A, et al. Alterations of white matter connectivity in first episode schizophrenia. Neurobiol. Dis. 2006;22:702–709. doi: 10.1016/j.nbd.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 38.Liu X, et al. Reduced white matter integrity and cognitive deficit in never-medicated chronic schizophrenia: a diffusion tensor study using TBSS. Behav. Brain Res. 2013;252:157–163. doi: 10.1016/j.bbr.2013.05.061. [DOI] [PubMed] [Google Scholar]
- 39.Castro-de-Araujo LFS, et al. Schizophrenia moderates the relationship between white matter integrity and cognition. Schizophr. Res. 2018;199:250–256. doi: 10.1016/j.schres.2018.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holleran L, et al. The relationship between white matter microstructure and general cognitive ability in patients with schizophrenia and healthy participants in the ENIGMA Consortium. Am. J. Psychiatry. 2020;177:537. doi: 10.1176/appi.ajp.2019.19030225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karlsgodt KH, et al. Diffusion tensor imaging of the superior longitudinal fasciculus and working memory in recent-onset schizophrenia. Biol. Psychiatry. 2008;63:512–518. doi: 10.1016/j.biopsych.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 42.Lim KO, et al. Voxelwise correlational analyses of white matter integrity in multiple cognitive domains in schizophrenia. Am. J. Psychiatry. 2006;163:2008–2010. doi: 10.1176/ajp.2006.163.11.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Subramaniam K, et al. White matter microstructure predicts cognitive training-induced improvements in attention and executive functioning in schizophrenia. Schizophr. Res. 2018;193:276–283. doi: 10.1016/j.schres.2017.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanaan R, et al. White matter microstructure in schizophrenia: Effects of disorder, duration and medication. Br. J. Psychiatry. 2009;194:236–242. doi: 10.1192/bjp.bp.108.054320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Navari S, Dazzan P. Do antipsychotic drugs affect brain structure? A systematic and critical review of MRI findings. Psychol. Med. 2009;39:1763–1777. doi: 10.1017/S0033291709005315. [DOI] [PubMed] [Google Scholar]
- 46.Buckley, P. E. & Evans, D. First-episode schizophrenia. A window of opportunity for optimizing care and outcomes. Postgrad. Med. Spec No, 5–19 (2006). [PubMed]
- 47.Green MF, Nuechterlein KH. The MATRICS initiative: developing a consensus cognitive battery for clinical trials. Schizophr. Res. 2004;72:1–3. doi: 10.1016/j.schres.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 48.Green MF, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol. Psychiatry. 2004;56:301–307. doi: 10.1016/j.biopsych.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 49.Zou Y, et al. Clinical reliability and validity of the Chinese version of measurement and treatment research to improve cognition in schizophrenia consensus cognitive battery. Chin. J. Psychiatry. 2009;42:29–33. [Google Scholar]
- 50.Smith SM. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andersson JL. Maximum a posteriori estimation of diffusion tensor parameters using a Rician noise model: why, how and but. Neuroimage. 2008;42:1340–1356. doi: 10.1016/j.neuroimage.2008.05.053. [DOI] [PubMed] [Google Scholar]
- 52.Asami T, et al. Cerebral white matter abnormalities and their associations with negative but not positive symptoms of schizophrenia. Psychiatry Res. 2014;222:52–59. doi: 10.1016/j.pscychresns.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheung V, et al. A diffusion tensor imaging study of structural dysconnectivity in never-medicated, first-episode schizophrenia. Psychol. Med. 2008;38:877–885. doi: 10.1017/S0033291707001808. [DOI] [PubMed] [Google Scholar]
- 54.Lei W, et al. White matter alterations in first episode treatment-naive patients with deficit schizophrenia: a combined VBM and DTI study. Sci. Rep. 2015;5:12994. doi: 10.1038/srep12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roalf DR, et al. White matter microstructure in schizophrenia: Associations to neurocognition and clinical symptomatology. Schizophr. Res. 2015;161:42–49. doi: 10.1016/j.schres.2014.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr. Bull. 2009;35:509–527. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Addington J, Addington D. Cognitive functioning in first-episode schizophrenia. J. Psychiatry Neurosci. 2002;27:188–192. [PMC free article] [PubMed] [Google Scholar]
- 58.Fioravanti M, Bianchi V, Cinti ME. Cognitive deficits in schizophrenia: an updated metanalysis of the scientific evidence. BMC Psychiatry. 2012;12:64. doi: 10.1186/1471-244X-12-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang H, et al. Meta-analysis of cognitive function in Chinese first-episode schizophrenia: MATRICS Consensus Cognitive Battery (MCCB) profile of impairment. General Psychiatry. 2019;32:e100043. doi: 10.1136/gpsych-2018-100043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McCleery A, et al. Cognitive functioning in first-episode schizophrenia: MATRICS Consensus Cognitive Battery (MCCB) Profile of Impairment. Schizophr. Res. 2014;157:33–39. doi: 10.1016/j.schres.2014.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sol¡s-Vivanco R, et al. Cognitive impairment in never-medicated individuals on the schizophrenia spectrum. JAMA Psychiatry. 2020;77:543–545. doi: 10.1001/jamapsychiatry.2020.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Juselius S, Kieseppä T, Kaprio J, Lönnqvist J, Tuulio-Henriksson A. Executive functioning in twins with bipolar I disorder and healthy co-twins. Arch. Clin. Neuropsychol. 2009;24:599–606. doi: 10.1093/arclin/acp047. [DOI] [PubMed] [Google Scholar]
- 63.Chang Y-L, et al. Level of executive function influences verbal memory in amnestic mild cognitive impairment and predicts prefrontal and posterior cingulate thickness. Cereb. Cortex. 2009;20:1305–1313. doi: 10.1093/cercor/bhp192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sasabayashi D, et al. Increased frontal gyrification negatively correlates with executive function in patients with first-episode schizophrenia. Cereb. Cortex. 2016;27:2686–2694. doi: 10.1093/cercor/bhw101. [DOI] [PubMed] [Google Scholar]
- 65.Nazeri A, et al. Alterations of superficial white matter in schizophrenia and relationship to cognitive performance. Neuropsychopharmacology. 2013;38:1954–1962. doi: 10.1038/npp.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Szeszko PR, et al. Clinical and neuropsychological correlates of white matter abnormalities in recent onset schizophrenia. Neuropsychopharmacology. 2008;33:976–984. doi: 10.1038/sj.npp.1301480. [DOI] [PubMed] [Google Scholar]
- 67.Abdolalizadeh A, et al. White matter microstructural properties associated with impaired attention in chronic schizophrenia: a multi-center study. Psychiatry Res. Neuroimaging. 2020;302:111105. doi: 10.1016/j.pscychresns.2020.111105. [DOI] [PubMed] [Google Scholar]
- 68.Sugranyes G, et al. Multimodal analyses identify linked functional and white matter abnormalities within the working memory network in schizophrenia. Schizophr. Res. 2012;138:136–142. doi: 10.1016/j.schres.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karbasforoushan H, Duffy B, Blackford JU, Woodward ND. Processing speed impairment in schizophrenia is mediated by white matter integrity. Psychol. Med. 2014;45:109–120. doi: 10.1017/S0033291714001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao X, et al. Reduced white matter integrity and facial emotion perception in never-medicated patients with first-episode schizophrenia: A diffusion tensor imaging study. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2017;77:57–64. doi: 10.1016/j.pnpbp.2017.03.025. [DOI] [PubMed] [Google Scholar]
- 71.Roalf DR, et al. White matter organization and neurocognitive performance variability in schizophrenia. Schizophr. Res. 2013;143:172–178. doi: 10.1016/j.schres.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nestor PG, et al. Comparing prefrontal gray and white matter contributions to intelligence and decision making in schizophrenia and healthy controls. Neuropsychology. 2010;24:121–129. doi: 10.1037/a0016981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wheeler AL, et al. Disrupted prefrontal interhemispheric structural coupling in schizophrenia related to working memory performance. Schizophr. Bull. 2014;40:914–924. doi: 10.1093/schbul/sbt100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nestor PG, et al. Neuropsychological variability, symptoms, and brain imaging in chronic schizophrenia. Brain Imaging Behav. 2013;7:68–76. doi: 10.1007/s11682-012-9193-0. [DOI] [PubMed] [Google Scholar]
- 75.Yamada S, et al. Widespread white matter microstructural abnormalities and cognitive impairment in schizophrenia, bipolar disorder, and major depressive disorder: Tract-based spatial statistics study. Psychiatry Res Neuroimaging. 2020;298:111045. doi: 10.1016/j.pscychresns.2020.111045. [DOI] [PubMed] [Google Scholar]
- 76.Wheeler AL, Voineskos AN. A review of structural neuroimaging in schizophrenia: from connectivity to connectomics. Front. Hum. Neurosci. 2014;8:653. doi: 10.3389/fnhum.2014.00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nestor PG, et al. Attentional networks and cingulum bundle in chronic schizophrenia. Schizophr. Res. 2007;90:308–315. doi: 10.1016/j.schres.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oouchi H, et al. Diffusion anisotropy measurement of brain white matter is affected by voxel size: underestimation occurs in areas with crossing fibers. AJNR Am. J. Neuroradiol. 2007;28:1102–1106. doi: 10.3174/ajnr.A0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dong Q, et al. Clinical applications of diffusion tensor imaging. J. Magn. Reson. Imaging. 2004;19:6–18. doi: 10.1002/jmri.10424. [DOI] [PubMed] [Google Scholar]
- 80.Jones D. The effect of gradient sampling schemes on measures derived from diffusion tensor MRI: a Monte Carlo study. Magn. Reson. Med. 2004;51:807–815. doi: 10.1002/mrm.20033. [DOI] [PubMed] [Google Scholar]
- 81.Jones D, Pierpaoli C. Confidence mapping in diffusion tensor magnetic resonance imaging tractography using a bootstrap approach. Magn. Reson. Med. 2005;53:1143–1149. doi: 10.1002/mrm.20466. [DOI] [PubMed] [Google Scholar]
- 82.Smith SM, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]


