Abstract
Purpose
To compare uncorrected and best-corrected visual acuity, low contrast acuity, residual refraction and ocular biometry after low cylinder power toric intraocular lens (IOL) or non-toric IOL implantation.
Patients and Methods
This was a non-interventional comparative study of visual outcomes after uncomplicated cataract or refractive lens exchange surgery with either a low cylinder (Low_Cyl) or non-toric (Non_Toric) IOL of similar design implanted (AcrySof® T2 IQ Toric IOL and AcrySof® IQ IOL). Subjects in both groups had to have been eligible for the low cylinder IOL based on biometry. They had to have uncorrected distance visual acuity (UDVA) of 20/32 (0.2 logMAR) or better at the time of their single diagnostic study visit. Clinical evaluation included the manifest refraction, visual acuity (VA), low contrast VA and ocular biometry.
Results
A total of 94 eyes were enrolled, 51 Low_Cyl and 43 Non_Toric. The mean manifest refractive cylinder was statistically significantly lower (~0.25 D) in the Low_Toric group (p < 0.01) and significantly more eyes had 0.25 D or less of refractive cylinder in that group (p = 0.03). The orientation of the preoperative anterior corneal astigmatism was a significant cofactor, with the difference between groups more evident when astigmatism was against the rule. Uncorrected high contrast visual acuity was statistically significantly better in the Low_Toric group (p = 0.02) as was the percentage of eyes with 20/20 visual acuity (p = 0.05). Uncorrected low contrast visual acuity was not statistically significantly different in mesopic or photopic conditions.
Conclusion
The low cylinder power toric IOL provided better uncorrected visual acuity and lower residual refractive cylinder than a similar non-toric IOL after cataract surgery.
Keywords: toric IOL, low cylinder, low contrast acuity, astigmatism correction
Plain Language Summary
At the time of cataract surgery patients typically have an intraocular lens (IOL) implanted to replace the cloudy lens that was removed. This is often chosen by the surgeon to provide clear vision at distance. Some IOLs can correct astigmatism, an optical error of the eye that reduces vision when glasses are not worn. These are called toric IOLs. This study was conducted to determine if correcting a very low amount of astigmatism made any difference to the vision of patients after surgery. A group with a lens to correct low amounts of astigmatism was compared to a very similar group that did not have their astigmatism corrected. Results suggested that the group with the lens to correct low astigmatism had slightly better visual acuity when not wearing glasses, though the differences were small (about a half a line on the eye chart, on average). The glasses prescription after surgery also showed that the astigmatism in the eyes with the low powered toric IOL was lower than for the IOL that did not correct astigmatism. This suggests that even in cases of patients with low astigmatism there may be some value in correcting it at the time of cataract surgery.
Introduction
Modern cataract and refractive lens exchange surgeries are appropriately considered refractive procedures. Current intraocular lens (IOL) power calculation formulas have significantly increased the likelihood of hitting the planned spherical equivalent refractive target in most eyes.1 Formulas for calculating cylinder power and orientation for toric IOLs have also improved, with most now including consideration of posterior corneal astigmatism (PCA).2,3 This has led to a corresponding improvement in clinical outcomes in astigmatic eyes.4
Significant factors that can adversely affect the postoperative refractive outcomes of toric IOLs remain. PCA is difficult to accurately measure, so average or calculated values are generally used as a proxy.5 This means that patients with atypical PCA values may not have optimal results. Accurate and precise lens orientation at the time of surgery is also important, as is the stability of the IOL once implanted.6 Lens misorientation, either due to calculation error or surgical error, can significantly alter the refractive effect of a toric IOL.7 The variability in surgically induced astigmatism is also recognized as a limiting factor in achieving optimal results.7 In general, these effects are not large, so good results with toric IOLs are achieved. However, they are often considered limiting factors in trying to correct lower levels of astigmatism; when attempting to correct lower magnitudes of astigmatism, the relative effect of small variances is higher.
Low levels of corneal astigmatism are often corrected at the time of cataract surgery with limbal arcuate or relaxing incisions (LRIs). However, results obtained with LRIs are reported to be more variable than those achieved with toric IOLs, though comparisons that are limited to very low levels of astigmatism are difficult to find in the literature.8
One IOL available for the correction of low cylinder is the AcrySof® T2 IQ Toric IOL (Alcon, Fort Worth, USA). The lens has a cylinder power of 1.0 D at the IOL plane, equivalent to about 0.68 D at the corneal plane for a nominal eye. This toric IOL is available as a monofocal in many regions of the world and the same optics have been incorporated into several multifocal and extended depth of focus IOL designs. We found only two articles in the literature reporting the clinical outcomes with this IOL, but neither included a control group.9,10 The current study was designed to assess the refractive and visual acuity outcomes in eyes implanted with the monofocal version of this low cylinder toric intraocular lens and to compare those outcomes to the outcomes in eyes with similar biometry that were implanted with a monofocal non-toric IOL of similar manufacture and design.
Patients and Methods
This study was a non-interventional comparative study of visual outcomes after successful bilateral cataract surgery or refractive lens exchange surgery with IOL implantation. The visual acuity and manifest refractions of subjects implanted with the T2 IOL, the Low_Toric group, were compared to a group of subjects with similar biometry who were implanted with a non-toric IOL of similar manufacture and design (AcrySof® IQ IOL, Alcon, Fort Worth, USA), the Non_Toric group. Regional ethics committee approval through the Regionale komiteer for medisinsk og helsefaglig forskningsetikk (REK), Norway was applied for and obtained before patients were enrolled.
All subjects signed an appropriate informed consent document. As the study was non-interventional, there was no requirement to register it as a clinical trial. The study was conducted in accordance with the tenets of the Declaration of Helsinki and good clinical practice. Data are not available for sharing.
Eligible test subjects had to have had previous uncomplicated implantation of one of the two IOLs above in one or both eyes. They all had to have been candidates for the T2 IOL, based on corneal astigmatism and toric IOL planning performed using the Barrett Toric IOL calculator. The Non_Toric group consisted of patients who declined the toric option, for financial or other reasons. Subjects were limited to those who had uncomplicated surgery with normal outcomes, and a best-corrected visual acuity (VA) of 20/32 (0.2 logMAR) or better in any enrolled eye, measured at the time of the diagnostic visit. For eyes implanted with the toric IOL, the VERION™ Image Guided System (Alcon, Fort Worth, USA) had to have been used for intraoperative IOL alignment. Subjects with previous corneal surgery or ocular pathology likely to compromise postoperative visual acuity were not included. All subjects had surgery by the same surgeon (KGG), using a 2.2 mm superior (12:00) corneal incision.
Subjects were identified based on a search of historical clinical records from the previous 3 years of surgery and if they met the criteria above they were asked to participate in a single postoperative diagnostic visit. Clinical evaluations included the manifest refraction and both corrected and uncorrected distance visual acuity. Uncorrected low contrast visual acuity was also tested in both photopic and mesopic conditions. The M&S Technologies Clinical Trial Suite (Niles, IL, USA) was used for visual acuity data collection. Preoperative ocular biometry was extracted from the subjects’ clinical records, and ocular biometry was also collected at the diagnostic visit using the same instrument (Lenstar LS900, Haag-Streit, Kõniz, Switzerland). One optometrist performed all the examinations. Clinical data were collected in Excel files and imported into MS Access for preliminary analysis (both Microsoft Corp., Redmond, WA, USA). Between-group comparisons were performed using the STATISTICA data analysis software system, version 12 (TIBCO Software Inc., Palo Alto, CA, USA). Subgroup analysis was conducted based on the categorization of the measured preoperative anterior corneal astigmatism as with the rule (WTR, steep meridian within 30 degrees of vertical), against-the-rule (ATR, steep meridian within 30 degrees of horizontal) or oblique (OBL, remaining eyes). Continuous variables were compared using an analysis of variance (ANOVA) while non-parametric variables were compared using the Chi-squared test. In both instances, statistical significance was based on p ≤ 0.05.
Results
A total of 94 eyes from 82 subjects met the relevant inclusion and exclusion criteria and were enrolled. The Low_Toric group included 51 eyes of 45 subjects while the Non_Toric group included 43 eyes of 37 subjects; the ratio of monocular to binocular enrolled subjects was not statistically significantly different between groups. Demographic, biometric and preoperative details are summarized in Table 1. There were significantly more male subjects in the Low_Toric group, but otherwise, the groups were well matched, particularly with regard to mean keratometry and corneal astigmatism. As expected, based on the inclusion criteria, there was a low range of corneal astigmatism in both groups. There were no adverse events identified at any of the diagnostic visits.
Table 1.
Demographics and Preoperative Details
| Low_Toric | Non_Toric | p | |
|---|---|---|---|
| Patients/eyes | 45/51 | 37/43 | 0.71 |
| Male/female | 23/28 | 11/32 | 0.05 |
| Age (Years) | 72 ± 5 (57 to 84) | 72 ± 9 (42 to 82) | 0.93 |
| MRSE (D) | −0.31 ± 2.43 (−4.88 to 3.63) | 0.57 ± 2.19 (−6.00 to 3.63) | 0.07 |
| Average keratometry (D) | 43.68 ± 1.53 (40.21 to 46.95) | 43.74 ± 1.26 (41.41 to 46.41) | 0.82 |
| Corneal astigmatism (D) | 0.63 ± 0.39 (0.07 to 1.46) | 0.75 ± 0.42 (0.10 to 1.45) | 0.16 |
| Anterior corneal astigmatism orientation (WTR/OBL/ATR) | 20/21/10 | 17/21/5 | 0.54 |
| Axial length (mm) | 23.73 ± 1.12 (21.82 to 26.56) | 23.47 ± 090 (21.66 to 25.78) | 0.23 |
| IOL sphere power (D) | 20.8 ± 3.8 (13.0 to 27.0) | 21.5 ± 3.3 (12.5 to 26.5) | 0.45 |
Note: Unless otherwise stated, values are: mean ± standard deviation (range).
Abbreviations: MRSE, mean refraction, spherical equivalent; D, diopter; mm, millimeter; IOL, intraocular lens; WTR, with the rule; OBL, oblique; ATR, against the rule.
The postoperative refractive data by group are summarized in Table 2. Follow-up time varied, but there was no statistically significant difference between the groups. Only two eyes (both in the Non_Toric group) were seen before 90 days postoperative. The mean manifest refractive cylinder was statistically significantly lower in the Low_Toric group, with about a 0.25 D difference between the groups; the clinical significance of such a difference might be debated. The only other statistically significant difference was in the percentage of eyes with 0.25 D or less of refractive cylinder; the percentage in the Low_Toric group was 23% higher than in the Non_Toric group. A higher percentage of Low_Toric eyes also had refractive cylinder ≤0.50 D, but this difference was not statistically significant (p = 0.09).
Table 2.
Postoperative Refractive Summary by Group
| Low_Toric | Non_Toric | p | |
|---|---|---|---|
| Follow-up time (days) | 522 ± 205 (132 to 791) | 452 ± 268 (35 to 903) | 0.16 |
| MRSE (D) | 0.04 ± 0.38 (−0.63 to 1.00) | 0.18 ± 0.49 (−1.50 to 1.25) | 0.13 |
| Manifest refractive cylinder (D) | 0.31 ± 0.28 (0.00 to 1.00) | 0.53 ± 0.38 (0.0 to 1.25) | < 0.01 |
| Eyes with absolute MRSE ≤ 0.50 D | 40 (75%) | 31 (72%) | 0.48 |
| Eyes with ≤ 0.25 D of cylinder | 33 (65%) | 18 (42%) | 0.03 |
| Eyes with ≤ 0.50 D of cylinder | 43 (84%) | 30 (70%) | 0.09 |
Note: Unless otherwise stated, values are: mean ± standard deviation (range).
Abbreviations: MRSE, mean refraction, spherical equivalent; D, diopter.
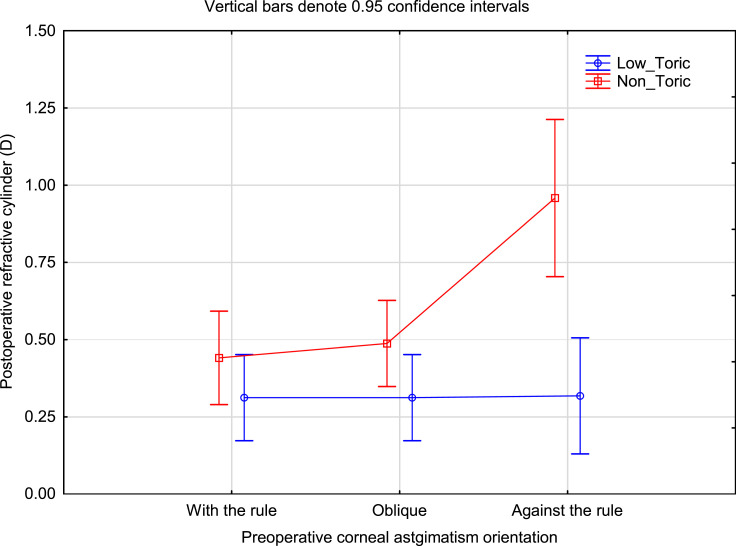
Figure 1 shows the mean postoperative refractive cylinder by group categorized by whether the preoperative corneal cylinder was WTR, OBL or ATR. There was a statistically significant difference by group (p < 0.01), and by orientation (p = 0.02). There was also a significant interaction effect, indicating that orientation had a statistically significantly different effect by group. This is particularly evident for ATR orientation, though it should be noted that there were a low number of eyes in the Non_Toric ATR category.
Figure 1.
Residual refractive cylinder by group and preoperative anterior corneal astigmatism orientation.
Abbreviation: D, diopter.
Table 3 shows the summary of the postoperative VA results by group. Uncorrected high contrast VA was statistically significantly better in the Low_Toric group, as was the percentage of eyes with 20/20 visual acuity. There were no statistically significant differences in best-corrected visual acuity or low contrast visual acuity in photopic or mesopic conditions. The difference between the uncorrected VA and the best-corrected VA was about a half line in the Low_Toric group and a full line in the Non_Toric group.
Table 3.
Postoperative Visual Acuity by Group
| Low_Toric | Non_Toric | p | |
|---|---|---|---|
| Uncorrected distance VA (logMAR) | 0.01 ± 0.12 (−0.18 to 0.34) | 0.07 ± 0.13 (−0.10 to 0.44) | 0.02 |
| Best-corrected distance VA (logMAR) | −0.05 ± 0.06 (−0.20 to 0.14) | −0.03 ± 0.05 (−0.10 to 0.08) | 0.07 |
| Uncorrected distance VA, low contrast photopic (logMAR) | 0.37 ± 0.18 (0.10 to 0.80) | 0.44 ± 0.18 (0.10 to 0.92) | 0.09 |
| Uncorrected distance VA, low contrast mesopic (logMAR) | 0.59 ± 0.13 (0.36 to 0.94) | 0.62 ± 0.16 (0.32 to 1.10) | 0.13 |
| Eyes with 20/20 or better uncorrected VA | 36 (71%) | 22 (51%) | 0.05 |
Note: Unless otherwise stated, values are: mean ± standard deviation (range).
Abbreviations: VA, visual acuity; logMAR, log of the minimum angle of resolution.
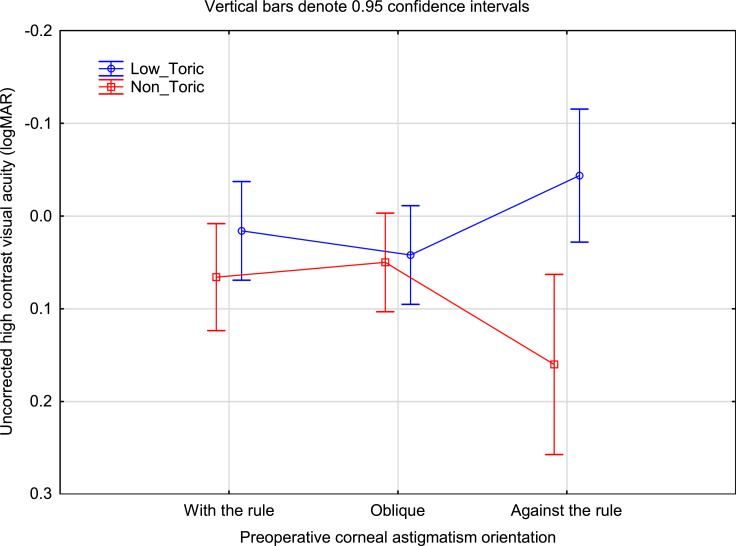
Figure 2 shows the mean uncorrected visual acuity by group and preoperative corneal cylinder orientation. As with the refractive cylinder data, the difference in the ATR orientation category was highest. Interestingly, the uncorrected VA in the Low_Toric group with ATR orientation appeared slightly better than for the Low_Toric group in the other orientations; no such difference was noted in the refractive cylinder data in Figure 1 (ie, refractive cylinder in the Low_Toric group appeared similar for all corneal cylinder orientations).
Figure 2.
Uncorrected high contrast VA by group and preoperative anterior corneal astigmatism orientation.
Abbreviation: logMAR, log of the minimum angle of resolution.
Table 4 shows the surgically induced astigmatism (SIA) data related to both groups, based on the vector difference between the preoperative and postoperative anterior corneal astigmatism. There was no statistically significant difference between groups in the overall magnitude of the SIA, but there was a difference in the y-coordinate of the centroid, with the y-coordinate magnitude in the Non_Toric group being slightly larger. This suggests a more oblique (45/135) orientation to the SIA, since the values are based on a double-angle plot.
Table 4.
SIA Data by Group
| Low_Toric | Non_Toric | p | |
|---|---|---|---|
| SIA vector magnitude (D) | 0.51 ± 0.29 (0.04 to 1.34) | 0.62 ± 0.37 (0.10 to 1.60) | 0.09 |
| SIA x coordinate (D, double angle) | −0.29 ± 0.40 (−1.28 to 0.63) | −0.15 ± 0.49 (−1.32 to 1.12) | 0.12 |
| SIA y coordinate (D, double angle) | −0.06 ± 0.33 (−0.82 to 0.53) | −0.23 ± 0.48 (−1.32 to 0.85) | 0.05 |
Note: Unless otherwise stated, values are: mean ± standard deviation (range).
Abbreviations: SIA, surgically induced astigmatism; D, diopter.
Discussion
We believe this is the first comparative study of the use of a very low cylinder power toric IOL as an alternative to a monofocal IOL without use of LRIs. The groups we included were well matched for biometry and we have shown a significant improvement in both residual refractive cylinder and uncorrected distance visual acuity. While the differences in low contrast visual acuity metrics were not statistically significantly different, the trend was towards a small improvement.
Most importantly, perhaps, was the fact that significant improvements were more obvious in the subgroup of patients with ATR anterior corneal astigmatism. We believe this is expected, given that the surgeon’s incision was at 90 degrees. Making an incision on the flat meridian is likely to increase the astigmatism on the cornea, which would remain untreated when using a non-toric monofocal IOL. Given this, it is reasonable to suspect that, for surgeons using a temporal incision, patients with WTR corneal astigmatism will have a better result when a low cylinder toric IOL is used instead of a non-toric IOL. However, we cannot ignore the fact that leaving ATR astigmatism uncorrected may be a contributing factor. Further research would be required to determine the relative contributions of these two factors.
Planning for low cylinder power correction is more difficult than higher correction because the variables that affect toric IOL outcomes, while generally small, have a relatively larger effect. This means that IOL cylinder power calculations and SIA calculation are more critical. In the current study, an IOL cylinder power calculator that compensated for effective lens position and posterior corneal astigmatism was used for surgical planning.11 IOL orientation was determined using image-based software, which has been demonstrated to provide more precise positioning than manual marks.12 SIA remains an issue that is difficult to address, as the corneal response to incisions varies significantly from patient to patient. However, improving the nominal value of the SIA included in toric IOL calculators may be of some help. For SIA, it may be that the method proposed by Holladay and Pettit might improve results.13 Again, this is something that would require further research. Conversely, IOL misorientation with higher cylinder power IOLs has a higher likelihood of resulting in a clinically significant residual astigmatic error.
Results here appear similar to those achieved by Aujla et al9 and Levitz et al.10 The preoperative corneal cylinder in these studies were comparable to the current study, as was the postoperative residual refractive astigmatism. Aujla et al showed a similar half-line difference between uncorrected and best-corrected VA with the low cylinder powered toric IOL.
Arguably the most common alternative to a low cylinder power toric IOL would be using LRIs on the steep meridian to flatten it. Mean results achieved can be similar to those reported here, but in general there is more variability in the outcomes with corneal incisions relative to toric IOLs, particularly when the incisions are made with a blade.8 Other studies have found that femtosecond LRIs can provide results similar to a toric IOL,14–16 but several authors have noted that the additional surgical time requirement, incision planning and laser system cost might argue for toric IOL use. A meta-analysis of studies that included LRIs made with both manual and femtosecond laser systems suggests that the likelihood of achieving a residual refractive cylinder of ≤0.50 D is higher with a toric IOL.17
There are limitations to this study. Perhaps most importantly, it was difficult to obtain a significant sample size of matched patients without a large range of follow-up times, but it was felt that the tradeoff for good biometric matches was important. The overall number of eyes was considered adequate, but the distribution of preoperative corneal astigmatism orientation in both groups meant that there were fewer ATR eyes. This limited the ability to further sub-analyze results by orientation. In addition, we collected only objective visual acuity and refractive data; patient satisfaction was not measured.
Conclusion
The use of a low cylinder power toric IOL provided improved uncorrected visual acuity and reduced residual refractive cylinder after cataract surgery, relative to a similar non-toric IOL.
Acknowledgments
Steffen Østenstad, MSc, of IFocus Øyeklinikk AS assisted with diagnostic testing and data collection/checking. Brad Hall, PhD, of Sengi Clinical, aided in the preparation of this manuscript.
Disclosure
Drs. Gundersen and Potvin are consultants to Alcon. This work was supported by as an investigator-initiated study grant funded by Alcon (IIT#49,520,381). Richard Potvin reports grants from Alcon, during the conduct of the study; personal fees from Alconand Carl Zeiss Meditec, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Kane JX, Chang DF. IOL power formulas, biometry, and intraoperative aberrometry: a review. Ophthalmology. 2020;S0161-6420(20)30789–2. doi: 10.1016/j.ophtha.2020.08.010 [DOI] [PubMed] [Google Scholar]
- 2.Canovas C, Alarcon A, Rosén R, et al. New algorithm for toric intraocular lens power calculation considering the posterior corneal astigmatism. J Cataract Refract Surg. 2018;44(2):168–174. doi: 10.1016/j.jcrs.2017.11.008 [DOI] [PubMed] [Google Scholar]
- 3.Ferreira TB, Ribeiro P, Ribeiro FJ, O’Neill JG. Comparison of astigmatic prediction errors associated with new calculation methods for toric intraocular lenses. J Cataract Refract Surg. 2017;43(3):340–347. doi: 10.1016/j.jcrs.2016.12.031 [DOI] [PubMed] [Google Scholar]
- 4.Gundersen KG, Potvin R. Clinical outcomes with toric intraocular lenses planned using an optical low coherence reflectometry ocular biometer with a new toric calculator. Clin Ophthalmol. 2016;10:2141–2147. doi: 10.2147/OPTH.S120414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferreira TB, Ribeiro P, Ribeiro FJ, O’Neill JG. Comparison of methodologies using estimated or measured values of total corneal astigmatism for toric intraocular lens power calculation. J Refract Surg. 2017;33(12):794–800. doi: 10.3928/1081597X-20171004-03 [DOI] [PubMed] [Google Scholar]
- 6.Kaur M, Shaikh F, Falera R, Titiyal JS. Optimizing outcomes with toric intraocular lenses. Indian J Ophthalmol. 2017;65(12):1301–1313. doi: 10.4103/ijo.IJO_810_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Potvin R, Kramer BA, Hardten DR, Berdahl JP. Factors associated with residual astigmatism after toric intraocular lens implantation reported in an online toric intraocular lens back-calculator. J Refract Surg. 2018;34(6):366–371. doi: 10.3928/1081597X-20180327-01 [DOI] [PubMed] [Google Scholar]
- 8.Leon P, Pastore MR, Zanei A, et al. Correction of low corneal astigmatism in cataract surgery. Int J Ophthalmol. 2015;8(4):719–724. doi: 10.3980/j.issn.2222-3959.2015.04.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aujla JS, Vincent SJ, White S, Panchapakesan J. Cataract surgery in eyes with low corneal astigmatism: implantation of the acrysof IQ toric SN6AT2 intraocular lens. J Ophthalmic Vis Res. 2014;9:324–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levitz L, Reich J, Roberts K, Hodge C. Evaluation of toric intraocular lenses in patients with low degrees of astigmatism. Asia Pac J Ophthalmol (Phila). 2015;4(5):245–249. doi: 10.1097/APO.0000000000000112 [DOI] [PubMed] [Google Scholar]
- 11.Ribeiro FJ, Ferreira TB, Relha C, Esteves C, Gaspar S. Predictability of different calculators in the minimization of postoperative astigmatism after implantation of a toric intraocular lens. Clin Ophthalmol. 2019;13:1649–1656. doi: 10.2147/OPTH.S213132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Webers VSC, Bauer NJC, Visser N, Berendschot TTJM, van den Biggelaar FJHM, Nuijts RMMA. Image-guided system versus manual marking for toric intraocular lens alignment in cataract surgery. J Cataract Refract Surg. 2017;43(6):781–788. doi: 10.1016/j.jcrs.2017.03.041 [DOI] [PubMed] [Google Scholar]
- 13.Holladay JT, Pettit G. Improving toric intraocular lens calculations using total surgically induced astigmatism for a 2.5 mm temporal incision. J Cataract Refract Surg. 2019;45(3):272–283. doi: 10.1016/j.jcrs.2018.09.028 [DOI] [PubMed] [Google Scholar]
- 14.Yoo A, Yun S, Kim JY, Kim MJ, Tchah H. Femtosecond laser-assisted arcuate keratotomy versus toric IOL implantation for correcting astigmatism. J Refract Surg. 2015;31(9):574–578. doi: 10.3928/1081597X-20150820-01 [DOI] [PubMed] [Google Scholar]
- 15.Titiyal JS, Khatik M, Sharma N, et al. Toric intraocular lens implantation versus astigmatic keratotomy to correct astigmatism during phacoemulsification. J Cataract Refract Surg. 2014;40(5):741–747. doi: 10.1016/j.jcrs.2013.10.036 [DOI] [PubMed] [Google Scholar]
- 16.Wortz G, Gupta PK, Goernert P, et al. Outcomes of femtosecond laser arcuate incisions in the treatment of low corneal astigmatism. Clin Ophthalmol. 2020;14:2229–2236. doi: 10.2147/OPTH.S264370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lake JC, Victor G, Clare G, Porfírio GJ, Kernohan A, Evans JR. Toric intraocular lens versus limbal relaxing incisions for corneal astigmatism after phacoemulsification. Cochrane Database Syst Rev. 2019;12(12):CD012801. doi: 10.1002/14651858.CD012801.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]