Abstract
Acute myocardial infarction (AMI) remains a leading cause of morbidity and mortality, Pioneering preclinical work reported by Peter Maroko and Eugene Braunwald in 1971 identified oxygen supply and demand are primary determinants of myocardial infarct size in the setting of a heart attack Since the 1950’s, advances in mechanical engineering led to the development of short-term circulatory support devices that range from pulsatile to continuous flow pumps. The primary objective of these pumps is to reduce native heart work, enhance coronary blood flow, and sustain systemic perfusion. Whether these pumps could reduce myocardial infarct size in the setting of AMI became an intense focus for preclinical investigation with variable animal models, experimental algorithms, and pump platforms being tested. In this review, we discuss the design of these preclinical studies, the evolution of mechanical support platforms, and attempts to translate these experimental methods into clinical trials.
Keywords: Acute myocardial infarction, ischemia-reperfusion injury, cardioprotection, mechanical circulatory support, preclinical models
Myocardial Ischemia-Reperfusion Injury Remains a Major Unresolved Target of Therapy
Acute myocardial infarction (AMI) remains a leading cause of morbidity and mortality, with an annual incidence of over 805,000 in the United States alone [1]. Beginning with the “open artery theory” in the 1970s, the field of STEMI management has been ruled by the fundamental principle that “time is muscle,” indicating that prolonged coronary occlusion leads to myocardial injury [2, 3]. For this reason, the well-established paradigm of contemporary management for STEMI focuses on rapid coronary reperfusion via balloon angioplasty and stenting to limit myocardial injury. However, despite timely reperfusion, nearly 10% of patients with acute myocardial infarction (MI) die during their index hospitalization, and 25% of survivors progress to develop chronic heart failure [4]. One explanation for these poor outcomes is that reperfusion of ischemic myocardium accelerates myocardial ischemia-reperfusion injury (IRI) leading to additional myocardial damage. Prior attempts to limit IRI via vascular conditioning or pharmacologic approaches have failed to show clear clinical benefit. Once a coronary artery becomes occluded, heart rate and myocardial contractility increase to compensate for reduced stroke volume, which decreases the myocardial oxygen supply-to-demand ratio and creates a vicious cycle of progressive myocardial damage.
Preclinical models of myocardial oxygen supply and demand mismatch in AMI
Pioneering preclinical work reported by Peter Maroko and Eugene Braunwald in 1971 identified oxygen supply and demand are primary determinants of myocardial infarct size in the setting of a heart attack [5]. Using an anesthetized canine model, these investigators performed a left thoracotomy, opened the pericardium, and surgically ligated the left anterior descending artery. Serial 20 minute occlusions of the artery were performed with one-hour intervals between occlusions to assess ischemic burden and myocardial damage by quantifying ST-segment elevations. To explore whether increasing myocardial oxygen consumption during coronary occlusion increases myocardial damage, they employed: 1) pharmacologic agents to drive heart rate and contractility (isoproterenol), 2) right atrial pacing, 3) inotropes (glucagon and ouabain), 4) sympathomimetics (bretylium), and 5) acute arterial hemorrhage. To test the effects of reduced myocardial oxygen consumption, investigators administered either methoxamine to increase systemic blood pressure or the negative inotrope propranolol. Compared to controls, all methods that increased myocardial oxygen consumption increased ST-segment elevations, whereas reducing myocardial oxygen consumption with either propranolol or methoxamine reduced ST-segment elevations and CPK levels. Major limitations of this study included: 1) the need for surgical thoracotomy to access the coronary vessel, 2) the inability to quantify myocardial infarct size, 3) the inability to assess myocardial oxygen consumption directly, and 4) the inability to test late term effects of initial drug therapy on infarct size and cardiac function. The investigators concluded that “measures designed for reduction of myocardial oxygen demands and improvement of coronary perfusion, when effected promptly after a patient has been brought to the hospital, might potentially reduce the ultimate size of the infarction.” At the time, 2 other cardiovascular pioneers, Charles Dotter and Andreas Greuntzig, were developing techniques for peripheral and coronary vascular angioplasty respectively. In 1977, the first coronary angioplasty was performed in Zurich and henceforth, coronary reperfusion to restore myocardial oxygen supply during an AMI became the cornerstone focus of STEMI management for the next 40 years.
Mechanically unloading the left ventricle and delaying coronary reperfusion limits infarct size
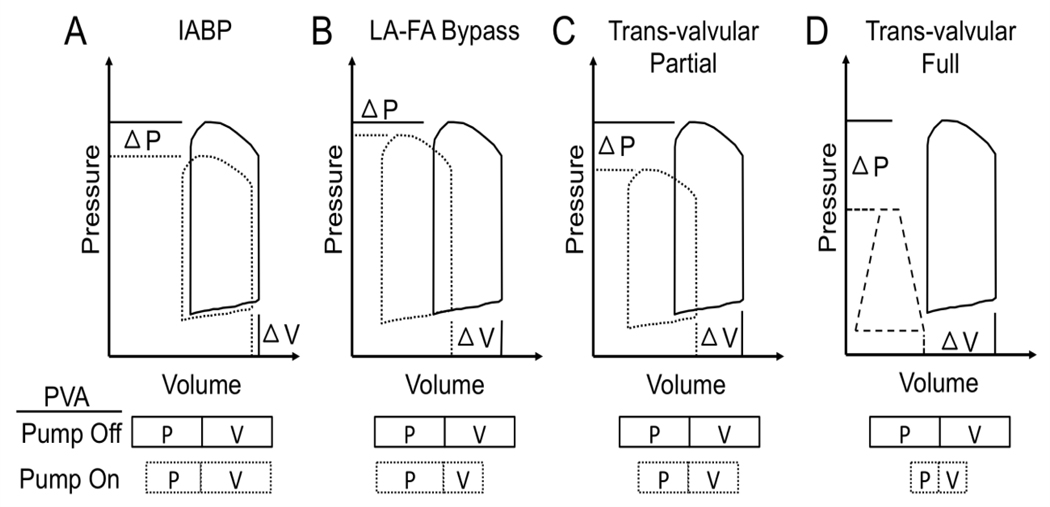
Prior attempts to limit ischemia-reperfusion injury via vascular conditioning or pharmacologic approaches have failed to show clear clinical benefit [6–7]. A critical barrier to these strategies is the mandate for rapid coronary reperfusion and therefore insufficient time for any beneficial impact prior to reperfusion. While these strategies were being tested in preclinical and clinical trials, a few investigators continued working on the first arm of Maroko and Braunwald’s conclusion, namely, reducing myocardial oxygen demand to reduce infarct size. Since the 1950’s, advances in mechanical engineering led to the development of short-term circulatory support devices that range from pulsatile to continuous flow pumps. The primary objective of these pumps is to reduce native heart work, enhance coronary blood flow, and sustain systemic perfusion. Whether these pumps could reduce myocardial infarct size in the setting of AMI became an intense focus for preclinical investigation with variable animal models, experimental algorithms, and pump platforms being tested (Table 1). Intra-aortic balloon counterpulsation pumps (IABPs) were among the first percutaneously delivered circulatory support pumps to be studied in preclinical models of infarct reduction. IABPs function by inflating during diastole and deflating during systole to create higher diastolic pressures in the aortic root and to reduce ventricular afterload during systole. The net effect of IABP activation is a reduction in LV pressure with minimal change in LV volume, thereby leading to minimal change in net PVA or myocardial oxygen consumption (Figure 1A) [8–9] Several studies have confirmed that intact native ventricular function is a major determinant of IABP effects [10–11]. For this reason, the more dysfunctional the LV, the less function an IABP becomes. The challenge with IABP studies in preclinical models is that major determinants of IABP function including aortic length, diameter, and compliance may vary considerably between species and relative to humans. Early preclinical studies with IABPs generated mixed results with respect to altering infarct size (Table 1). All 4 of the referenced IABP studies were performed using an open thoracotomy and a surgical ligature around the left anterior descending artery (LAD), which may alter intra-cardiac loading conditions once the pericardium is incised [12–15]. Furthermore, with the exception of the study by Ledoux and Smalling, all prior studies initiated IABP support after LAD ligation. Ledoux and Smalling were the first to identify that IABP insertion before, not after, LAD reperfusion reduced infarct size. Based largely on the findings of this study, the Counterpulsation to Reduce Infarct Size Pre-PCI Acute Myocardial Infarction (CRISP-AMI) study explored the utility of initiating IABP therapy immediately before coronary reperfusion in patients with acute anterior myocardial infarction [16]. The study failed to demonstrate any reduction in myocardial infarct size measured by cardiac MRI among IABP recipients compared to subjects receiving coronary reperfusion without IABP therapy. One explanation for this observation is that in CRISP AMI, a majority of patients were treated beyond 3 hours from time of symptom onset to IABP insertion and/or reperfusion, which may have impacted any potential for infarct salvage with IABP therapy [17–18].
Table 1.
Summary of preclinical studies of acute mechanical left ventricular support devices to reduce infarct size
| Animal Model | Year | Duration of Ischemia (min) | Duration of Reperfusion (min) | Mechanical Support Before or After Reperfusion | Occluded Vessel | Method of Occlusion | Device | Reduction in Infarct Size? | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Canine | 1978 | 480 | X | Before | LAD | Ligation | IABP | Yes | Roberts & Gay [6] |
| Baboons | 1979 | 1440 | X | Before | LAD | Ligation | IABP | No | Haston & McNamara [7] |
| Porcine | 1980 | 1440 | X | Before | LAD | Ligation | IABP | No | Laas & Replogle [8] |
| Porcine | 2008 | 60 | 240 | Before vs After | LAD | Ligation | IABP | Yes / No | Ledoux & Smalling [9] |
| Canine | 1983 | 240 | X | Before | LAD | Ligation | LA-FA Bypass | Yes | Catinella & Spencer [12] |
| Porcine | 2013 | 120 | 120 | Before | LAD | Balloon angioplasty | TandemHeart | Yes | Kapur & Karas [13] |
| Canine | 1989 | 120 | 60 | Before | LAD | Ligation | Hemopump | Yes | Merhige & Wampler [14] |
| Canine | 1992 | 120 | 60 | Before | LAD | Snare ligation | Hemopump/IABP | Yes | Smalling & Amirian [15] |
| Canine | 2005 | 120 | 240 | Before vs After | LAD | Snare ligation | Hemopump | Yes / No | Achour & Smalling [16] |
| Sheep | 2003 | 60 | 120 | Before vs After | LAD | Ligation | Impella 5.0 | Yes | Meyns & Flameng [17] |
| Porcine | 2015 | 90 | 120 | Before | LAD | Balloon angioplasty | Impella CP | Yes | Kapur & Karas [18] |
| Porcine | 2015 | 120 | 120 | Before | LCx | Ligation | Impella LD | Yes | Sun & Wang [20] |
| Porcine | 2018 | 90 | 120 | Before | LAD | Balloon angioplasty | Impella CP | Yes | Esposito & Kapur [21] |
Figure 1.
Impact of acute mechanical circulatory support pumps on myocardial oxygen consumption. A) Compared to baseline, intra-aortic balloon pumps reduce LV pressure, not volume, thereby creating a small reduction in pressure-volume area (PVA), which directly correlates with myocardial oxygen consumption; B) Left atrial to femoral artery (LA-FA) bypass pumps such as the TandemHeart device reduce PVA by significantly decreasing LV volume, not pressure; C-D) Trans-valvular pumps such as the Hemopump, Impella, or HeartMate percutaneous heart pump, reduce PVA by decreasing both LV pressure and volume. The magnitude of PVA reduction directly correlates with the magnitude of flow through a trans-valvular pump. Full lines represent ‘Pump Off’. Hashed lines represent ‘Pump On’. PVA is illustrated by the boxes encompassing the product of LV pressure (P) and volume (V).
Another mechanical circulatory support platform studies in the early 1980’s was left atrial-to-femoral artery bypass. Using an extracorporeal rotary flow pump, a drainage cannula was placed into the left atrium and a return cannula placed in the femoral artery. Oxygenated blood was removed from the left atrium and delivered to the systemic arterial circulation. The net effect of this support system is minimal change in LV pressure, but a significant reduction in LV volume leading to reduced PVA and myocardial oxygen consumption (Figure 1B) [19–20]. Catinella and Spencer tested this approach by applying the LA-FA bypass circuit 15 minutes after surgical LAD ligation [21]. Four hours later, compared to controls, myocardial infarct size was reduced in the LA-FA bypass group. The clinical utility of LA-FA bypass was further explored in a porcine model where balloon occlusion of the LAD for 120 minutes was followed by 120 minutes of reperfusion without mechanical support [22]. In the mechanically supported group, percutaneous LA-FA bypass was initiated after 120 minutes of ischemia, and LAD occlusion was prolonged for an additional 30 minutes (total of 150 minutes of LAD occlusion), followed by 120 minutes of reperfusion with device support. Compared to controls, LA-FA bypass significantly reduced infarct size in the group despite 30 additional minutes of LAD occlusion (Figure 2). These findings extended the observations of Ledoux and Smalling by suggesting that mechanical unloading of the LV and delaying coronary reperfusion reduces infarct size. In 2014, the TandemHeart to Reduce Infarct Size (TRIS) trial was initiated and proposed insertion of the TandemHeart LA-FA bypass circuit before reperfusion in patients presenting with anterior STEMI. The trial was terminated in 2015 due to lack of enrollment. While the TandemHeart is able to effectively unload the LV, the failure of this trial reflected concerns among the interventional community about the feasibility of trans-septal puncture prior to reperfusion in STEMI.
Figure 2.
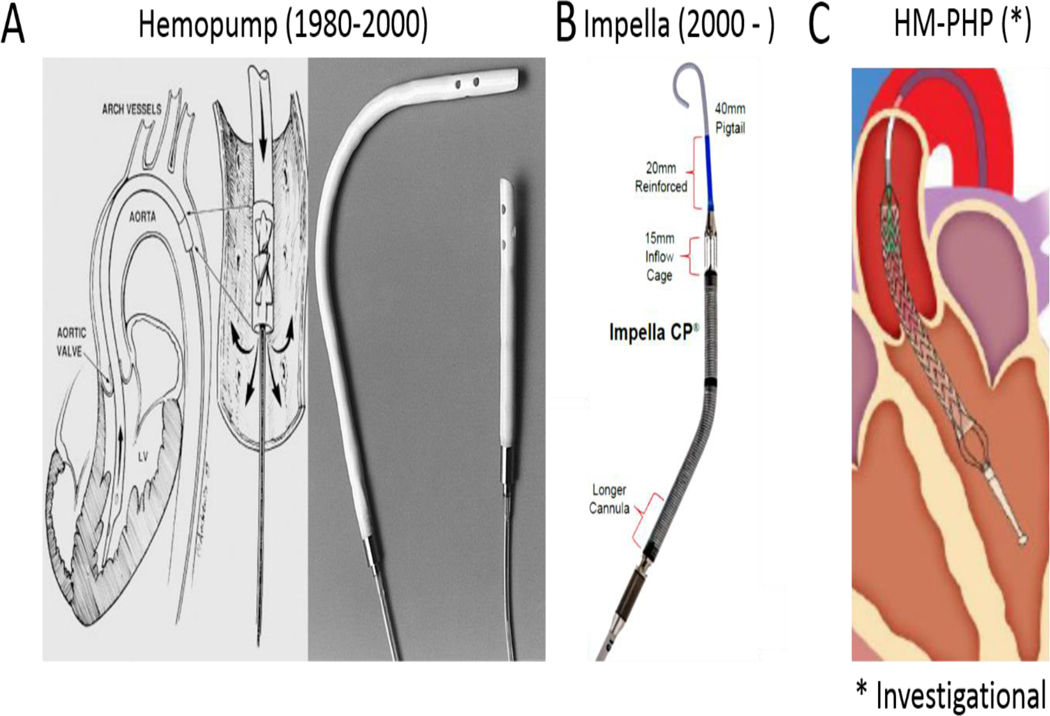
Transvalvular Pumps. A) The Hemopump has a trans-valvular impeller connected to an extra-corporeal motor by a driveline; B) The Impella has a trans-valvular impeller connected to an intracorporeal motor without a driveline; C) The investigational HeartMate Percutaneous Heart Pump (HM-PHP) has a self-expanding trans-valvular impeller connected to an extra-corporeal motor by a driveline.
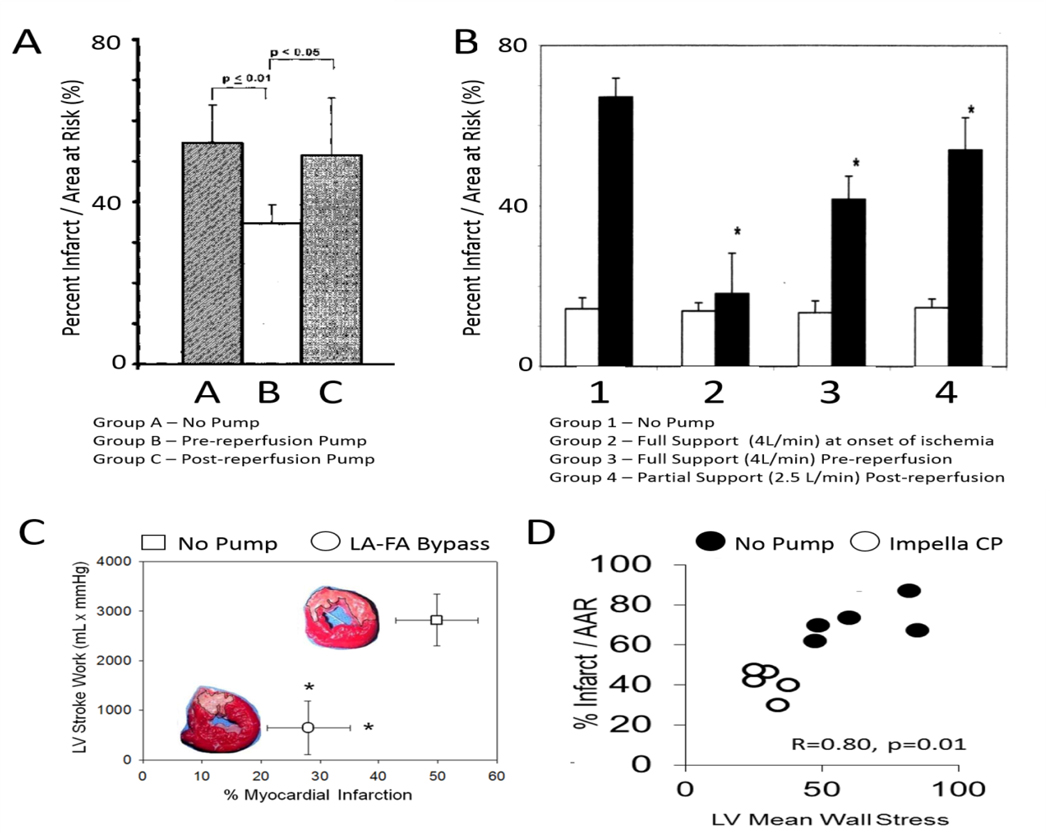
Over the same time period, a third class of acute circulatory support devices, known as trans-valvular pumps were being developed. Trans-valvular pumps employed micro-axial impellers to transfer rotational kinetic energy to blood and thereby generate flow. These pumps were deployed across the aortic valve and displace blood directly from the LV to the aorta. In contrast to LA-FA bypass, trans-valvular pumps reduce both LV pressure and volume, thereby significantly reducing PVA and myocardial oxygen consumption. The magnitude of PVA reduction is determined, in part, b the magnitude of flow generated by the trans-valvular pump (Figures 1C-D) [23]. One of the earliest trans-valvular pumps was the Hemopump (Nimbus Inc), which was a micro-axial impeller attached by a driveline to an extracorporeal motor (Figure 2). Initial preclinical testing in a canine model of surgical LAD ischemia and reperfusion showed reduced cardiac workload due to systolic and diastolic unloading with a concomitant increase in perfusion to ischemic myocardium [24]. Based on these preliminary studies, Smalling and colleagues compared the effect of IABPs versus the Hemopump in a canine model of surgical LAD ischemia and reperfusion [25]. The IABP and Hemopump were active throughout the entire period of LAD occlusion and reperfusion. Compared to controls without mechanical support, both the IABP and Hemopump reduced infarct size by 57% and 65% respectively. A follow up study in 2005 using a similar canine model of surgical LAD ischemia and reperfusion injury confirmed that initiation of Hemopump support within 15 minutes before, not after, reperfusion reduced infarct size [26] (Figure 3). Clinical translation of these exciting studies was limited by a risk of potential adverse effects of the Hemopump including vascular complications, hemolysis, and the need for a driveline and externalized motor.
Figure 3.
A) Infarct reduction before, not after, reperfusion with the Hemopump; B) Infarct reduction before, not after, reperfusion with full Impella support (>4 liters/minute of flow); C) Infarct reduction with the TandemHeart LA-FA bypass pump before reperfusion; D) Infarct reduction with the Impella CP before reperfusion.
In 2015, the SHIELD 1 trial tested the clinical utility of the HeartMate Percutaneous Heart Pump (HM-PHP) device in 50 patients undergoing high risk PCI (Figure 2). In this study, the HM-PHP increased cardiac index and mean arterial pressure [27]. The HM-PHP shares the external motor and drive-line connected to a trans-valvular impeller from the Hemopump design. However, in contrast the Hemopump, the HM-PHP device can be deployed into the LV via a 14Fr sheath and employs a self-expanding 24Fr impeller across the aortic valve, which allows the device to achieve flows above 4 liters/minute without the need for surgical access. The HM-PHP is under investigation in the United States as part of the SHIELD-2 trial. However, this study was paused in 2017 due to isolated instances of pump stoppage. The HM-PHP device is not currently available for commercial use.
In parallel to the development of the Hemopump, another trans-valvular pump known as the Impella (Abiomed Inc) was introduced into clinical practice in the early 2000’s. In contrast to the Hemopump, the Impella microaxial impellers were connected to an intracorporeal motor without the need for an externalized driveline (Figure 2). In 2003, Meyns and colleagues employed a sheep model of surgical LAD ischemia and reperfusion injury to test whether full or partial trans-valvular support with a surgically implanted Impella 5.0 LP pump reduced infarct size [28] (Figure 3). These investigators observed that initiation of full support at the time of reperfusion with flow rates of over 4 liters/minutes had a greater reduction in infarct size compared to partial support (2.5 liters/minute). Using aortic and coronary sinus blood samples, they further reported that reduced myocardial oxygen consumption during Impella support correlated directly with reduced myocardial infarct size. In 2012, a percutaneously delivered Impella CP pump was introduced into clinical practice and allowed for flows of 3.5 liters/ minute without the need for surgery. In 2015, this novel Impella device was employed in a non-surgical swine model of LAD ischemia and reperfusion to test whether first unloading the LV and extending the delay to reperfusion by 60 minutes (primary unloading) would reduce infarct size [29] (Figure 3). Compared to animals receiving primary reperfusion alone, primary unloading reduced infarct size, increased signaling through the reperfusion injury salvage kinase (RISK) pathway and increased levels of the cardioprotective cytokine stromal derived factor one alpha (SDF1a). This was the first report to introduce the concept of mechanical conditioning whereby LV unloading and delayed reperfusion activates a cardioprotective signaling program within the myocardium. Since then, several laboratories have confirmed that primary unloading with variable periods of ‘mechanical conditioning’ before reperfusion in swine and canine models [30–31]. Furthermore, compared to primary reperfusion, primary unloading with the Impella CP for 30 minutes before reperfusion in swine triggers a cardioprotective shift in myocardial gene expression, preserves mitochondrial integrity, and leads to a durable reduction in LV scar size as quantified by cardiac magnetic resonance imaging 28 days after the initial ischemic injury [32]. Collectively, these preclinical studies led to a clinical first-in-human study known as the Door To Unloading With Impella CP System in Acute Myocardial Infarction to Reduce Infarct Size (DTU): A Prospective Feasibility Study (NIH CLINICAL TRIAL: NCT03000270). This is a multi-center, prospective, randomized, two-arm feasibility trial to assess the potential role of unloading with the Impella CP prior to revascularization in reducing infarct size. The study design includes 1:1 randomization between: 1) 30 minutes of unloading with Impella CP prior to primary percutaneous coronary intervention (PPCI); and 2) initiation of Impella CP unloading followed immediately by PPCI. In addition to evaluating safety, infarct size at 3–5 days and 30 days will be evaluated using cardiac magnetic resonance imaging. This study is actively underway in the United States.
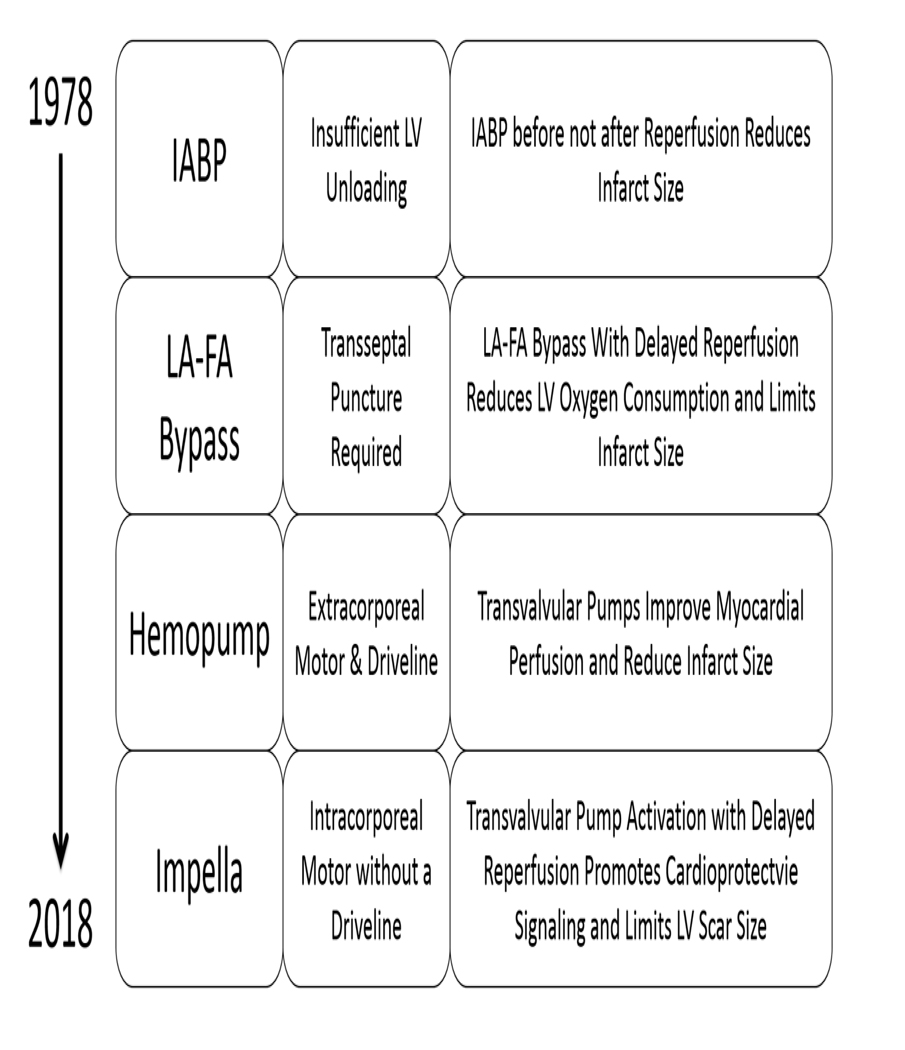
In conclusion, reducing myocardial infarct size remains a major unmet need with broad-reaching implications for long-term survival and morbidity associated with heart failure after an acute myocardial infarction. Over the past 50 years, mechanical circulatory support devices have evolved from balloon counterpulsation pumps to extracorporeal rotary flow pumps to intracorporeal micro-axial flow pumps that can be rapidly implanted without the need for surgery (Figure 4). Cumulative experience from laboratories around the world testing these devices in preclinical models of ischemia-reperfusion injury suggest that LV unloading prior to reperfusion (Primary Unloading) may be the most efficient method to achieve the two objectives proposed by Maroko and Braunwald in 1971 to reduce infarct size, namely “reduction of myocardial oxygen demands and improvement of coronary perfusion.” Whether Primary Unloading translates to improved clinical outcomes remains to be determined.
Figure 4.
Summary of key lessons over the past four decades of preclinical studies exploring infarct reduction with acute mechanical left ventricular support devices
Acknowledgements
This work was supported by a grant from the National Institutes of Health (RO1HL139785-01) to N.K.K.
Disclosures:
Institutional research grants, consulting, and speaker honoraria to N.K.K. from Abbott, Abiomed, Boston Scientific, Maquet, Medtronic, and MD Start
Compliance with Ethical Standards
No funding was received for this report. Conflict of interest: Navin Kapur, MD has received institutional grant support from Abbott, Abiomed, Boston Scientific, Maquet/Getinge, Medtronic and MD Start. Dr. Kapur receives consulting/speaking honoraria from Abbott, Abiomed, Boston Scientific, and MD Start.
Footnotes
All other authors do not have a conflict of interest. No animal or human studies were performed in this report.
References:
- 1.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, P Muntner; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018. March 20;137(12):e67–e492. [DOI] [PubMed] [Google Scholar]
- 2.Maroko PR, Braunwald E. Modification of myocardial infarction size after coronary occlusion. Ann Intern Med. 1973. November;79(5):720–33. [DOI] [PubMed] [Google Scholar]
- 3.Reimer KA, Lowe JE, Rasmussen MM, Jennings RB. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation. 1977. November;56(5):786–94. [DOI] [PubMed] [Google Scholar]
- 4.Ezekowitz JA, Kaul P, Bakal JA, Armstrong PW, Welsh RC, McAlister FA. Declining in-hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction. J Am Coll Cardiol. 2009. January 6;53(1):13–20. [DOI] [PubMed] [Google Scholar]
- 5.Maroko PR, Kjekshus JK, Sobel BE, Watanabe T, Covell JW, Ross J Jr, Braunwald E. Factors influencing infarct size following experimental coronary artery occlusions. Circulation. 1971. January;43(1):67–82. [DOI] [PubMed] [Google Scholar]
- 6.Heusch G, Rassaf T. Time to Give Up on Cardioprotection? A Critical Appraisal of Clinical Studies on Ischemic Pre-, Post-, and Remote Conditioning. Circ Res. 2016. August 19;119(5):676–95. [DOI] [PubMed] [Google Scholar]
- 7.Hausenloy DJ, Garcia-Dorado D, Bøtker HE, Davidson SM, Downey J, Engel FB, Jennings R, Lecour S, Leor J, Madonna R, Ovize M, Perrino C, Prunier F, Schulz R, Sluijter JPG, Van Laake LW, Vinten-Johansen J, Yellon DM, Ytrehus K, Heusch G, Ferdinandy P. Novel targets and future strategies for acute cardioprotection: Position Paper of the European Society of Cardiology Working Group on Cellular Biology of the Heart. Cardiovasc Res. 2017. May 1;113(6):564–585. [DOI] [PubMed] [Google Scholar]
- 8.Annamalai SK, Buiten L, Esposito ML, Paruchuri V, Mullin A, Breton C, Pedicini R, O’Kelly R, Morine K, Wessler B, Patel AR, Kiernan MS, Karas RH, Kapur NK. Acute Hemodynamic Effects of Intra-aortic Balloon Counterpulsation Pumps in Advanced Heart Failure. J Card Fail. 2017. August;23(8):606–614. [DOI] [PubMed] [Google Scholar]
- 9.Schreuder JJ, Maisano F, Donelli A, Jansen JR, Hanlon P, Bovelander J, Alfieri O. Beat-to-beat effects of intraaortic balloon pump timing on left ventricular performance in patients with low ejection fraction. Ann Thorac Surg. 2005. March;79(3):872–80. [DOI] [PubMed] [Google Scholar]
- 10.Sintek MA, Gdowski M, Lindman BR, Nassif M, Lavine KJ, Novak E, Bach RG, Silvestry SC, Mann DL, Joseph SM. Intra-Aortic Balloon Counterpulsation in Patients With Chronic Heart Failure and Cardiogenic Shock: Clinical Response and Predictors of Stabilization. J Card Fail. 2015. November;21(11):868–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu S, Kambhampati S, Sciortino CM, Russell SD, Schulman SP. Predictors of intra-aortic balloon pump hemodynamic failure in non-acute myocardial infarction cardiogenic shock. Am Heart J. 2018. May;199:181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts AJ, Alonso DR, Combes JR, Jacobstein JG, Post MR, Cahill PT, Ho SL, Abel RM, Subramanian VA, Gay WA Jr. Role of delayed intraaortic balloon pumping in treatment of experimental myocardial infarction. Am J Cardiol. 1978. June;41(7):1202–8. [DOI] [PubMed] [Google Scholar]
- 13.Haston HH, McNamara JJ. The effects of intraaortic balloon counterpulsation on myocardial infarct size. Ann Thorac Surg. 1979. October;28(4):335–41. [DOI] [PubMed] [Google Scholar]
- 14.LeDoux JF, Tamareille S, Felli PR, Amirian J, Smalling RW. Left ventricular unloading with intra-aortic counter pulsation prior to reperfusion reduces myocardial release of endothelin-1 and decreases infarction size in a porcine ischemia-reperfusion model. Catheter Cardiovasc Interv. 2008. October 1;72(4):513–21. [DOI] [PubMed] [Google Scholar]
- 15.Laas J, Campbell CD, Takanashi Y, Pick RL, Replogle RL. Failure of intra-aortic balloon pumping to reduce experimental myocardial infarct size in swine. J Thorac Cardiovasc Surg. 1980. July;80(1):85–93. [PubMed] [Google Scholar]
- 16.Patel MR, Smalling RW, Thiele H, Barnhart HX, Zhou Y, Chandra P, Chew D, Cohen M, French J, Perera D, Ohman EM. Intra-aortic balloon counterpulsation and infarct size in patients with acute anterior myocardial infarction without shock: the CRISP AMI randomized trial. JAMA. 2011. September 28;306(12):1329–37. [DOI] [PubMed] [Google Scholar]
- 17.Eitel I, Desch S, Fuernau G, Hildebrand L, Gutberlet M, Schuler G, Thiele H. Prognostic significance and determinants of myocardial salvage assessed by cardiovascular magnetic resonance in acute reperfused myocardial infarction. J Am Coll Cardiol. 2010. June 1;55(22):2470–9. [DOI] [PubMed] [Google Scholar]
- 18.Francone M, Bucciarelli-Ducci C, Carbone I, Canali E, Scardala R, Calabrese FA, Sardella G, Mancone M, Catalano C, Fedele F, Passariello R, Bogaert J, Agati L. Impact of primary coronary angioplasty delay on myocardial salvage, infarct size, and microvascular damage in patients with ST-segment elevation myocardial infarction: insight from cardiovascular magnetic resonance. J Am Coll Cardiol. 2009. December 1;54(23):2145–53. [DOI] [PubMed] [Google Scholar]
- 19.Kapur NK, Paruchuri V, Pham DT, Reyelt L, Murphy B, Beale C, Bogins C, Wiener D, Nilson J, Esposito M, Perkins S, Perides G, Karas RH. Hemodynamic effects of left atrial or left ventricular cannulation for acute circulatory support in a bovine model of left heart injury. ASAIO J. 2015. May-Jun;61(3):301–6. [DOI] [PubMed] [Google Scholar]
- 20.Esposito ML, Shah N, Dow S, Kang S, Paruchuri V, Karas RH, Kapur NK. Distinct Effects of Left or Right Atrial Cannulation on Left Ventricular Hemodynamics in a Swine Model of Acute Myocardial Injury. ASAIO J. 2016. Nov/Dec;62(6):671–676. [DOI] [PubMed] [Google Scholar]
- 21.Catinella FP, Cunningham JN Jr, Glassman E, Laschinger JC, Baumann FG, Spencer FC. Left atrium-to-femoral artery bypass: effectiveness in reduction of acute experimental myocardial infarction. J Thorac Cardiovasc Surg. 1983. December;86(6):887–96. [PubMed] [Google Scholar]
- 22.Kapur NK, Paruchuri V, Urbano-Morales JA, Mackey EE, Daly GH, Qiao X, Pandian N, Perides G, Karas RH. Mechanically unloading the left ventricle before coronary reperfusion reduces left ventricular wall stress and myocardial infarct size. Circulation. 2013. July 23;128(4):328–36. [DOI] [PubMed] [Google Scholar]
- 23.Kapur NK, Paruchuri V, Pham DT, Reyelt L, Murphy B, Beale C, Bogins C, Wiener D, Nilson J, Esposito M, Perkins S, Perides G, Karas RH. Hemodynamic effects of left atrial or left ventricular cannulation for acute circulatory support in a bovine model of left heart injury. ASAIO J. 2015. May-Jun;61(3):301–6. [DOI] [PubMed] [Google Scholar]
- 24.Merhige ME, Smalling RW, Cassidy D, Barrett R, Wise G, Short J, Wampler RK. Effect of the hemopump left ventricular assist device on regional myocardial perfusion and function. Reduction of ischemia during coronary occlusion. Circulation. 1989. November;80(5 Pt 2):III158–66. [PubMed] [Google Scholar]
- 25.Smalling RW, Cassidy DB, Barrett R, Lachterman B, Felli P, Amirian J. Improved regional myocardial blood flow, left ventricular unloading, and infarct salvage using an axial-flow, transvalvular left ventricular assist device. A comparison with intra-aortic balloon counterpulsation and reperfusion alone in a canine infarction model. Circulation. 1992. March;85(3):1152–9. [DOI] [PubMed] [Google Scholar]
- 26.Achour H, Boccalandro F, Felli P, Amirian J, Uthman M, Buja M, Smalling RW. Mechanical left ventricular unloading prior to reperfusion reduces infarct size in a canine infarction model. Catheter Cardiovasc Interv. 2005. February;64(2):182–92. [DOI] [PubMed] [Google Scholar]
- 27.Maly J, Ivak P, Netuka I, Herman A, Kettner J, Sood P, Jorde UP. Initial experience with the HeartMate percutaneous heart pump in circulatory failure. J Heart Lung Transplant. 2017. September;36(9):1016–1019. [DOI] [PubMed] [Google Scholar]
- 28.Meyns B, Stolinski J, Leunens V, Verbeken E, Flameng W. Left ventricular support by catheter-mounted axial flow pump reduces infarct size. J Am Coll Cardiol. 2003. April 2;41(7):1087–95. [DOI] [PubMed] [Google Scholar]
- 29.Kapur NK, Qiao X, Paruchuri V, Morine KJ, Syed W, Dow S, Shah N, Pandian N, Karas RH. Mechanical Pre-Conditioning With Acute Circulatory Support Before Reperfusion Limits Infarct Size in Acute Myocardial Infarction. JACC Heart Fail. 2015. November;3(11):873–82. [DOI] [PubMed] [Google Scholar]
- 30.Saku K, Kakino T, Arimura T, Sakamoto T, Nishikawa T, Sakamoto K, Ikeda M, Kishi T, Ide T, Sunagawa K. Total Mechanical Unloading Minimizes Metabolic Demand of Left Ventricle and Dramatically Reduces Infarct Size in Myocardial Infarction. PLoS One. 2016. April 28;11(4):e0152911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun X, Li J, Zhao W, Lu S, Guo C, Lai H, Wang C. Early Assistance With Left Ventricular Assist Device Limits Left Ventricular Remodeling After Acute Myocardial Infarction in a Swine Model. Artif Organs. 2016. March;40(3):243–51. [DOI] [PubMed] [Google Scholar]
- 32.Esposito M, Zhang Y, Qiao X, Reyelt L, Morine K, Annamalai S, Bogins C, Natov P, Patel A, Rowin E, Karas R, Kapur N. Primary LV Unloading With a Trans-Valvular Axial Flow Pump Reduces Infarct Size by Increasing Myocardial Levels of Stromal Derived Factor One Alpha (SDF-1a) in Acute Myocardial Infarction and Limits the Development of Ischemic Heart Failure. J Am Coll Cardiol. 2018. 71: p. A2660. [Google Scholar]