SUMMARY
Objective:
Magnetic resonance imaging (MRI) is a widely used imaging modality for studies of knee osteoarthritis (OA). Compared to radiography, MRI offers exceptional soft tissue imaging and true three-dimensional (3D) visualization. However, MRI is expensive both due to the cost of acquisition and evaluation of the images. The goal of our study is to develop a new method to address the cost of MRI by combining innovative acquisition methods and automated post-processing software.
Methods:
Ten healthy volunteers were scanned with three different MRI protocols: A standard 3D dual-echo steady state (DESS) pulse sequence, an accelerated DESS (DESSAcc), acquired at approximately half the time compared to DESS, and a multi-echo time DESS (DESSMTE), which is capable of producing measurements of T2 relaxation time. A software tool was used to measure cartilage volume. Accuracy was quantified by comparing DESS to DESSAcc and DESSMTE and precision was measured using repeat readings and acquisitions. T2 precision was determined using duplicate DESSMTE acquisitions. Intra-class correlation coefficients (ICCs), root-mean square standard deviation (RMSSD), and the coefficient of variation (CoV) were used to quantify accuracy and precision.
Results:
The accuracies of DESSAcc and DESSMTE were CoV = 3.7% and CoV = 6.6% respectively, while precision was 3.8%, 3.0%, and 3.1% for DESS, DESSAcc and DESSMTE. T2 repositioning precision was 5.8%.
Conclusion:
The results demonstrate that accurate and precise quantification of cartilage volume is possible using a combination of substantially faster MRI acquisition and post-processing software. Precise measurements of cartilage T2 and volume can be made using the same acquisition.
Keywords: Osteoarthritis, Cartilage, Knee, Magnetic resonance imaging, Accelerated imaging, Segmentation software
Introduction
Knee osteoarthritis (OA) is a common and debilitating disease with a high social and economic impact1. Both conventional radiography and magnetic resonance imaging (MRI) are used to assess structural changes of knee OA with complementary advantages2. Unlike radiography, MRI directly shows most tissues associated with knee OA, such as cartilage, bone marrow lesions (BMLs), menisci, and effusion/synovitis. It is a true three-dimensional (3D) modality with exceptional contrast between the relevant anatomical structures of the OA knee. The principle drawbacks of MRI are the time and expense associated with both the acquisition and the reading of the image, a substantial limit to study power and best experimental design.
For research studies of knee OA, MRI image sets can be evaluated by semi-quantitative scoring systems to assess disease-related structures3. Fully-quantitative software-based methods have also been reported to measure cartilage4,5, BMLs6,7, osteophytes8 and other structures. Unlike semi-quantitative scoring, quantitative methods can provide objective measures of the area, volume, and thickness of diseased and/or normal features. Furthermore, software-based quantitative assessments can be made substantially faster than qualitative readings as computer algorithms are often largely automated, reducing the need for reader effort. Our laboratory has developed several software tools that can produce measurements of cartilage, BML and osteophyte volume in just over 30 min per knee for all three measures5,7,8. Faster assessment translates directly into lower costs for clinical studies of knee OA.
Accelerated MRI techniques provide another approach for reducing the cost of knee OA assessment. Unlike radiography, MR images are not initially acquired in a form that readily resembles the imaged object; instead, the raw data are acquired in k-space which is the Fourier transform of the imaged object. An MRI scanner can be viewed as a machine that Fourier transforms the object placed within it, and for this reason, the final image is obtained by performing an inverse Fourier transform. MRI signals are most often acquired in the form of a k-space line, so that a rectangular k-space (2D imaging), or box-shaped k-space region (3D imaging) is filled, one k-space line at a time, over the duration of a scan. This way of filling k-space is typically referred to as Cartesian sampling. Most accelerated MRI methods function by skipping some of the k-space locations that would normally be acquired. By sampling less data, a given scan can be completed faster9. These methods can be used to reduce the acquisition time, but at a cost to image quality.
The goal of this study is to investigate a unique approach for MRI assessment of knee OA that integrates fast acquisition and post-processing software, along with T2 mapping. We hypothesize that the software tool used to measure cartilage volume mitigates the inevitable loss of image quality resulting from these types of acquisitions. Specifically, the study was designed to measure the precision and accuracy of a faster and more information-dense MRI protocol, and is intended to be a first proof-of-principle study to investigate these methods for knee OA.
Methods
Study sample
We imaged 10 healthy (non-OA) volunteers, following informed consent using an IRB-approved and HIPAA-compliant protocol.
MRI protocols
MR imaging was performed on a 3.0T scanner (Tim Trio, Siemens Medical Solutions, Erlangen, Germany) using an 8-channel knee coil (Invivo Corporation, Gainesville, Florida). A single knee of each subject was scanned using three different MRI protocols, all based on the 3D dual-echo steady state (DESS) pulse sequence.
With DESS, two different types of images are obtained jointly: A ‘fast imaging with steady-state free precession’ (FISP) image and an ‘inverted-FISP’ (PSIF) image. These two images are added in magnitude to create the final DESS image. The two types of signals, FISP and PSIF, are created by the pulse sequence and sampled side-by-side every repetition time (TR). Studies have demonstrated that DESS has similar cartilage volume measurement precision to other pulse sequences10.
Using a regular DESS sequence, the FISP and PSIF are collected over a relatively long period, called readout window, which may extend over many milliseconds. In contrast, in our implementation11 the readout window has much-reduced duration and is repeated several times, in quick succession. Compared to the regular DESS sequence, the change comes at no penalty in scan time and essentially no penalty in signal-to-noise ratio (SNR). While a longer readout generally provides better SNR, several lower-SNR measurements can be combined to reach essentially the same SNR level as the reference case. However, having several individual measurements allows T2 to be estimated11.
A prior method, sometimes called quantitative DESS (qDESS), has also been proposed to generate T2 maps from DESS signals12. qDESS relies on the ratio of PSIF to FISP signals to evaluate T2, but assumptions on T1 and flip angle are required. In contrast, the present multi-echo method uses PSIF and FISP signal change to evaluate T2 mostly immune to errors in expected T1 and/or flip angle value11. By sampling several echoes during TR, with higher bandwidth, both R2 and R2′ can be evaluated from fitting FISP and PSIF decay curves11. Given that T2 = 1/R2 and T2* = 1/(R2+R2′), maps of T2 and T2* can be generated. This approach differs from previous methods such as qDESS that rely on PSIF to FISP ratios to evaluate T212.
In a first protocol, a conventional sagittal 3D DESS sequence was used with the following parameters: resolution = 0.37 (superior/inferior) × 0.46 (anterior/posterior) × 0.70 (left/right) mm3, TR = 16.3 ms, echo time (TE) = 5.3 ms and flip angle 25°. The second protocol used an accelerated DESS (DESSAcc) sequence with identical imaging parameters as DESS but with fewer k-space lines acquired, and therefore is faster (non-uniform sampling with k-space center fully acquired, overall acceleration of 1.6 in each phase-encoding direction, least-square algorithm for reconstruction). In a third protocol, a multi-echo time DESS (DESSMTE) sequence was employed, with imaging parameters identical to the conventional DESS but with modifications to the pulse sequence to make it multi-echo11. All three protocols involved a water-only excitation pulse, for fat suppression (1-2-1 design, 2.38 ms duration).
Compared to the regular DESS sequence, the DESSMTE scan can be acquired with no increase in scan time and essentially no additional noise to the image and provides images with better geometrical fidelity and lower distortions. Of potentially greater significance for knee OA is that the individual measurements obtained can be used to generate quantitative maps of T2, which have been shown to provide useful bio-markers for early knee OA13. To quantify T2 and volume repositioning reproducibility, the DESSMTE was performed twice where the subjects were removed and placed back into the MRI scanner in-between scans. We also assessed the intra-reader DESSMTE “re-segmentation” precision using independent segmentations of the same images. The acquisition times were 11.0, 5.8, and 10.2 min for the DESS, DESSAcc, and DESSMTE respectively.
Image analysis and reader procedure
DESS images were generated by adding a magnitude FISP image to a magnitude PSIF image, and these were analyzed using a previously validated semi-automated software method that quantifies the cartilage volume at specific locations with respect to a 3D coordinate system centered on a single point in the weight-bearing region of the femur. For our study, we used the region defined as z = 0.8, Δz = 0.1, θ = 270°, and Δθ = 100° according to the published method5. The reader (LS) applied the software in a fully blinded method and performed a second reading of all scans after 2 weeks to determine the reader precision. T2 values were calculated using the DESSMTE scans in the segmented regions but constrained to locations within 2.5 mm of the cartilage-bone margin.
Statistical analysis
Accuracy was determined by a comparison of the cartilage volumes between the experimental DESS protocols (DESSAcc and DESSMTE) and the conventional DESS acquisition. Precision was determined by duplicate measurements by a reader (LS) of the scans for each protocol. Average T2 values were determined in the segmented regions on the DESSMTE image sets. The reproducibility of T2 was determined by a comparison of the average T2 values between the two repositioned DESSMTE acquisitions. Accuracy and precisions were characterized using intra-class correlation coefficients, root-mean square standard deviation (RMSSD) and the coefficient of variation (CoV). 95% confidence intervals (95% CI) were calculated using student t-distribution.
Results
Figure 1 shows examples of the three different pulse sequences. The average (SD) age of our subjects was 32.5 (9.1), five were female (50%), and one was African American (10%). Figure 2(a) and (b) show graphs of the cartilage volumes measured from DESSAcc and DESSMTE vs conventional DESS. Figure 2(c) is a graph comparing cartilage T2 measurements made with the two DESSMTE acquisitions. Table I provides the corresponding ICC, RMSSD, and CoV values and reader reproducibility. The average reader time was 12.0, 12.2, and 10.4 min/scan for the DESS, DESSAcc, and DESSMTE respectively.
Fig. 1.
Examples of (a) conventional DESS, (b) DESSAcc, and (c) DESSMTE.
Fig. 2.
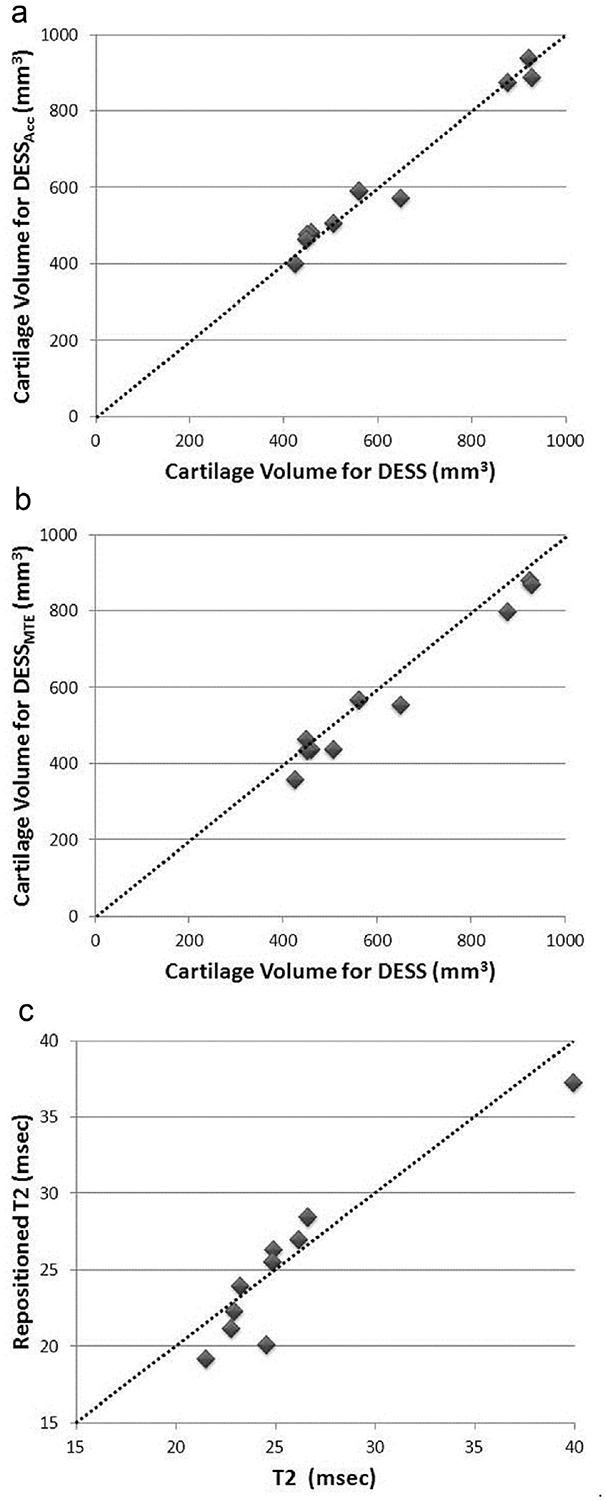
(a). Plot of the cartilage volume from DESSAcc vs the conventional DESS to demonstrate the accuracy of accelerated DESS. The identity line is shown as dashed line. (b). Plot of the cartilage volume from DESSMTE vs the conventional DESS to demonstrate the accuracy of the DESSMTE acquisition. Comparison against the identity line, shown as dashed line, suggests a systematic downward shift of the DESSMTE volume compared to DESS. (c). Plot of T2 precision for the two DESSMTE acquisitions. The identity line is shown as dashed line.
Table I.
Precision and accuracy results
| DESSACC vs DESS accuracy (volume) | DESSMTE vs DESS accuracy (volume) | DESSMTE repositioning precision (volume) | DESS reader precision (volume) | DESSACC reader precision (volume) | DESSMTE reader precision (volume) | DESSMTE repositioning precision (T2) | DESSMTE re-segmentation precision (T2) | |
|---|---|---|---|---|---|---|---|---|
| ICC | 0.99 | 0.98 | 0.99 | 0.99 | 0.99 | 0.99 | 0.92 | 0.99 |
| RMSSD | 23.0 mm3 | 39.6 mm3 | 18.3 mm3 | 23.6 mm3 | 18.7 mm3 | 17.8 mm3 | 1.46 ms | 0.33 ms |
| CoV | 3.7% | 6.6% | 3.2% | 3.8% | 3.0% | 3.1% | 5.8% | 1.3% |
| 95% CI | (−20.3 mm3, 28.3 mm3) | (16.6 mm3, 70.0 mm3) | (−9.2 mm3, 27.4 mm3) | (−19.5 mm3, 29.7 mm3) | (−25.4 mm3, 13.5 mm3) | (−2.0 mm3, 20.5 mm3) | (−0.83 ms, 2.13 ms) | (−0.53 ms, 0.08 ms) |
The accuracy for DESSAcc was excellent (ICC = 0.99, CoV = 3.4%) suggesting that DESSAcc can be used as an accurate substitute for conventional DESS. DESSMTE accuracy was lower compared to DESSAcc. However the data indicate a systematic decrease in segmented volume of the DESSMTE scans compared to DESS. The precision was excellent for all three protocols and the reader times demonstrate that both DESSAcc and DESSMTE can be assessed as efficiently as the conventional DESS.
Discussion
Accurate and precise measurements of femoral cartilage are possible using DESSAcc, an MRI acquisition protocol that requires approximately half the acquisition time as conventional DESS. The DESSMTE offers precise measurements of both T2 and cartilage volume with no additional scan time compared to DESS. The sequence is a reasonably-simple modification of basic gradient-echo sequences, and we will strive to make it available through the Siemens ‘work in progress’ (WIP) mechanism. A CoV of 5.8% is consistent with T2 precision reported in the literature13. A much reduced re-segmentation precision suggests that the error is dominated by noise associated with the image acquisition. Similar average reader times for DESS and the two experimental protocols imply that further acceleration may also be possible without additional burden to the reader. We did observe a decrease in accuracy for DESSMTE, however this appears to be due to a systematic shift [Fig. 2(b)], (also evident from the 95% CI values) which could be corrected in future studies should it prove to be consistent. The excellent reader and repositioning reproducibility values for DESSMTE suggest that reduced precision is not the cause.
Ultimately a comprehensive study will be necessary to determine an optimal “protocol” that minimizes the total cost while maintaining performance. For example, it may be possible to reduce the acquisition time to an even smaller fraction of the conventional scan, but with a substantial increase in the reader time. Even less total (acquisition plus reader) time may be possible if some loss of precision and or accuracy is acceptable. Such issues will be best addressed by considering the individual costs of the acquisition and readers separately and the needs of the user. We would anticipate that this methodology would be used first in clinical trials and other studies of knee OA where a small loss of precision may be acceptable if it comes with a substantial reduction in costs. A decrease in responsiveness to the measurement might be offset by a reduction of per-subject cost allowing for an increased number of subjects and, therefore, a more highly powered study.
In the future it will be possible to combine DESSAcc and DESSMTE into a single fast protocol that produces images appropriate for both cartilage volume and T2 measurements. It may also be possible to use such an acquisition to produce T2-weighted images with which BMLs and effusion/synovitis can be quantitatively assessed with software methods. The ultimate goal of this effort will be to define a single fast acquisition protocol that can produce appropriate images for measuring many structural features of the OA knee.
Images produced by accelerated MRI have additional noise that increases as the scan time decreases. Such images may not be ideal for traditional clinical evaluation particularly for higher levels of acceleration. We do not consider this a limitation since the post processing software can be designed to overcome the reduction in image quality. The method will focus on producing the best measurements of knee OA-related structures rather than providing an MRI of the knee that can be used in place of the conventional scan. As with Dual X-ray Absorptiometry (DXA) the images will not be of diagnostic quality but will deliver the clinician or researcher with a set of quantitative measurements.
There are several limitations to our study. The number of subjects was low (N = 10) and we used healthy volunteers rather than diseased individuals. Knees with thinning cartilage may present increased difficulties to the segmentation software. The study is cross-sectional; a longitudinal assessment would be more relevant for clinical trials of OA. Nevertheless, we do provide a proof-of-principle validation and if successful, this approach could dramatically lower the cost of imaging for future studies of knee OA.
Going forward, we expect to test the method in a longitudinal study using subjects with knee OA. There are numerous potential modifications to both the acquisition and post-processing software than can be explored to determine an optimal ‘protocol’ for low-cost MRI. Testing the method using OA subjects will also permit validation of measuring other OA-related structures including BMLs and osteophytes; we have validated software tools for these features7,8. Finally, we expect to investigate further the possibility of additional reductions to cost by integrating the methods with a peripheral/extremity MRI scanner.
Conclusions
We have demonstrated that combining accelerated and multi-echo time MRI with automated post processing software is a promising approach for addressing the high cost of MRI in studies of knee OA.
Acknowledgements
We acknowledge Charles Ratzlaff for help reviewing the manuscript, Lori Chibnik for help with the statistical analysis, and Quinley Miao for her invaluable assistance with the image analysis.
Role of funding source
This study was supported by the National Institutes of Health grants R01AR056664, R01CA149342, R01EB010195, R21EB019500 and P41EB015898.
Footnotes
Conflict of interest statement
The authors declare that they have no conflicting interests.
References
- 1.Hunter DJ, Schofield D, Callander E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol 2014;10:437–41. [DOI] [PubMed] [Google Scholar]
- 2.Guermazi A, Hayashi D, Eckstein F, Hunter DJ, Duryea J, Roemer FW. Imaging of osteoarthritis. Rheum Dis Clin North Am 2013;39:67–105. [DOI] [PubMed] [Google Scholar]
- 3.Hunter DJ, Guermazi A, Lo GH, Grainger AJ, Conaghan PG, Boudreau RM, et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score). Osteoarthritis Cartilage 2011;19:990–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckstein F, Glaser C. Measuring cartilage morphology with quantitative magnetic resonance imaging. Semin Musculoskelet Radiol 2004;8:329–53. [DOI] [PubMed] [Google Scholar]
- 5.Duryea J, Iranpour-Boroujeni T, Collins JE, Vanwynngaarden C, Guermazi A, Katz JN, et al. Local area cartilage segmentation: a semiautomated novel method of measuring cartilage loss in knee osteoarthritis. Arthritis Care Res (Hoboken) 2014;66: 1560–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roemer FW, Khrad H, Hayashi D, Jara H, Ozonoff A, Fotinos-Hoyer AK, et al. Volumetric and semiquantitative assessment of MRI-detected subchondral bone marrow lesions in knee osteoarthritis: a comparison of contrast-enhanced and non-enhanced imaging. Osteoarthritis Cartilage 2010;18:1062–6. [DOI] [PubMed] [Google Scholar]
- 7.Ratzlaff C, Guermazi A, Collins J, Katz JN, Losina E, Vanwyngaarden C, et al. A rapid, novel method of volumetric assessment of MRI-detected subchondral bone marrow lesions in knee osteoarthritis. Osteoarthritis Cartilage 2013;21:806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hakky M, Jarraya M, Ratzlaff C, Guermazi A, Duryea J. Validity and responsiveness of a new measure of knee osteophytes for osteoarthritis studies: data from the osteoarthritis initiative. Osteoarthritis Cartilage 2015;23:2199–205. [DOI] [PubMed] [Google Scholar]
- 9.Madore B. Fast imaging techniques In: Kwong R, Ed. Cardiovascular Magnetic Resonance Imaging. Totowa, New Jersey: Humana Press; 2008:211–36. [Google Scholar]
- 10.Eckstein F, Kunz M, Schutzer M, Hudelmaier M, Jackson RD, Yu J, et al. Two year longitudinal change and test-retest-precision of knee cartilage morphology in a pilot study for the osteoarthritis initiative. Osteoarthritis Cartilage 2007;15: 1326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng C-C, Mei C-S, Duryea J, Chung H-W, Chao T-C, Panych LP, et al. Dual-pathway multi-echo sequence for simultaneous frequency and T2 mapping. J Magn Reson 2016;265:117–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welsch GH, Scheffler K, Mamisch TC, Hughes T, Millington S, Deimling M, et al. Rapid estimation of cartilage T2 based on double echo at steady state (DESS) with 3 Tesla. Magn Reson Med 2009;62:544–9. [DOI] [PubMed] [Google Scholar]
- 13.Baum T, Joseph GB, Karampinos DC, Jungmann PM, Link TM, Bauer JS. Cartilage and meniscal T2 relaxation time as noninvasive biomarker for knee osteoarthritis and cartilage repair procedures. Osteoarthritis Cartilage 2013;21: 1474–84. [DOI] [PMC free article] [PubMed] [Google Scholar]


